Abstract
Dengue vaccines are currently being tested in clinical trials but public health specialists are already working on delivery strategies for a rapid roll-out to the people who need them most. Gozde Zorlu and Fiona Fleck report.
“The goal is to make the dengue vaccine available as early as 2015.”
Michael Watson
“The proof of a safe and efficacious dengue vaccine has yet to be made.”
Joachim Hombach
For Luiz Jacintho da Silva, director of the Dengue Vaccine Initiative, the spread of dengue is a matter of the gravest concern. “Over the past few decades the disease has spread dramatically,” says da Silva, noting in particular the toll it has taken on the urban poor living in the shanty towns of Asia and Latin America.
Dengue, a mosquito-borne viral infection, causes severe flu-like symptoms, but can develop into potentially deadly haemorrhagic fever. It is carried mainly by the Aedes aegyptii mosquito, which breeds in water-filled containers such as discarded tyres, tin cans or oil drums. The estimated incidence of the disease has increased 30-fold over the past 50 years with 50 million dengue infections now occurring worldwide each year.
Because dengue is caused by four serologically related but distinct viruses, an effective dengue vaccine needs to induce a protective immune response against all four viruses simultaneously. In drug industry parlance it must be tetravalent. Until now this hurdle has been insurmountable, but French vaccine company Sanofi-Pasteur announced this year that it has a viable tetravalent dengue vaccine undergoing phase III trials in Australia.
This means that in smaller trials the vaccine has been shown capable of producing an immune response and that it is now a matter of confirming those results by demonstrating protection against the disease and monitoring side-effects in a larger group. “The goal is to make the dengue vaccine available as early as 2015,” says Michael Watson, vice president of global immunization policy at Sanofi-Pasteur.
“In the past, when new vaccines were developed, it took years for them to reach people in developing countries on a large scale. The vaccine for hepatitis B took more than 10 years and one for HiB (Haemophilus influenzae type b) also took a long time, despite a high disease burden,” says Joachim Hombach, acting head of WHO's Initiative for Vaccine Research.
“A future dengue vaccine would try to repeat what has been done with vaccines for rotavirus and pneumococcal disease, when the time it took between licensing and introduction of the vaccine in developing countries was massively reduced,” Hombach says.
The Dengue Vaccine Initiative, a Bill and Melinda Gates-funded project launched in February of this year, headed by da Silva, works towards this goal. “We are working with public health programmes, vaccine producers and international organizations, such as WHO, to make sure the dengue vaccine will have an early introduction,” da Silva says.
Sanofi-Pasteur is keen to move forward with the preparations for roll-out and – instead of going the usual route of so-called travel vaccines where wealthy westerners get the vaccine first and the endemic populations are the last to benefit – it wants the vaccine to go straight to the endemic countries. As laudable as this intention may be, it raises several issues regarding distribution, notably with regard to supply and demand.
“There will not be sufficient vaccine for all in the beginning,” says da Silva. This means that there are going to be some difficult decisions about who gets it first.
Unfortunately, to make those decisions, Sanofi-Pasteur and its partners need epidemiological information that is currently unavailable. “Available epidemiological data, with few exceptions, are inadequate,” says da Silva. “They are barely sufficient to make educated guesses on vaccination policies.” One of the reasons for this is the way dengue tends to blend into the background. “The disease can be easily misdiagnosed due to the fact that its clinical features are common to a host of other infectious diseases,” da Silva says.
Another reason for the paucity of data is the lack of disease surveillance in endemic countries. According to Ciro de Quadros, executive vice president of the Sabin Vaccine Institute, Africa is a matter of particular concern in this regard. “No countries have perfect data on dengue,” says WHO epidemiologist and Cambodia field officer Steve Bjorge, who points out that most countries rely on clinical diagnosis supplemented by sentinel sampling of small numbers of cases for laboratory serotyping. While such information can give an impression of the severity and importance of diseases and tell us something about trends over time, it is insufficient for detailed epidemiological analysis.
Given the amount of effort and funds needed to launch a targeted – let alone a blanket – immunization programme at the global level, sentinel sampling is not enough. Da Silva believes that better reporting can be achieved by making new rapid diagnostic tests for dengue available at the point of care, but this would represent an additional cost to already under-funded health systems.
Because of the different epidemiological profiles for dengue, it will also be necessary to develop a rationale for vaccine usage tailored to each country. “In Latin America dengue is not common in children,” says Hombach, while it often is in other regions, notably Asia. Where it is not a disease commonly found among children, it makes less sense to include it as part of the Expanded Programme on Immunization (EPI), which has been mooted as an obvious delivery system. “If it is not delivered along with the EPI vaccines, there would need to be a special delivery system,” Hombach says. These epidemiological challenges are not the only considerations. There are also complex country-by-country regulatory issues to be dealt with and financing concerns for many of the low-income endemic countries.
Sanofi-Pasteur’s Watson says that he is well aware of these challenges but takes the view that this is all the more reason for starting to think about roll-out now rather than later. “The time between now and when the vaccine will be available is actually very, very short and there is an awful lot to do,” he says. Da Silva agrees: “We cannot wait for the vaccine to be available, we have to work now.” But work on what exactly? There is broad consensus on the need for better surveillance, but when it comes to complex matters of regulatory approval, or an eventual delivery system and schedule, in the absence of an approved vaccine agreement is harder to come by.
For Watson the bottom line is that a vaccine without a vaccination programme is of little use to anyone. He points to mistakes made in the past, when important vaccines took years to reach the people who needed them. But the contrary argument can be made just as forcefully: there can’t be a vaccination programme without a vaccine and for the time being – at least for the next couple of years – we do not have a vaccine. Says Hombach: “The proof of a safe and efficacious dengue vaccine has yet to be made.” After all, vaccines have failed phase III clinical trials in the past.
Once a dengue vaccine is available there will be a great demand. However, in terms of public health economics, spending on such a vaccine will need to be justified. Says Hombach: “Dengue causes a considerable economic burden, which makes the vaccine highly demanded by the affected populations. In contrast, it causes a rather low mortality compared with other diseases, such as malaria and HIV/AIDS, which means that dengue is ranked lower by the global public health community.”
That is why de Quadros says the Sabin Institute will focus on “the social communication side to raise awareness of both the population and policy-makers on the importance of the use of the vaccine, once it becomes available”.
Like Watson, Hombach is concerned that lessons are learnt from past mistakes and says that the most obvious way of doing this is to avoid confusion and wasteful false starts. “It’s a major commitment for countries to introduce the vaccine,” Hombach says. “And once you’re in, you’re in. You can’t have it one year, then drop it the next.” Nor is Hombach entirely convinced that dengue will be the next big push. “It is unclear whether dengue or malaria will be the next vaccine-preventable disease,” he says. “Currently they are neck and neck in the race. A vaccine for falciparum malaria is on track, and licensure could be around 2015.”
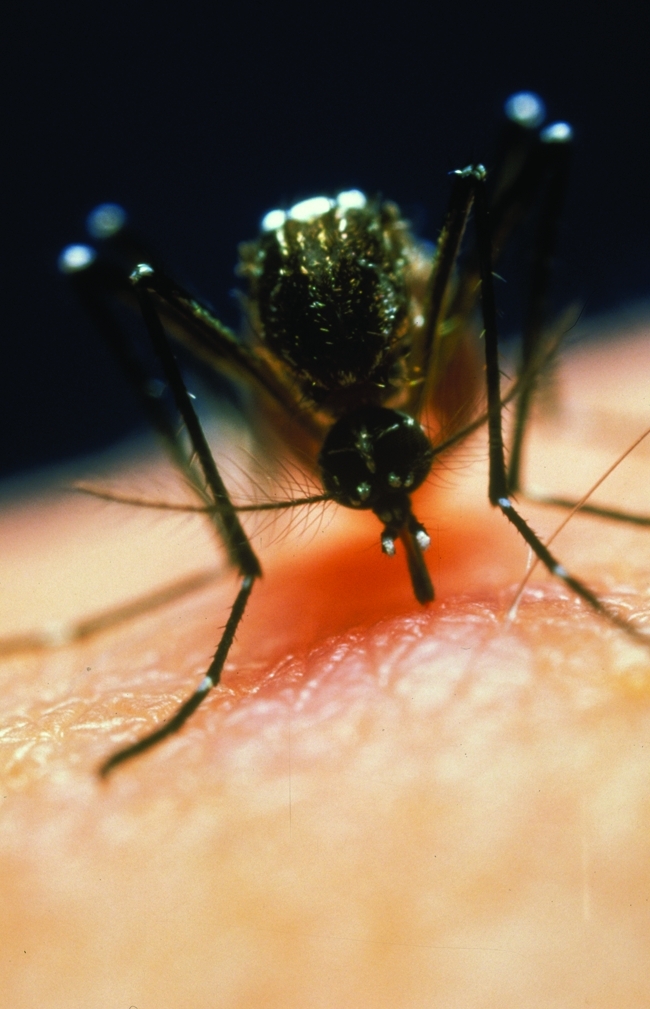
Aedes aegyptii mosquito, the vector for dengue
WHO/TDR/Stammers
A health worker inspects tyres for water and mosquito eggs
WHO/TDR/Crump



