Abstract
Objective
To develop a new algorithm for the presumptive diagnosis of severe disease associated with human immunodeficiency virus (HIV) infection in children less than 18 months of age for the purpose of identifying children who require antiretroviral therapy (ART).
Methods
A conditional probability model was constructed and non-virologic parameters in various combinations were tested in a hypothetical cohort of 1000 children aged 6 weeks, 6 months and 12 months to assess the sensitivity, specificity, and positive and negative predictive values of these algorithms for identifying children in need of ART. The modelled parameters consisted of clinical criteria, rapid HIV antibody testing and CD4+ T-lymphocyte (CD4) count.
Findings
In children younger than 18 months, the best-performing screening algorithm, consisting of clinical symptoms plus antibody testing plus CD4 count, showed a sensitivity ranging from 71% to 80% and a specificity ranging from 92% to 99%. Positive and negative predictive values were between 61% and 97% and between 95% and 96%, respectively. In the absence of virologic tests, this alternate algorithm for the presumptive diagnosis of severe HIV disease makes it possible to correctly initiate ART in 91% to 98% of HIV-positive children who are at highest risk of dying.
Conclusion
The algorithms presented in this paper have better sensitivity and specificity than clinical parameters, with or without rapid HIV testing, for the presumptive diagnosis of severe disease in HIV-positive children less than 18 months of age. If implemented, they can increase the number of HIV-positive children successfully initiated on ART.
Résumé
Objectif
Développer un nouvel algorithme pour le diagnostic présomptif d’une maladie grave associée à l’infection par le virus de l’immunodéficience humaine (VIH) chez les enfants âgés de moins de 18 mois afin d’identifier ceux qui nécessitent une thérapie antirétrovirale (TAR).
Méthodes
Un modèle de probabilité conditionnelle a été créé et des paramètres non virologiques dans différentes combinaisons ont été testés dans une cohorte hypothétique de 1 000 enfants âgés de 6 semaines, 6 mois et 12 mois afin d’évaluer la sensibilité, la spécificité et les valeurs prédictives positives et négatives de ces algorithmes dans l’identification des enfants nécessitant une TAR. Les paramètres modélisés comprenaient des critères cliniques, un test rapide de dépistage des anticorps anti-VIH et la numération des lymphocytes T CD4+ (CD4).
Résultats
Chez les enfants âgés de moins de 18 mois, l’algorithme de tramage qui a présenté les meilleurs résultats réunissait les symptômes cliniques, le dépistage des anticorps et la numération des lymphocytes CD4. Il a montré une sensibilité comprise entre 71 et 80%, et une spécificité comprise entre 92 et 99%. Les valeurs prédictives positives et négatives étaient comprises entre 61 et 97% et entre 95 et 96%, respectivement. En l’absence de tests virologiques, cet autre algorithme de diagnostic présomptif d’une maladie grave associée au VIH permet de correctement mettre en place la TAR chez 91 à 98% des enfants séropositifs qui présentent le risque le plus important de mourir.
Conclusion
Les algorithmes décrits dans cet article ont une meilleure sensibilité et spécificité que les paramètres cliniques, avec ou sans dépistage rapide du VIH, pour le diagnostic présomptif d’une maladie grave chez les enfants séropositifs âgés de moins de 18 mois. S’ils sont appliqués, ils peuvent augmenter le nombre d’enfants séropositifs pour lesquels une TAR est mise en place avec succès.
Resumen
Objetivo
Desarrollar un nuevo algoritmo para el diagnóstico de sospecha de la enfermedad grave asociada la infección por el virus de la inmunodeficiencia humana (VIH) en niños menores de 18 meses, con el objetivo de identificar a aquellos niños que necesiten someterse a un tratamiento antirretrovírico (TAR).
Métodos
Se construyó un modelo de probabilidad condicional y se probaron diversas combinaciones de parámetros no víricos en una cohorte hipotética de 1000 niños con edades de 6 semanas, 6 meses y 12 meses para valorar la sensibilidad, la especificidad y los valores predictivos positivos y negativos de dichos algoritmos en la identificación de los niños que necesitan someterse al tratamiento antirretrovírico. Los parámetros del modelo consistieron en criterios clínicos, pruebas rápidas de anticuerpos del VIH y recuentos de linfocitos T CD4+.
Resultados
En los niños menores de 18 meses, el mejor algoritmo de control, consistente en los síntomas clínicos unidos a las pruebas de anticuerpos y al recuento CD4, mostró una sensibilidad de entre un 71% y un 80% y una especificidad de entre un 92% y un 99%. Se registraron unos valores predictivos positivos y negativos de entre un 61% y un 97% y de entre un 95% y un 96%, respectivamente. En caso de carecer de pruebas víricas, este algoritmo alternativo para el diagnóstico provisional de la infección grave por el VIH permite iniciar de manera adecuada el TAR en el 91-98% de los niños VIH positivos con mayor riesgo de muerte.
Conclusión
Los algoritmos que se presentan en este artículo muestran una mejor sensibilidad y especificidad que los parámetros clínicos, con o sin pruebas rápidas del VIH, para el diagnóstico de sospecha de los niños VIH positivos con enfermedad grave de menos de 18 meses de edad. Si se aplican, conseguirán aumentar el número de niños positivos para el VIH sometidos a un correcto tratamiento antirretroviral.
ملخص
الغرض
إعداد خوارزمية جديدة للتشخيص الترجيحي للمرض الوخيم المرتبط بعدوى فيروس العوز المناعي البشري في الأطفال أقل من عمر 18 شهراً بهدف تحديد الأطفال المحتاجين للمعالجة بمضادات الفيروسات القهقرية.
الطريقة
أُعِدَ نموذج احتمال شرطي وجرى اختبار متثابتات غير فيروسية من مختلف التواليف في مجموعة أترابية فرضيّة مكونة من 1000 طفل في عمر 6 أسابيع، و 6 شهور، و 12 شهراً لقياس الحساسية، والنوعية، والقيم التكهنية الإيجابية والسلبية لهذه الخوارزميات لتحديد الأطفال المحتاجين إلى المعالجة بمضادات الفيروسات القهقرية. وتكوّنت المتثابتات النموذجية من المعايير السريرية، والاختبار السريع لمضادات فيروس العوز المناعي البشري، وعدد خلايا اللمفاويات التائية CD4+.
النتائج
في الأطفال أقل من عمر 18 شهراً، تكوّنت أفضل خوارزمية للأداء من الأعراض السريرية إضافة إلى اختبار الأضداد وعدد الخلايا CD4، وأظهرت حساسية تراوحت بين 71% و 80%، ونوعية تراوحت بين 92% و 99%. وكانت القيم التكهنية الإيجابية بين 61% و 97%، والقيم التكهنية السلبية بين 95% و 96%. وفي غياب الاختبارات الفيروسية، تمكّننا هذه الخوارزمية البديلة من التشخيص الترجيحي لمرض فيروس العوز المناعي البشري الوخيم حتى يمكننا الشروع الصحيح في المعالجة بمضادات الفيروسات القهقرية في 91% إلى 98% من الأطفال الإيجابيين لفيروس العوز المناعي البشري المعرضين لأشد مخاطر الوفاة.
الاستنتاج
الخوارزميات المعروضة في هذا البحث لديها حساسية ونوعية أفضل من المتثابتات السريرية، سواء مع وجود أو عدم وجودة اختبار سريع لفيروس العوز المناعي البشري، للتشخيص الترجيحي للمرض الوخيم في الأطفال الإيجابيين لفيروس العوز المناعي البشري أقل من عمر 18 شهراً. وبتطبيق هذه الخوارزميات، يمكن زيادة أعداد الأطفال المصابين بفيروس العوز المناعي البشري الذين يبدأون بنجاح المعالجة بمضادات الفيروسات القهقرية.
Резюме
Цель
Разработать новый алгоритм постановки предположительного диагноза тяжелой формы заболевания, связанного с инфекцией вирусом иммунодефицита человека (ВИЧ) у детей в возрасте до 18 месяцев с целью выявления детей, нуждающихся в антиретровирусной терапии (АРТ).
Методы
Была разработана модель условной вероятности и проведено тестирование невирусологических параметров в различных сочетаниях в гипотетической когорте из 1000 детей в возрасте 6 недель, 6 месяцев и 12 месяцев, с целью оценки чувствительности, специфичности и положительной и отрицательной прогностической ценности этих алгоритмов для выявления детей, нуждающихся в АРТ. Моделируемые параметры включали в себя клинические критерии, экспресс-тест на ВИЧ-антитела и определение абсолютного количества лимфоцитов CD4 + T-лимфоцитов (CD4).
Результаты
У детей в возрасте до 18 месяцев наиболее результативный алгоритм скрининга, включающего в себя клинические симптомы, тест на антитела плюс подсчет CD4 лимфоцитов, показал чувствительность в пределах от 71 до 80% и специфичность в пределах от 92 до 99%. Положительная и отрицательная прогностическая ценность составляла от 61 до 97% и от 95% до 96%, соответственно. При отсутствии вирусологических тестов этот альтернативный алгоритм постановки предположительного диагноза тяжелой формы заболевания, связанного с ВИЧ-инфекцией, позволяет принимать правильное решение о начале АРТ у 91–98% ВИЧ-положительных детей с наиболее высоким риском смертельного исхода.
Вывод
Алгоритмы, представленные в этой статье, обладают более высокой чувствительностью и специфичностью, чем клинические параметры, с проведением или без проведения экспресс-теста на ВИЧ, для постановки предположительного диагноза тяжелой формы заболевания у ВИЧ-положительных детей в возрасте до 18 месяцев. В случае применения они могут повысить численность ВИЧ-положительных детей, успешно получающих АРТ.
摘要
目的
旨在开发一种新算法,用于未满18个月幼儿中与艾滋病病毒感染相关的严重疾病的推定诊断,从而确定需要进行抗逆转录病毒疗法的幼儿。
方法
构建了条件概率模型,并对年龄为6周、6个月和12个月的1000名幼儿组成的假设组群进行了各种组合的非病毒性参数测试,以评定这些算法在确定需要进行抗逆转录病毒疗法的儿童方面的敏感性、特异性以及阳性和阴性预测值。模拟的参数由临床标准、快速艾滋病病毒抗体检测和CD4+ T淋巴细胞数量构成。
结果
在未满18个月的幼儿中,由临床症状、抗体检测和CD4数量构成的表现最好的筛选算法显示,敏感性为71%-80%,而特异性则为92%-99%。阳性和阴性分别为61%-97%和95%-96%。在缺乏病毒测试的情况下,这种严重艾滋病推定诊断的替代算法能够有效地推动91%-98%的处在死亡风险极高的艾滋病病毒测试呈阳性幼儿的抗逆转录病毒治疗。
结论
无论有无快速艾滋病病毒检测,与临床参数相比,本文中展示的算法在对未满18个月的艾滋病病毒检测呈阳幼儿中严重疾病的推定诊断方面均显示出较好的敏感性和特异性。如果实施该算法,则能够增加艾滋病病毒检测呈阳性幼儿实施抗逆转录病毒疗法的人数。
Introduction
Important strides have been made in recent years in the prevention of mother-to-child transmission (PMTCT) of the human immunodeficiency virus (HIV). Donor investment in PMTCT programmes has been substantial and governments have made a commitment to roll out the programmes. Yet despite these advances, in developing countries approximately 15% of all new cases of HIV infection are diagnosed in children.1 Between one half and two thirds of the children who become infected with HIV will die before their second birthday2 unless they are diagnosed and placed on antiretroviral therapy (ART).3 In sub-Saharan Africa, only around 15% of HIV-exposed infants are tested for HIV in the first two months of life, and the majority of infected children less than 2 years of age die without having their HIV status definitively confirmed through virologic tests.4,5
The World Health Organization (WHO) recommends giving ART to all infants less than 24 months of age with a confirmed diagnosis of HIV infection (i.e. with positive virologic tests results), even if they have no symptoms.4 Antibody tests cannot provide the definitive diagnosis of HIV infection in very young children because maternal IgG antibodies cross the placenta into the fetal circulation during pregnancy and remain detectable for up to 18 months after birth.6 Thus, a virologic method, typically nucleic acid testing to detect viral desoxyribonucleic acid (DNA) or ribonucleic acid (RNA), is required. Such tests are much more complex and costly than more common HIV laboratory assays and have proven difficult to implement in national treatment programmes worldwide.7–10
In resource-constrained settings, lack of access to virologic tests leads to reliance on the presumptive diagnosis of HIV infection for case management. In fact, in its revised 2010 guidelines WHO recommends initiating ART in all children less than 18 months of age with a presumptive clinical diagnosis of severe HIV disease, established on the basis of a defined set of clinical symptoms in infants with antibodies against HIV.4 Historically, however, health-care providers have been reluctant to treat children with a presumptive diagnosis due to various concerns, including but not limited to: initiating HIV negative infants on potentially toxic antiretrovirals, stigma for infants with unclear HIV status, or physician comfort with moderately predictive diagnostic tools. Clinical screening parameters alone, without antibody testing, have demonstrated low sensitivity (20–23%), although their specificity is reasonably high (92–94%).11–15 Thus, efforts to improve the management of children with a presumptive diagnosis of severe HIV disease have focused on two goals: (i) identifying a set of clinical criteria with high enough sensitivity to detect severe HIV disease so that more affected children receive ART, and (ii) identifying HIV-positive children at high risk of death15 and in need of ART without delay.
In our model we have used a set of modified clinical criteria developed by Iliff et al. to define severe HIV disease. The criteria include the following: pneumonia, current diarrhoea, ear discharge, very low weight, generalized lymphadenopathy, oral thrush, and tuberculosis in either the infant or in a sibling or parent of the infant. These criteria, based on the results of a clinical trial of approximately 14 000 infants in Zimbabwe, have shown a sensitivity and specificity ranging from 19.8% to 67.7% and from 94.0% to 90.4%, respectively, depending on the child’s age. They are “modified” in the sense that they resemble WHO’s criteria for the Integrated Management of Childhood Illness15 but exclude persistent diarrhoea (> 14 days) and parotid swelling and include underweight (i.e. weight below the median for age). By adding underweight to the algorithm, Iliff et al. achieved a sensitivity of 65% at 6 weeks, 79% at 6 months and 86% at 12 months, respectively. This innovation is important, as HIV-positive children lose weight early and consistently.16 According to some, the modified clinical criteria are diagnostically superior to the WHO criteria.15
WHO’s 2010 guidelines for presumptively diagnosing severe HIV disease call for the use a rapid HIV antibody test in addition to clinical symptoms. In facilities that practice PMTCT or provide HIV care and treatment, rapid HIV antibody tests are uniformly available; CD4 count can usually be performed on site or is accessible to maternal and child health facilities, but not as widely as antibody tests. When compared with nucleic acid testing (gold standard), CD4 count has shown a sensitivity of 87% and a specificity of 78% for the presumptive diagnosis of severe HIV disease in 3-month-old children.17 However, the problem with using only antibody tests or CD4 count to presumptively diagnose severe disease is that in very young children their individual predictive values are unacceptably low. Besides, not all facilities can perform a CD4 count.15
No published reports until now have explored the combined use of rapid HIV antibody testing and the modified clinical criteria, with or without CD4 count, for the presumptive diagnosis of severe HIV disease in very small children. We propose a screening algorithm based on a combination of these different parameters and hypothesize that such an algorithm can effectively identify HIV-infected children less than 18 months of age who need ART. We quantify how the algorithm improves on the test characteristics of the criteria described in WHO’s 2010 guidelines and suggest additional modifications to further improve test performance. In short, the objective of this study is to develop, for the benefit of clinicians, a means of effectively identifying children under 18 months of age who are in need of ART in settings where virologic tests are not available.
Methods
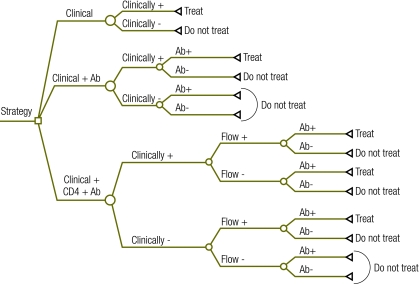
We constructed a conditional probability model to compare the test performance characteristics of three algorithms based on non-virologic parameters to presumptively diagnose severe HIV disease (Fig. 1). The algorithms were as follows: (i) modified clinical criteria, as described earlier, alone; (ii) modified clinical criteria plus HIV antibody testing; (iii) modified clinical criteria plus HIV antibody testing plus CD4 count. Each algorithm was evaluated for diagnostic accuracy against polymerase chain reaction (PCR), the WHO gold standard. The model considered ART eligibility at 6 weeks, 6 months and 12 months of age. A hypothetical cohort of 1000 children was analysed at each of these ages to generate the test characteristics (sensitivity, specificity and positive and negative predictive values). Model outcomes are based on conditional probabilities and hence are independent of the number of individuals simulated. Whenever possible the model was populated with data specific to sub-Saharan Africa. If the literature provided no suitable model input values, we used data collected by the Centre for Infectious Disease Research in Zambia (CIDRZ), whose ART and PMTCT programmes are described in detail in another paper.18 We constructed the model using the Tree Age Pro Suite software package (TreeAge Software, Inc., Williamstown, United States of America) and used Excel (Microsoft Corp., Redmond, USA) to aggregate the data. Table 1 provides a list of model inputs and sources.
Fig. 1.
Screening algorithms for identifying infants exposed to the human immunodeficiency virus (HIV) who are eligible for antiretroviral therapy
Ab, HIV antibody testing; CD4, CD4+ T lymphocyte.
Table 1. Values used as inputs in modelling of algorithms for presumptively diagnosing severe disease associated with human immunodeficiency virus (HIV) infection in small children at different ages.
| Variable | Infant age |
References | ||
|---|---|---|---|---|
| 6 weeks | 6 months | 12 months | ||
| Prevalence of HIV infection among HIV-exposed infants (%) | 11.8 | 14.5 | 18.4 | 19–21 |
| Sensitivity of clinical algorithm for HIV status (%) | 19.8 | 48.4 | 67.7 | 15 |
| Specificity of clinical algorithm for HIV status (%) | 94.0 | 84.4 | 90.4 | 15 |
| Seroverted infants without HIV infection (%) | 1.5 | 50 | 94 | 22–26 |
| Sensitivity of CD4 testing in clinically negativea infants (%) | 64.3 | 43.2 | 37.1 | 26, CIDRZ |
| Specificity of CD4 testing in clinically negativea infants (%) | 99 | 99 | 99 | 24,25 |
| Sensitivity of CD4 testing in clinically positiveb infants (%) | 81.8 | 78.9 | 65.1 | 26, CIDRZ |
| Specificity of CD4 testing in clinically positiveb infants (%) | 99 | 99 | 99 | 24,25 |
CD4, CD4+ T lymphocyte; CIDRZ, Centre for Infectious Disease Research in Zambia.
a Clinically negative infants are those who weigh more than the median weight for their age and who have one or fewer of the signs or symptoms included in the modified clinical criteria.
b Clinically positive infants are those who weigh less than the median weight for their age and who have two or more of the signs or symptoms included in the modified clinical criteria.
Henceforth throughout this paper, “antibody-positive” will designate positivity to an HIV antibody test; “clinically-positive” will designate less than the median weight for age and two or more of the signs or symptoms of the modified clinical criteria; and “CD4-positive” will apply to children with a CD4 count below the 2006 WHO threshold for initiating ART (CD4 ≤ 25% of total lymphocytes at 6 weeks and 6 months of age and CD4 ≤ 20% of total lymphocytes at 12 months of age).10
Some subpopulations of HIV-infected infants are at a particularly high risk of dying. According to data from Zimbabwe, of HIV-positive children whose diagnosis is confirmed by PCR at 6 weeks, those who are clinically positive are 50% more likely to die before reaching 6 months of age than those who are clinically negative.15 Furthermore, young CD4-positive infants who are not started on ART have a risk of dying by 6 months of age 50% higher than CD4-negative infants.19 Clinically positive or CD4-positive infants therefore constitute a high-risk subgroup among HIV-positive infants. We explored the usefulness of the screening algorithms for identifying these high-risk infants.
Model inputs
Prevalence of HIV infection
We assumed a prevalence of HIV infection of 11.8% among HIV-exposed infants 6 to 8 weeks old. This is the reported perinatal rate of mother-to-child HIV transmission in settings where single-dose nevirapine is routinely administered to mothers during labour and to children within 72 hours of birth.20,21 Thus, we used this prevalence on the assumption that minimal antiretroviral prophylaxis services are available to mothers and infants. It is an underestimate of the prevalence of HIV infection in places where ART is not available or not optimally implemented, and it is an overestimate of the prevalence where more complex prophylactic regimens or ART are routinely administered.
We assumed that the prevalence of HIV infection was higher among infants between 6 and 12 month of age than among younger infants because of potential transmission during breastfeeding. This increase in HIV prevalence in older infants is offset to some degree by higher early mortality among HIV-positive infants, which was accounted for in the prevalence estimates.20,22
Seroreversion in HIV-negative children
In HIV-negative (HIV−) children maternal antibodies against HIV decay and disappear over time. By 6 weeks of age 1.5% of infants have seroreverted. Because seroreversion takes an average of 11.6 months,23,24 a positive antibody test in a child younger than 18 months is not indicative of HIV infection (potential false positive). On the other hand, a negative antibody test indicates that the child is not infected (likely a true negative). We used seroreversion rates from a cohort of South African children to approximate the age-dependent test performance of rapid HIV testing.24,25 While nearly all seroreversion studies date from the early 1990s and measured the rate of decay of maternal IgG antibodies with enzyme-linked immunosorbent assays or Western blots, the values found in those studies closely match more recent rapid HIV antibody test results in infants (Table 2).27 In this study we assumed that infants were given a rapid HIV antibody test.
Table 2. Seroversion rates in children without human immunodeficiency virus (HIV) infection who initially tested positive on rapid HIV antibody testing, by age group.
| Age (months) | Seroreverted (%) |
|---|---|
| < 3 | 06.7 |
| 3–6 | 34.7 |
| 6–9 | 79.5 |
| 9–12 | 94.1 |
| 12–18 | 95.0 |
Sensitivity and specificity of the modified clinical criteria
The modified clinical criteria consist of nine HIV-associated clinical signs and symptoms. A child with poor weight gain in the presence of any two symptoms included among the modified clinical criteria is considered clinically positive, as indicated earlier. The sensitivity of the modified clinical criteria increases with age. It increases from 19.8% at 6 weeks to 67.7% at 1 year, whereas specificity drops slightly, from 94% to 90.4%. The positive predictive value increases from 51% to 54% and the negative predictive value, from 78.8% to 85.5%.15
Sensitivity and specificity of CD4 cell count
We obtained CD4 counts for HIV-infected children from CIDRZ data, which are specific to infants enrolled in CIDRZ programmes in Zambia from 2004–2007.16 The values in this cohort closely match the values given in the literature for other sub-Saharan African cohorts and values used in previously published modelling analyses.28 For our model, CIDRZ data were stratified by age into 0–3 months, 4–9 months and 10–15 months to compose the 6-week, 6-month and 12-month age groups, respectively. More than 99% of HIV-negative children have a CD4 count above the recommended threshold for treatment eligibility. This translates into a specificity of 99% for CD4 count in our model.25,26 The 1% of HIV uninfected infants whose CD4 count is below WHO thresholds for treatment eligibility are categorized as false positives.
High-risk infant and sensitivity analyses
Several of the clinical signs and symptoms in the modified clinical criteria are also symptoms of stage III/IV disease in HIV-infected infants and could therefore be used to identify the infants at greatest risk of dying within 6 months. We assumed a correlation coefficient of 0.5 between a diagnosis of severe HIV disease according to the modified clinical criteria and a diagnosis of stage III/IV HIV disease according to WHO criteria. In sensitivity analyses this correlation coefficient ranged from 0.5 to 1.0.
Results
The test characteristics of the three screening algorithms used to presumptively diagnose severe HIV disease are summarized in Table 3. The performance of each algorithm depends on the model inputs. The percentage of children correctly treated (true positives plus true negatives divided by total tested) varies from 79% in our worst-performing algorithm to 96% in our best-performing one, respectively.
Table 3. Test characteristics of three screening algorithms for presumptively diagnosing severe human immunodeficiency virus (HIV) disease in infants, by infant age at testing.
| Characteristic | MCC only | MCC + RAT | MCC + RAT + CD4 count |
|---|---|---|---|
| 6 weeks | |||
| SE | 19.5 | 19.5 | 71.2 |
| SP | 94.0 | 94.1 | 93.9 |
| PPV | 27.0 | 30.7 | 60.9 |
| NPV | 91.4 | 89.7 | 96.1 |
| 6 months | |||
| SE | 48.3 | 48.3 | 71.0 |
| SP | 84.4 | 92.2 | 92.2 |
| PPV | 34.5 | 51.1 | 60.6 |
| NPV | 90.6 | 91.3 | 94.9 |
| 12 months | |||
| SE | 67.9 | 67.8 | 79.8 |
| SP | 90.4 | 99.5 | 99.5 |
| PPV | 61.6 | 96.9 | 97.3 |
| NPV | 92.6 | 93.2 | 95.6 |
CD4, CD4+ T lymphocyte; MCC, modified clinical criteria; NPV, negative predictive value; PPV, positive predictive value; RAT, rapid antibody test; SE, sensitivity; SP, specificity.
Table 4 shows the performance of each screening algorithm when used to determine which infants are at high risk of dying and eligible for ART. Sensitivity analyses in Table 4 represent the correlation between the results obtained with the modified clinical criteria and WHO staging. As shown, by using the best-performing screening combination (modified clinical criteria + CD4 count + HIV antibody testing) infants at high risk of dying can be correctly treated without virologic testing 93% of the time at 6 weeks, 91% at 6 months and 98% at 12 months, respectively.
Table 4. Test characteristics and sensitivity analyses of three algorithms for identifying infants with the human immunodeficiency virus (HIV) at high risk of dying and in need of antiretrovirals, by infant age at testing.
| Characteristic | MCC only |
MCC + RAT |
MCC + RAT + CD4 count |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Analysis |
Analysis |
Analysis |
|||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |||
| 6 weeks | |||||||||||
| qa | 0.50 | 0.75 | 1.00 | 0.50 | 0.75 | 1.00 | 0.50 | 0.75 | 1.00 | ||
| SE (%) | 43.4 | 33.3 | 27.4 | 43.4 | 33.3 | 27.4 | 83.2 | 91.3 | 99.9 | ||
| SP (%) | 94.4 | 94.3 | 94.2 | 94.5 | 94.4 | 94.3 | 94.0 | 94.0 | 94.1 | ||
| PPV (%) | 30.3 | 30.3 | 30.3 | 30.7 | 30.7 | 30.7 | 60.9 | 60.9 | 60.9 | ||
| NPV (%) | 96.7 | 95.0 | 93.4 | 96.8 | 95.0 | 93.4 | 98.0 | 99.1 | 99.9 | ||
| 6 months | |||||||||||
| qa | 0.50 | 0.75 | 1.00 | 0.50 | 0.75 | 1.00 | 0.50 | 0.75 | 1.00 | ||
| SE (%) | 80.5 | 73.7 | 68.0 | 81.4 | 73.7 | 68.0 | 83.1 | 91.2 | 99.9 | ||
| SP (%) | 85.4 | 85.3 | 85.2 | 92.7 | 92.6 | 92.5 | 92.3 | 92.4 | 92.5 | ||
| PPV (%) | 34.5 | 34.5 | 34.5 | 51.1 | 51.1 | 51.1 | 60.6 | 60.6 | 60.6 | ||
| NPV (%) | 97.9 | 96.9 | 95.9 | 98.1 | 97.1 | 96.2 | 97.5 | 98.8 | 99.9 | ||
| 12 months | |||||||||||
| qa | 0.50 | 0.75 | 1.00 | 0.50 | 0.75 | 1.00 | 0.50 | 0.75 | 1.00 | ||
| SE (%) | 91.9 | 88.0 | 85.0 | 91.9 | 88.6 | 84.9 | 88.5 | 94.2 | 99.9 | ||
| SP (%) | 91.0 | 90.9 | 90.9 | 99.5 | 99.5 | 99.5 | 99.5 | 99.5 | 99.5 | ||
| PPV (%) | 61.6 | 61.6 | 61.6 | 96.9 | 96.9 | 96.9 | 97.3 | 97.3 | 97.3 | ||
| NPV (%) | 98.6 | 97.9 | 97.2 | 98.7 | 98.2 | 97.5 | 97.8 | 98.9 | 99.9 | ||
CD4, CD4+ T lymphocyte; MCC, modified clinical criteria; NPV, negative predictive value; PPV, positive predictive value; RAT, rapid antibody test; SE, sensitivity; SP, specificity.
a Coefficient of correlation between the results of clinical screening and of World Health Organization clinical staging.
Discussion
International guidelines do not recommend using clinical algorithms without concurrent HIV antibody testing to presumptively diagnose severe HIV disease in children less than 18 months of age. We included this among our algorithms to determine how much of an improvement in test performance can be expected when routine rapid HIV antibody testing is added to the clinical criteria. In children 6 weeks of age test performance remains essentially unchanged when rapid HIV testing is added to routine clinical screening. This is not surprising because infants in this age group still have high titres of circulating maternal antibodies. In children 12 months of age test specificity can improve by nearly 10% and positive predictive value by nearly 30% by adding rapid HIV antibody testing to routine clinical screening as recommended in WHO’s 2010 guidelines for the presumptive diagnosis of severe HIV infection in infants.
Prognostic markers should display high sensitivity for identifying a specific condition as well as enough specificity to exclude individuals without the condition. Of our modelled screening algorithms, the optimal non-virologic diagnostic combination for determining ART eligibility in infants appears to consist of the modified clinical criteria plus rapid HIV testing plus CD4 count. With the addition of CD4 count to the currently recommended rapid HIV antibody test plus clinical screening, test sensitivity at 6 weeks of age can improve from 19.5% to 71.2%, test specificity from 94.1% to 93.9%, positive predictive value from 30.7% to 60.9% and negative predictive value from 89.7% to 96.1%. The benefits of including CD4 count to the current recommended screening parameters at 12 months of age are less dramatic but still noteworthy. The proposed modified clinical criteria plus antibody testing with or without CD4 count satisfy established thresholds for low-cost diagnostic tests for use in children under 18 months of age (> 70% test sensitivity and > 90% test specificity)28 and respond to WHO’s request for novel tools for use in settings where nucleic acid tests are not available.4
The predictive power of this optimal diagnostic algorithm in children 6 weeks of age is primarily determined by the high sensitivity of CD4 testing and the poor sensitivity of rapid HIV antibody testing. For this age group, the algorithm’s predictive power derives from the rapid HIV antibody test. In settings where CD4 count and nucleic acid testing are not available, applying the modified clinical criteria plus rapid antibody tests is superior to using only the current WHO clinical criteria for the Integrated Management of Childhood Illness.15 Adding rapid HIV antibody testing to the modified clinical criteria is particularly beneficial when diagnosing older infants (6- and 12-month-olds), as the positive predictive value increases from 34.5% to 51.1% and from 61.6% to 96.9%, respectively.
Modelled test characteristics are most heavily influenced by three variables: the prevalence of HIV infection in each age group, the time to seroreversion in HIV-negative children and immunological status (CD4 count) in HIV-positive infants. Uncertainty around the input for the prevalence of HIV infection is low. Modelled prevalence data are specific to sub-Saharan African, concur with internal CIDRZ analyses and closely match the inputs used in previous analyses.28 The rates of maternal antibody decay and the performance characteristics of rapid HIV antibody tests are not well documented in African infants. Data from South African and Ugandan cohorts of children born to HIV-infected mothers suggest that rapid HIV test characteristics are similar to the well studied outcomes from enzyme-linked immunosorbent assays and Western blots.23,24,27 Large uncertainty does surround the distribution of CD4 counts among HIV-infected infants. Decreasing the specificity of CD4 testing would increase the number of infants with false-positive results. For model inputs we used CIDRZ data for infants enrolled in care and treatment programmes in Zambia. These data closely agree with information from outside experts and with published data on the subject.16,25,26,28
Benefits to high-risk children
According to data from South Africa, HIV-related mortality among HIV-exposed infants peaks at 2 to 3 months postpartum.29 By training health-care staff to apply the modified clinical criteria and rapid HIV antibody test, more children at high risk of dying can be identified and treated. These tools are currently readily accessible and can be applied to assess all children. The tools allow staff to quickly identify infants at high risk of dying whose mothers’ HIV status is unknown or undocumented. They can then try to obtain a definitive diagnosis in these children.
According to WHO guidelines, any infant born to an HIV-positive mother or any HIV-positive infant should be placed on co-trimoxazole prophylaxis at 6 weeks of age.30 The rapid HIV antibody test administered at 6 weeks can identify all HIV-exposed infants and ensure that co-trimoxazole prophylaxis is started as early as possible in children who need it. In older HIV-exposed children who are no longer breastfeeding, a negative rapid HIV antibody test is basis enough to discontinue co-trimoxazole prophylaxis.
In countries that are scaling up the use of nucleic acid testing, screening algorithms will allow health-care providers to start therapy on infants with presumptively severe HIV disease while they await the results of nucleic acid tests. In high-volume clinics and hospitals, combined use of rapid HIV testing and modified clinical criteria can reduce the number of nucleic acid tests needed and identify most HIV-positive children who are eligible for ART.15
Study limitations
The test characteristics of the modelled diagnostic algorithms were obtained by using a combination of Zambia-specific data and the best assumptions that could be made from the literature regarding unknown parameters. Hence, actual test performance could vary depending on factors such as seroreversion rate, rate of HIV transmission from mother to child, and non-HIV-related immune parameters (e.g. CD4 counts in HIV-uninfected, immune-competent infants). Iliff et al. have presented a large body of data substantiating the utility of the modified clinical criteria. Operational research is currently under way to determine the exact test efficacy of this model in Zambia and further studies are needed to assure the generalized applicability of the diagnostic algorithm herein discussed.
Conclusion
Health staff in countries lacking access to nucleic acid tests need to rely on a presumptive diagnosis of severe HIV disease to initiate ART in young children. In practice, therapy is often delayed until the age of 18 months, when antibody tests can provide a definitive diagnosis. The optimal algorithm discussed in this paper has better sensitivity and specificity than other algorithms for the presumptive diagnosis of severe HIV disease and, if implemented, will increase the number of HIV-infected infants placed on ART in settings where nucleic acid tests are not available.
Funding:
Funding was provided by Stanford University School of Medicine, the Elizabeth Glaser Pediatric AIDS Foundation, the Centre for Infectious Disease Research in Zambia and the Zimbabwe Vitamin A for Mothers and Babies Project.
Competing interests:
None declared.
References
- 1.AIDS epidemic update: December 2009 Geneva: Joint United Nations Programme on HIV/AIDS & World Health Organization; 2009.
- 2.Marinda E, Humphrey JH, Iliff PJ, Mutasa K, Nathoo KJ, Piwoz EG, et al. ZVITAMBO Study Group Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr Infect Dis J. 2007;26:519–26. doi: 10.1097/01.inf.0000264527.69954.4c. [DOI] [PubMed] [Google Scholar]
- 3.Sherman GG, Matsebula TC, Jones SA. Is early HIV testing of infants in poorly resourced prevention of mother to child transmission programmes unaffordable? Trop Med Int Health. 2005;10:1108–13. doi: 10.1111/j.1365-3156.2005.01495.x. [DOI] [PubMed] [Google Scholar]
- 4.Antiretroviral therapy of HIV infection in infants and children: towards universal access [Internet]. Geneva: World Health Organization; 2010. Available from: http://www.who.int/hiv/pub/guidelines/art/en/ [accessed 12 April 2011]. [PubMed]
- 5.Children and AIDS: fourth stocktaking report New York: United Nations Children’s Fund; 2009. Available from: http://www.who.int/hiv/pub/paediatric/en/ [accessed 15 April 2011].
- 6.Moodley D, Bobat RA, Coutsoudis A, Coovadia HM. Predicting perinatal human immunodeficiency virus infection by antibody patterns. Pediatr Infect Dis J. 1995;14:850–2. doi: 10.1097/00006454-199510000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Creek TL, Sherman GG, Nkengasong J, Finkbeiner T, Fowler MG, Rivadeneira E, et al. Infant human immunodeficiency virus diagnosis in resource-limited settings: issues, technologies, and country experiences. Am J Obstet Gynecol. 2007;197:S64–91. doi: 10.1016/j.ajog.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Towards universal access: scaling up priority interventions in the health sector Geneva: World Health Organization & Joint United Nations Programme on HIV/AIDS & United Nations Children’s Fund; 2008. Available from: www.who.int/hiv/mediacentre/2008progressreport/en/index.html [accessed 12 April 2011].
- 9.Sherman GG, Cooper PA, Coovadia AH, Puren AJ, Jones SA, Mokhachane M, et al. Polymerase chain reaction for diagnosis of human immunodeficiency virus infection in infancy in low resource settings. Pediatr Infect Dis J. 2005;24:993–7. doi: 10.1097/01.inf.0000187036.73539.8d. [DOI] [PubMed] [Google Scholar]
- 10.Antiretroviral therapy of HIV infection in infants and children: towards universal access [Internet]. Geneva: World Health Organization; 2006. Available from: http://www.who.int/hiv/pub/guidelines/en/ [accessed 12 April 2011].
- 11.Horwood C, Liebeschuetz S, Blaauw D, Cassol S, Qazi S. Diagnosis of paediatric HIV infection in a primary health care setting with a clinical algorithm. Bull World Health Organ. 2003;81:858–66. [PMC free article] [PubMed] [Google Scholar]
- 12.Jones SA, Sherman GG, Coovadia AH. Can clinical algorithms deliver an accurate diagnosis of HIV infection in infancy? Bull World Health Organ. 2005;83:559–60. [PMC free article] [PubMed] [Google Scholar]
- 13.Lulseged S, Mengitu Y, Qazi S, Mason E. Validation of the HIV component of the Integrated Management of Childhood Illness (IMCI) algorithm in Addis Ababa, Ethiopia. Comm Dis Bull Afr Reg. 2004;2:9–10. [Google Scholar]
- 14.van Gend CL, Haadsma ML, Sauer PJ, Schoeman CJ. Evaluation of the WHO clinical case definition for pediatric HIV infection in Bloemfontein, South Africa. J Trop Pediatr. 2003;49:143–7. doi: 10.1093/tropej/49.3.143. [DOI] [PubMed] [Google Scholar]
- 15.Iliff P, Ntozini R, Nathoo K, Piwoz E, Moulton L, Humphrey J, ZVITAMBO Study Group Making a working clinical diagnosis of HIV infection in infants in Zimbabwe. Trop Med Int Health. 2008;13:1459–69. doi: 10.1111/j.1365-3156.2008.02178.x. [DOI] [PubMed] [Google Scholar]
- 16.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, Chintu N, Stringer EM, Chi EH, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298:1888–99. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 17.Rouet F, Inwoley A, Ekouevi DK, Viho I, Becquet R, Sakarovitch C, NRS 1201/1202 Ditrame Plus Study Group CD4 percentages and total lymphocyte counts as early surrogate markers for pediatric HIV-1 infection in resource-limited settings. J Trop Pediatr. 2006;52:346–54. doi: 10.1093/tropej/fml024. [DOI] [PubMed] [Google Scholar]
- 18.Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A, Chi BH, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–93. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 19.HIV Paediatric Prognostic Markers Collaborative Study Group Short-term risk of disease progression in HIV-1-infected children receiving no antiretroviral therapy or zidovudine monotherapy: a meta-analysis. Lancet. 2003;362:1605–11. doi: 10.1016/S0140-6736(03)14793-9. [DOI] [PubMed] [Google Scholar]
- 20.Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 21.Namukwaya Z, Mudiope P, Kekitiinwa A, Musoke P, Matovu J, Kayma S, et al. The impact of maternal highly active antiretroviral therapy and short-course combination antiretrovirals for prevention of mother-to-child transmission on early infant infection rates at the Mulago national referral hospital in Kampala, Uganda, January 2007 to May 2009. J Acquir Immune Defic Syndr. 2011;56:69–75. doi: 10.1097/QAI.0b013e3181fdb4a8. [DOI] [PubMed] [Google Scholar]
- 22.Ginsburg AS, Miller A, Wilfert CM. Diagnosis of pediatric human immunodeficiency virus infection in resource-constrained settings. Pediatr Infect Dis J. 2006;25:1057–64. doi: 10.1097/01.inf.0000243157.16405.f0. [DOI] [PubMed] [Google Scholar]
- 23.Chantry CJ, Cooper ER, Pelton SI, Zorilla C, Hillyer GV, Diaz C. Seroreversion in human immunodeficiency virus-exposed but uninfected infants. Pediatr Infect Dis J. 1995;14:382–7. doi: 10.1097/00006454-199505000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Louisirirotchanakul S, Kanoksinsombat C, Likanonsakul S, Sunthornkachit R, Supanit I, Wasi C. Patterns of anti-HIV IgG3, IgA and p24Ag in perinatally HIV-1 infected infants. Asian Pac J Allergy Immunol. 2002;20:99–104. [PubMed] [Google Scholar]
- 25.Zijenah LS, Katzenstein DA, Nathoo KJ, Rusakaniko S, Tobaiwa O, Gwanzura C, et al. T lymphocytes among HIV-infected and -uninfected infants: CD4/CD8 ratio as a potential tool in diagnosis of infection in infants under the age of 2 years. J Transl Med. 2005;3:6. doi: 10.1186/1479-5876-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Embree J, Bwayo J, Nagelkerke N, Njenga S, Nyange P, Ndinya-Achola J, et al. Lymphocyte subsets in human immunodeficiency virus type 1-infected and uninfected children in Nairobi. Pediatr Infect Dis J. 2001;20:397–403. doi: 10.1097/00006454-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Homsy J, Downing R, Finkbeiner T, Kalamya J, Opio C, Mugumya R, et al. Rapid HIV testing prior to DNA-PCR for early screening of HIV infection in infants in Uganda (CROI abstract #668). Proceedings of the 14th Conference on Retroviruses and Opportunistic Infections; 2007 Feb 24–28; Los Angeles, United States; 2007. [Google Scholar]
- 28.Aledort JE, Ronald A, Le Blancq S, Ridzon R, Landay A, Rafael ME, et al. Reducing the burden of HIV/AIDS in infants: the contribution of improved diagnostics. Nature. 2006;444:19–28. doi: 10.1038/nature05443. [DOI] [PubMed] [Google Scholar]
- 29.Bourne DE, Thompson M, Brody LL, Cotton M, Draper B, Laubscher R, et al. Emergence of a peak in early infant mortality due to HIV/AIDS in South Africa. AIDS. 2009;23:101–6. doi: 10.1097/QAD.0b013e32831c54bd. [DOI] [PubMed] [Google Scholar]
- 30.Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents and adults [Internet]. Geneva: World Health Organization; 2006. Available from: http://www.who.int/hiv/pub/guidelines/ctx/en/index.html [accessed 15 April 2011].