Lanthanide luminescence offers several advantages for fluorescence-based biological assays: 1) large Stoke’s shifts (>150 nm) and multiple, narrow emission bands (<10 nm at half-maximum) allow efficient spectral separation of emission signals; 2) long luminescence lifetimes (micro- to millisecond) enable time-resolved detection methods to remove scattering and autofluorescence background; and 3) relative insensitivity to photobleaching allows for prolonged detection.[1] Terbium and europium probes typically incorporate the metal ion into an organic chelating ligand that contains a sensitizing chromophore. When excited with near-UV light in the absorption band, the chromophore transfers energy via intersystem crossing to the triplet excited state and intramolecular transfer to the emissive level of the chelated metal.[1, 2] Direct conjugation of lanthanide probes to antibodies, oligonucleotides and proteins has enabled the development of sensitive, time-resolved fluorescence resonance energy transfer (TR-FRET) assays of biomolecular interactions in purified biochemical preparations, cellular extracts, and on cell surfaces.[3–8] Recent efforts have sought to develop lanthanide probes for live cell imaging applications using time-resolved microscopy with pulsed, near-UV single photon excitation or two-photon excitation.[9–15]
Here we report a facile method of imparting terbium luminescence to proteins for in vitro TR-FRET-based assays and live cell imaging applications. We prepared heterodimeric molecules consisting of a protein binding ligand, trimethoprim (TMP), linked to a series of luminescent terbium complexes, including carbostyril 124-linked polyaminocarboxylates and a 2-hydroxyisophthalamide-based complex (Scheme 1). The TMP-terbium complex conjugates (TMP-TCs) exhibit characteristic terbium luminescence and bind with high affinity to Escherichia coli dihydrofolate reductase (eDHFR) fusion proteins in vitro. The specific labelling of eDHFR expressed on the surface of living mammalian cells with TMP-TCs could be visualized using a sensitive time-resolved, fluorescence microscope capable of rapid image acquisition.
Scheme 1.
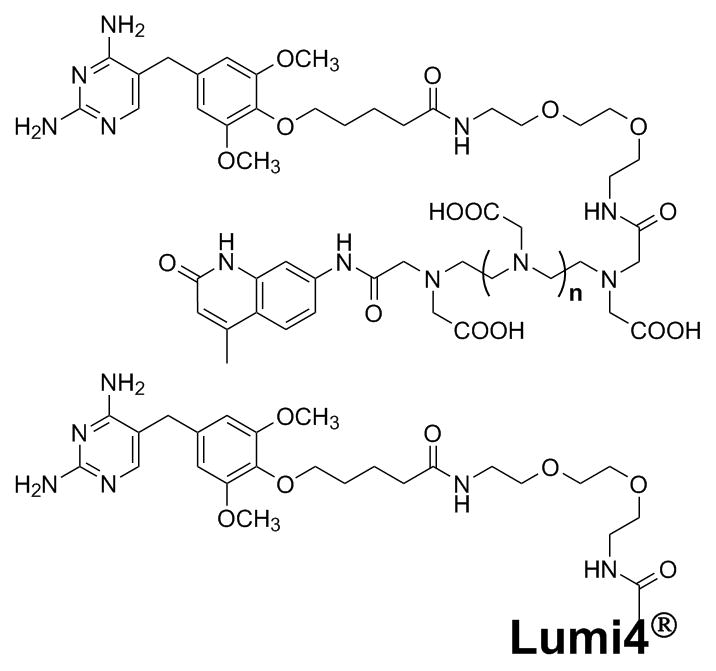
Conjugates of trimethoprim (TMP) to sensitised terbium chelate complexes. Top: cs124-polyaminocarboxylates (n=1, diethylenetriamine pentaacetic acid (TMP-cDTPA); n=2, triethylenetetraamine hexaacetic acid (TMP-cTTHA)). Bottom: Lumi4®-Tb, a proprietary 2-hydroxyisophthalamide terbium complex (TMP-Lumi4).
The specific, high affinity (KD = ca. 1 nM) interaction between TMP and eDHFR has been exploited to develop the LigandLink™ Universal Labeling Technology (Active Motif, Inc., Carlsbad, CA) that makes it possible to tag eDHFR fusion proteins in wild-type mammalian cells with cell-permeable TMP-fluorophore conjugates.[16, 17] We sought to leverage the TMP-eDHFR interaction for labelling proteins with lanthanide probes. For the first generation of TMP-TCs, we selected terbium complexes that were known to have good brightness (high extinction coefficients and quantum yields), could be conjugated without disrupting their terbium binding characteristics or luminescence, and could be synthesized relatively easily. Selvin and coworkers have developed and extensively characterized complexes of the chromophore carbostyril-124 (cs124) linked to diethylenetriamine pentaacetic acid (DTPA) and triethylenetetraamine hexaacetic acid (TTHA).[18–21] Terbium complexes of cs124-DTPA and cs124-TTHA have relatively high extinction coefficients (ε= ca. 10,000 M−1 cm−1 at 343 nm) and quantum yields in water of 0.32 and 0.40, respectively.[21] Moreover, the complexes exhibited similar brightness and lifetimes when conjugated to peptides or proteins.[18, 20] Therefore, we prepared heterodimers of cs124-DTPA and cs124-TTHA linked to TMP via a 15-atom linker (Scheme 1), reasoning that the conjugation strategy would preserve the essential characteristics of the parent complexes. Raymond and co-workers reported an extremely bright (ε = ca. 28,000 M−1 cm−1 at 354 nm, QY = 0.59) multidentate 2-hydroxyisophthalamide (IAM) terbium chelate.[22] We covalently linked TMP to a proprietary analog of IAM complex (Lumi4) that has similar brightness and a luminescence lifetime (ca. 2.7 ms) that remains unchanged upon conjugation to proteins (unpublished data personally communicated from Lumiphore, Inc.). Each of the TMP-TCs that we prepared exhibited characteristic terbium luminescence when complexed with the metal (Supporting Figure 1, online).
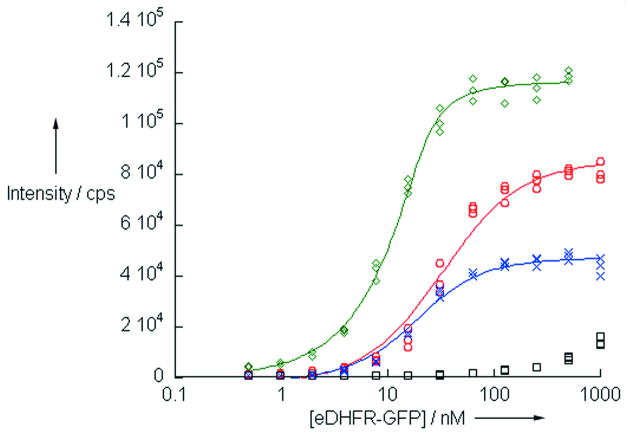
Figure 1.
Intramololecular, time-resolved, fluorescence resonance energy transfer (TR-FRET) between eDHFR-bound TMP-TCs and GFP. Increasing concentrations of purified eDHFR-GFP were titrated against a constant concentration (20 nM) of each compound. Sensitized GFP emission (520 nm) was detected after a time delay of 100 μs, upon pulsed excitation with near-UV light (ca. 340 nm): green diamonds, TMP-Lumi4; red circles, TMP-cTTHA; blue x’s, TMP-cDTPA. Addition of 1 μM TMP reduced the signal, confirming FRET (shown for TMP-cTTHA, black squares). Lines represent non-linear least squares fit to the data.
For both in vitro and live cell applications, TMP-TCs must necessarily bind with high affinity to eDHFR fusion proteins. In order to determine whether the TMP-TCs could bind to eDHFR and serve as FRET donors to green fluorescent protein (GFP), we titrated a purified eDHFR-GFP fusion protein against a fixed concentration (20 nM) of the different TMP-TCs. Using a time-resolved fluorescence plate reader, we detected sensitized, long-lifetime (>100 μs) emission of GFP that increased with increasing protein concentration (Figure 1). Addition of excess TMP substantially reduced the signal, indicating that intramolecular TR-FRET occurred between the eDHFR-bound conjugates and GFP. The relative intensities of sensitised GFP emission at binding saturation were positively correlated to the reported quantum yields of the complexes. A non-linear, least-squares fit of the data revealed the dissociation constants for binding to eDHFR of TMP-cDTPA, TMP-cTTHA and TMP-Lumi4 to be 9 ± 1.3 nM, 22 ± 3.0 nM and 1.8 ± 0.3 nM, respectively. The measured affinities were higher than a previously reported value for binding of a TMP-fluorescein conjugate to eDHFR (KD = ca. 30 nM)[17] and approach the value of free TMP.
We next sought to determine whether TMP-TCs could be used to label eDHFR fusion proteins in living mammalian cells. NIH3T3 fibroblast cells were transiently co-transfected with two plasmid DNA vectors; one that expressed plasma membrane-targeted eDHFR and another that expressed nucleus-localized cyan fluorescent protein (CFP), included as a positive control for transfection. The cells were incubated ca. 20 h in growth medium containing 100 μM TMP-cTTHA, washed, and imaged using an epi-fluorescence microscope capable of pulsed UV excitation and time-resolved detection. No specific labelling of plasma membrane-localized eDHFR was observed in cells that expressed nucleus-localized CFP (Figure 2a, b). Non-specific luminescence was detected in all cells, possibly indicating endocytosis of the compound and trapping in lysosomes. When similar experiments were performed with lower concentrations and/or shorter incubation times, long-lifetime luminescence could not be detected in cells incubated with any of the TMP-TCs.
Figure 2.
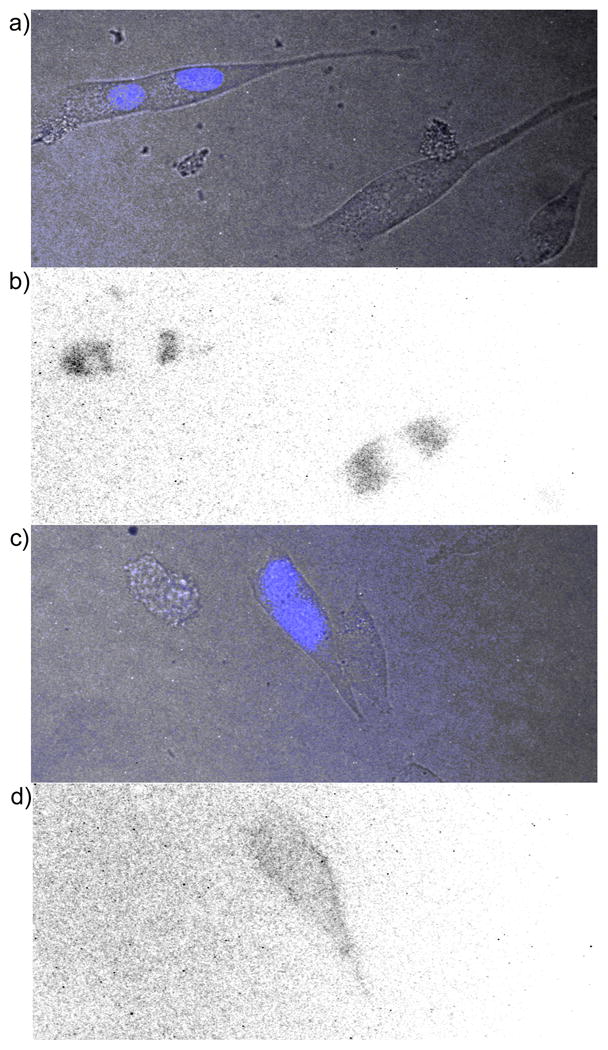
Time-resolved microscopy of NIH3T3 cells treated with TMP-TCs. a) Overlay of bright field and prompt fluorescence (λex = 480± 20 nm, λem = 535 ± 25 nm) images of cells transiently expressing nucleus-localized CFP and plasma membrane-localized eDHFR. b) Inverse, time-resolved fluorescence image of cells in a) showing non-specific luminescence. Cells were incubated 20 h in media containing TMP-cTTHA (100 μM), washed with PBS, mounted in media without compound, and imaged in time-resolved mode (λex = 350± 25 nm, λem = 550± 10 nm, delay = 80 μs, exposure time = 1420 μs, no. exposure cycles = 660). c) Overlay image of cells transiently expressing nucleus-localized CFP and cell surface-localized eDHFR. d) Inverse, time-resolved fluorescence image of cells in c) showing membrane luminescence in transfected cell. Cells were incubated in media containing 1 μM TMP-Lumi4 (10 min.), washed, and imaged as in b).
While intracellular labelling of eDHFR with the TMP-TCs was not possible, we were able to successfully label eDHFR expressed on the cell surface. NIH3T3 fibroblasts were co-transfected with the nucleus-localized CFP expression plasmid and a vector that expressed eDHFR on the extracellular surface of the plasma membrane (pDisplay-eDHFR). Ca. 24 h after transfection, the cells were incubated in growth medium containing 1 μM TMP-Lumi4 for 10 min., washed, and imaged. A distinct membrane luminescence was observed only in cells that expressed nucleus-localized CFP when the cells were imaged in time-resolved mode (Figure 2c, d). The membrane fluorescence could only be detected for approximately 20 min. after washing due to dissociation of the TMP-Lumi4 from eDHFR and diffusion into the medium. A control experiment established that the membrane fluorescence was dependent on the specific labelling of the eDHFR fusion protein with TMP-Lumi4. Pre-incubation of the cells expressing membrane-targeted eDHFR in medium containing 10 μM TMP, followed by incubation in medium containing 1 μM TMP-Lumi4 resulted in no membrane staining. We were only able to detect cell-surface labelling of eDHFR with TMP-Lumi4, and not with TMP-cDTPA or TMP-cTTHA.
Here we have shown that the high affinity, non-covalent interaction between TMP and eDHFR provides an effective means for imparting terbium luminescence to recombinant fusion proteins. TMP-TCs exhibited characteristic luminescence and high affinity for eDHFR, and they proved to be efficient sensitizers of GFP emission in an intramolecular TR-FRET assay. TMP-Lumi4 was particularly effective, binding to eDHFR-GFP with ca. 2 nM affinity and exhibiting >100-fold increase in FRET signal upon binding saturation. As FRET donors to GFP, TMP-TCs could be used to detect interactions between eDHFR and GFP fusion proteins. This would be particularly useful when protein-specific antibodies are unavailable, or in situations where direct conjugation of proteins with terbium complexes is problematic, such as assays of cell lysates. As prepared, the TMP-TCs reported here are cell-impermeable, and can only be used to label proteins on cell surfaces. However, physical methods such as scrape loading or bead loading that are commonly used to load macromolecules into living cells could conceivably be used to deliver TMP-TCs to intracellularly expressed eDFHR fusion proteins.[23]
EXPERIMENTAL SECTION
The complete details of TMP-LC syntheses and characterization, plasmid vector construction, eDHFR-GFP fusion protein expression and purification, cell culture conditions as well as the instrumental configurations and experimental details of binding assays and cellular microscopy are reported in Supporting Information.
Footnotes
We thank Dr. Ana Sanz of Active Motif, Inc. for providing purified eDHFR-GFP protein. Lumi4®-Tb is a registered trademark of Lumiphore, Inc. This research was supported by the National Institutes of Health (GM081030-01). L.W.M. is a recipient of a Chicago Biomedical Consortium Catalyst Award, funded with support from the Searle Funds at the Chicago Community Trust.
Contributor Information
Harsha E. Rajapakse, Department of Chemistry, University of Illinois at Chicago, 845 W. Taylor St., Chicago, IL 60607 (USA)
Dr. D. Rajasekhar Reddy, Department of Chemistry, University of Illinois at Chicago, 845 W. Taylor St., Chicago, IL 60607 (USA)
Shabnam Mohandessi, Department of Chemistry, University of Illinois at Chicago, 845 W. Taylor St., Chicago, IL 60607 (USA).
Dr. Nathaniel G. Butlin, Lumiphore, Inc., 4677 Meade St., Suite 216, Richmond, CA 94804 (USA)
Prof. Dr. Lawrence W. Miller, Department of Chemistry, University of Illinois at Chicago, 845 W. Taylor St., Chicago, IL 60607 (USA).
References
- 1.Pandya S, Yu J, Parker D. Dalton Trans. 2006:2757. doi: 10.1039/b514637b. [DOI] [PubMed] [Google Scholar]
- 2.Hemmila I, Laitala V. J Fluoresc. 2005;15:529. doi: 10.1007/s10895-005-2826-6. [DOI] [PubMed] [Google Scholar]
- 3.Cha A, Snyder GE, Selvin PR, Bezanilla F. Nature. 1999;402:809. doi: 10.1038/45552. [DOI] [PubMed] [Google Scholar]
- 4.Ghose S, Trinquet E, Laget M, Bazin H, Mathis G. J Alloys Compd. 2008;451:35. [Google Scholar]
- 5.Jia Y, Quinn CM, Gagnon AI, Talanian R. Anal Biochem. 2006;356:273. doi: 10.1016/j.ab.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Maurel D, Comps-Agrar L, Brock C, Rives ML, Bourrier E, Ayoub MA, Bazin H, Tinel N, Durroux T, Prezeau L, Trinquet E, Pin JP. Nat Methods. 2008;5:561. doi: 10.1038/nmeth.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maurel D, Kniazeff J, Mathis G, Trinquet E, Pin JP, Ansanay H. Anal Biochem. 2004;329:253. doi: 10.1016/j.ab.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Hovinen J, Guy PM. Bioconjugate Chem. 2009;20:404. doi: 10.1021/bc800370s. [DOI] [PubMed] [Google Scholar]
- 9.Hanaoka K, Kikuchi K, Kobayashi S, Nagano T. J Am Chem Soc. 2007;129:13502. doi: 10.1021/ja073392j. [DOI] [PubMed] [Google Scholar]
- 10.Law GL, Wong KL, Man CWY, Wong WT, Tsao SW, Lam MHW, Lam PKS. J Am Chem Soc. 2008;130:3714. doi: 10.1021/ja710418d. [DOI] [PubMed] [Google Scholar]
- 11.Manning HC, Goebel T, Thompson RC, Price RR, Lee H, Bornhop DJ. Bioconjugate Chem. 2004;15:1488. doi: 10.1021/bc049904q. [DOI] [PubMed] [Google Scholar]
- 12.Palsson LO, Pal R, Murray BS, Parker D, Beeby A. Dalton Trans. 2007:5726. doi: 10.1039/b710717j. [DOI] [PubMed] [Google Scholar]
- 13.Poole RA, Bobba G, Cann MJ, Frias JC, Parker D, Peacock RD. Org Biomol Chem. 2005;3:1013. doi: 10.1039/b418964g. [DOI] [PubMed] [Google Scholar]
- 14.Chauvin AS, Comby S, Song B, Vandevyver CDB, Bunzli JCG. Chem Eur J. 2008;14:1726. doi: 10.1002/chem.200701357. [DOI] [PubMed] [Google Scholar]
- 15.Yu JH, Parker D, Pal R, Poole RA, Cann MJ. J Am Chem Soc. 2006;128:2294. doi: 10.1021/ja056303g. [DOI] [PubMed] [Google Scholar]
- 16.Calloway NT, Choob M, Sanz A, Sheetz MP, Miller LW, Cornish VW. Chembiochem. 2007;8:767. doi: 10.1002/cbic.200600414. [DOI] [PubMed] [Google Scholar]
- 17.Miller LW, Cai YF, Sheetz MP, Cornish VW. Nat Methods. 2005;2:255. doi: 10.1038/nmeth749. [DOI] [PubMed] [Google Scholar]
- 18.Chen JY, Selvin PR. Bioconjugate Chem. 1999;10:311. doi: 10.1021/bc980113w. [DOI] [PubMed] [Google Scholar]
- 19.Li M, Selvin PR. J Am Chem Soc. 1995;117:8132. [Google Scholar]
- 20.Li M, Selvin PR. Bioconjugate Chem. 1997;8:127. doi: 10.1021/bc960085m. [DOI] [PubMed] [Google Scholar]
- 21.Xiao M, Selvin PR. J Am Chem Soc. 2001;123:7067. doi: 10.1021/ja0031669. [DOI] [PubMed] [Google Scholar]
- 22.Petoud S, Cohen SM, Bunzli JC, Raymond KN. J Am Chem Soc. 2003;125:13324. doi: 10.1021/ja0379363. [DOI] [PubMed] [Google Scholar]
- 23.McNeil PL, Warder E. J Cell Sci. 1987;88:669. doi: 10.1242/jcs.88.5.669. [DOI] [PubMed] [Google Scholar]