Summary
Objectives
To compare fatigue failure modes and reliability of hand-veneered and over-pressed implant-supported three-unit zirconium-oxide fixed-dental-prostheses(FDPs).
Methods
Sixty-four custom-made zirconium-oxide abutments (n=32/group) and thirty-two zirconium-oxide FDP-frameworks were CAD/CAM manufactured. Frameworks were veneered with hand-built up or over-pressed porcelain (n=16/group). Step-stress-accelerated-life-testing (SSALT) was performed in water applying a distributed contact load at the buccal cusp-pontic-area. Post failure examinations were carried out using optical (polarized-reflected-light) and scanning electron microscopy (SEM) to visualize crack propagation and failure modes. Reliability was compared using cumulative-damage step-stress analysis (Alta-7-Pro, Reliasoft).
Results
Crack propagation was observed in the veneering porcelain during fatigue. The majority of zirconium-oxide FDPs demonstrated porcelain chipping as the dominant failure mode. Nevertheless, fracture of the zirconium-oxide frameworks was also observed. Over-pressed FDPs failed earlier at a mean failure load of 696 ± 149 N relative to hand-veneered at 882 ± 61 N (profile I). Weibull-stress-number of cycles-unreliability-curves were generated. The reliability (2-sided at 90% confidence bounds) for a 400N load at 100K cycles indicated values of 0.84 (0.98-0.24) for the hand-veneered FDPs and 0.50 (0.82-0.09) for their over-pressed counterparts.
Conclusions
Both zirconium-oxide FDP systems were resistant under accelerated-life-time-testing. Over-pressed specimens were more susceptible to fatigue loading with earlier veneer chipping.
Keywords: zirconium-oxide, fatigue, chipping, Weibull-reliability, fixed-partial-denture, implants, over-pressed, hand-veneered, core, porcelain
1. Introduction
Porcelain fused to metal (PFM) fixed-dental-prostheses (FDPs) are used extensively as dental restorations due to their long-term mechanical performance 1, 2. However, because of their metal framework, they are not able to mimic the translucency and transparency of the natural tooth structure 3.
Metal free all-ceramic restorations can satisfy increasing aesthetic demands and offer improved biocompatibility 3–5. In 1988, Yttrium-oxide-Tetragonal Zirconia Polycrystal (Y-TZP) was introduced for fixed-dental-prostheses, a bio-ceramic with high flexural strength (~900–1200 MPa) and fracture toughness (~5–10 MPa·m1/2) 6. The mechanical properties of zirconium-oxide ceramic are achievable due to its phase transformation toughening effect, which makes this biomaterial more resistant to crack propagation 7, 8. Nowadays, Y-TZP is widely used for frameworks of anterior and posterior FDPs 9–11. Interest in this ceramic is increasing because of the improvements in CAD/CAM technologies 12.
However, good mechanical properties of zirconium-oxide do not reflect in the clinical performance of veneered Y-TZP FDPs, which have shown good structural stability only in short-term clinical studies 13, 14. The lack of translucency of high-density polycrystalline zirconium-oxide ceramic 15 makes the use of veneering porcelain with inferior material properties necessary to achieve an aesthetic outcome. The most critical failure associated with these types of restorations is chipping or fracture of the veneering porcelain 9, 10, 14, which required the replacement of the restoration in many documented instances 3. Therefore, further efforts are needed to investigate the variables affecting the long-term clinical performance of zirconium-oxide FDPs, such as framework and coping design, fatigue behavior, veneering procedures and surface treatment of the framework 16–18. Chipping can be attributed to potential flaws and artifacts generated during the veneering technique. Different veneering procedures, involving various porcelain application methods, heating temperatures, firing profiles, and glazing steps, might lead to distinguished mechanical function of FDPs. Therefore, it is critical to focus on the influence of the veneering procedure on the long-term mechanical performance of bilayered all-ceramic restorations 19, 20, such as zirconium-oxide FDPs. However, to date, only limited data are available concerning the damage modes and reliability of zirconium-oxide three-unit FDP restorations 21, 22.
Two types of veneering methods are available for all zirconium-oxide restorations: hand built-up and over-pressing 23. The objective of this study was to compare the influence of the two veneering techniques on the mechanical performance of three-unit zirconium-oxide FDPs. Mouth motion contact fatigue testing 24 with distributed load was used to simulate chewing and failure modes were observed in both groups in relation to failure loads and number of cycles.
2. Materials and Methods
2.1. Specimen preparation
Thirty-two implant-supported zirconium-oxide mandibular three-unit FDP specimens were fabricated (Procera, Nobel Biocare, Gothenburg, Sweden) (Fig. 1). A master model was obtained from a human lower dentition missing the right second premolar, first and second molar locations. Two implant analogs were positioned at the center of the missing premolar and second molar. A wax-up of a three-unit FDP was created based on temporary implant abutments. The wax-up was duplicated and maintained in a master cast providing standardized specimen dimensions from which polyvinyl silioxane impression matrices were prepared to permit standardized veneer application. To define ideal implant abutment dimensions the wax-up was cut back, allowing for sufficient zirconium-oxide framework and for average veneering porcelain thicknesses of 1.2 mm on occlusal, 1 mm on buccal and lingual, and 0.5 mm on the bottom surfaces. All specimens had similar thickness of veneer and core thickness in a particular area by using a standard model FDP. Therefore, in the loading area, the veneer and core thicknesses were almost identical in all specimens. Wax-ups of the premolar and molar abutments were scanned and sixty-four custom-made zirconium-oxide abutments were CAD/CAM manufactured (Procera, Nobel Biocare, Gothenburg, Sweden). Implant positions at the master model were scanned as well as the intended FDP dimensions (NobelProcera Scanner, Gothenburg, Sweden). Thirty-two zirconium-oxide frameworks, consisting of the second premolar and first and second molar, were CAD/CAM fabricated. For the fabrication of the zirconium-oxide FDP frameworks as well as the abutments identical CAD files were used to fabricate dimensionally standardized specimens. Before veneering, air-particle abrasion was performed on the external surface of all frameworks with 100 micron Al2O3 at 1.0 bar pressure and a distance of 10 mm. Transpa Clear (NobelRondo, Nobel Biocare, Gothenburg, Sweden) was used for a thin wash bake at 940°C. Sixteen FDPs were hand built-up with veneering porcelain (NobelRondo porcelain, Nobel Biocare, Gothenburg, Sweden) by a specialized technician using the polyvinyl siloxane matrices to establish contours and thickness. The remaining sixteen FDPs received pressed porcelain veneering (NobelRondo Press, Nobel Biocare, Gothenburg, Sweden). The hand-veneering procedure involved two firings at 910 and 900°C, with a preheat temperature of 575°C followed by a rise temperature of 45°C/min and holding time (HT) of 1 min according to manufacturer’s information. Two glaze firings at 890°C (HT 1 min) and 850°C (HT 2 min) were performed. For each firing, the technician kept the furnace door closed until 520°C, which is around 50°C below the porcelain Tg temperature to allow for slow cooling. For the over-pressing method, the veneer was waxed up on the framework taking into account the same porcelain thickness of the hand-veneering method. A master silicone casting mold was realized to obtain standardized veneering wax layers for the remaining frameworks. The over-pressing method consisted of one unique firing at 1060°C, with a preheating at 700°C followed by a rise temperature of 60°C/min according to manufacturer’s information. During the cooling phase the furnace door was locked and the pressure remained. Then, two glaze firings were performed similar to the hand-veneered group allowing for slow cooling. The custom-made zirconium-oxide abutments were screw-retained to Replace-straight-Groovy titanium implants (13 mm in length) (Nobel Biocare, Gothenburg, Sweden) with a torque of 32 Ncm. Ethanol was used to clean the abutments and the internal surfaces of the copings. The abutments were cemented into the FDPs copings with glass-ionomer cement (Ketac Cem, 3M-ESPE), according to manufacturer’s instructions. A load of 15 N was applied until setting of the dental cement to control cement thickness. The specimens were embedded in a polymethylmethacrylate (PMMA; Densply Caulk, Milford, DE, US) base, covering the implants within 2 mm of the implant shoulder. The embedded FDPs were stored in water at 37°C for at least 14 days before mechanical testing 25.
Figure 1.
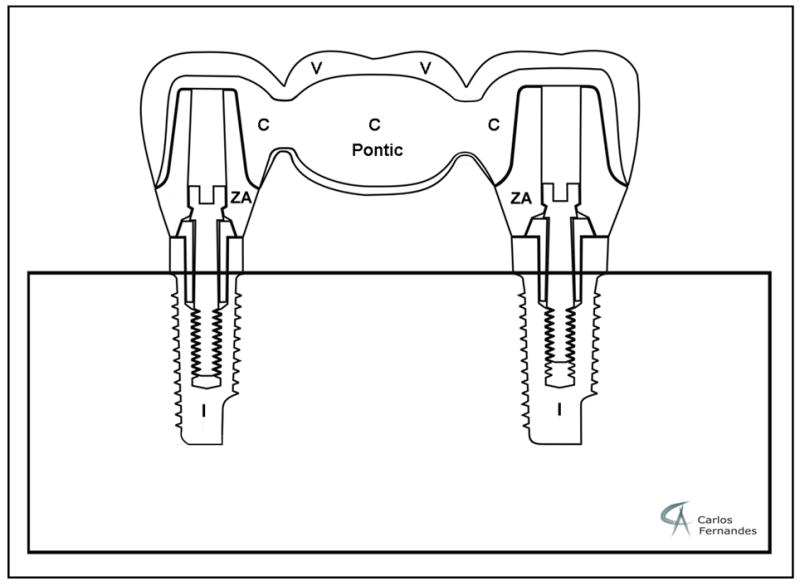
Schematic drawing of an implant-supported FDP specimen (mandibular zirconium-oxide FDP). Each specimen consisted of two dental titanium implants (I), two custom-made zirconium-oxide abutments (ZA), two implant screws (32 N/cm), CAD/CAM fabricated zirconium-oxide framework (C) and veneering porcelain (V). (Drawing Dr. Carlos Fernandes)
2.2. Mechanical testing
All specimens were mechanically tested until failure occurred. Based on clinical observations, failure was defined by chipping of the veneering porcelain or chipping in combination with the fracture of the framework.
Static failure load values of the FDPs were obtained by performing mechanical tests on two hand-veneered and two over-pressed restorations using a universal testing machine (Model 5566, Instron Corporation, Canton, MA) with a tungsten carbide spherical indenter (d = 6.25 mm) 26. The load was vertically applied with a rate of 1 mm/min up to failure at the buccal cusp pontic area of the FDPs inclined at 30 degrees (Fig. 2) to simulate clinical occlusal loading conditions 27. The mean failure load was 1316 N for veneering porcelain chipping and 1744 N for framework fracture of the hand-veneered FDPs, and 1241 N and 1680 N for the over-pressed FDPs, respectively. Material-specific step-stress-profiles were designed based on the load-to-failure values. Fatigue testing was performed using the step-stress accelerated life testing method (SSALT), which distributes specimens across different load to cycle profiles 28. According to Nelson 28, three profiles (mild, medium, and aggressive) are required to predict fatigue life using accelerated lifetime testing. To develop the three distinguished SSALT profiles, the mean static failure load for veneering porcelain chipping of hand-veneered and over-pressed FDPs was computed (1279(±57)N). The step-stress profiles for each test group started at a step load of 5 to 15% of the mean static failure load (Fig. 3). Each specimen was loaded using one of the three profiles characterized by increments of increasing load and number of cycles, until defined failure occurred 29. For both hand-veneered and over-pressed groups, specimens were distributed among the profiles as follows: seven specimens were tested with profile I, four with profile II and three with profile III. All specimens were tested until failure occurred. It was taken into account that the applied loads could exceed maximum clinical loading values (approximately 900N) 30. A mouth-motion simulator (ELF 3300, EnduraTEC Division of Bose, Minnetonka, MN, US) was employed for fatigue testing. The load was vertically applied at the buccal cusp pontic area of the FDPs inclined at a 30 degree angle (Fig. 2) in water 31 using a tungsten carbide spherical indenter (d = 6.25 mm) 26 with an aluminum foil spacer of 0.8 mm thickness to allow load distribution 27. The aluminum foil was replaced every 5000 cycles to avoid foil wear out. The buccal side of the pontic area in which the load was applied was inclined by twelve degrees with respect to the horizontal plane (Fig. 2), generating a slight sliding (≤1 mm) of the indenter on the occlusal surface during loading.
Figure 2.
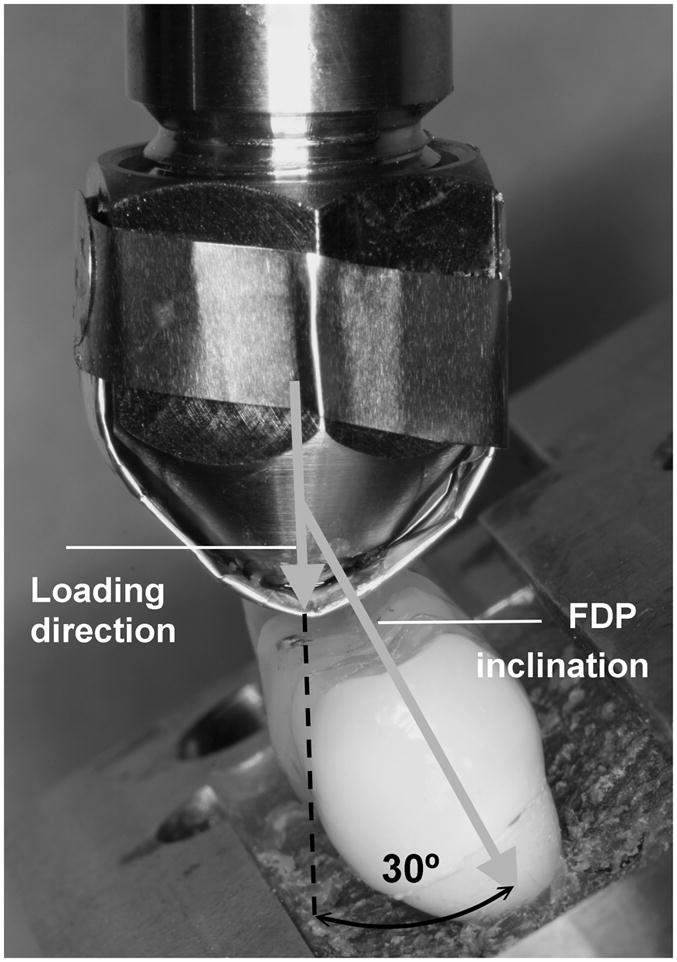
Mechanical loading was applied on each FDP using a spherical indenter with an aluminum foil spacer of 0.8 mm thickness to allow load distribution. All specimens were inclined by 30 degrees with respect to the loading direction.
Figure 3.
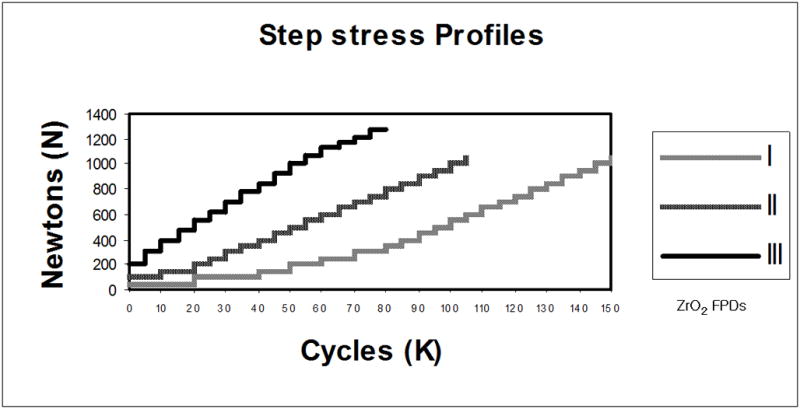
Step-stress accelerated life test (SSALT) profiles. The three profiles were characterized as 1) ‘mild’, 2) ‘medium’ and 3) ‘aggressive’ reflecting the varying ratio of load application and number of cycles.
2.3. Failure modes/loads analysis
For each FDP, failure modes were observed in relation to their corresponding failure loads and cycles. Fracture patterns were identified with both polarized-reflected-light microscopy (Leica MZ APO; Leica, Bensheim, Germany) and SEM (S-3500 N, Hitachi Instruments, San Jose, CA, USA). Superficial cracks were detected on epoxy-resin replicas (Epofix, Struers, Copenhagen, Denmark), created from impressions of the FDPs using polyvinylsiloxane (Virtual 380, extra light body and heavy body, Ivoclar-Vivadent, Mississauga, Ontario, US). Samples were then embedded in epoxy-resin (Epofix, Struers, Copenhagen, Denmark) to allow for either longitudinally or transversally sectioning (Isomet 1000, Buehler, Lake Bluff, IL). Internal cracks, which would have remained undetected due to the opacity of the FDPs, were investigated on the sectioned specimens.
A statistical software (SigmaPlot 11.0, Ashburn, VA, US) was used to perform a t-test (α=0.05) for the comparison of failure loads and cycles between over-pressed and hand-veneered FDPs tested with Profile I. The comparison was performed only for specimens in Profile I (n=7/group), since the sample size in Profiles II (n=4/group) and III (n=3/group) was too small to justify t-test analysis. Weibull-reliability analysis was performed using accelerated life testing software (Alta Pro 7, Reliasoft, Tuscon, AZ), to calculate the reliability of both hand-veneered and over-pressed FDPs. The applied Weibull-reliability analysis was developed to allow for reliability calculations for small sample sizes 32.
2.4. Micro-indentation testing
To determine the mechanical properties of the veneering porcelains, fracture toughness (MPa·m1/2) of the veneering porcelain was measured in the occlusal pontic area of three over-pressed and three hand-veneered FDP sections using Vickers micro-indentation (St. Joseph, MI, USA). Twelve indentations were performed for each specimen with a load of 9.8 N at a dwell time of 5 s. To avoid interactions, indentations were placed at a distance of at least twice the crack length from artifacts and porcelain borders 33. Fracture toughness was computed using validated formulas, for which the crack length was measured from the center of the indentation 34, 35. In highly brittle materials, it is typical that cracks are not only generated at the apex but also from the sides of the indentation impression. Therefore, a circle enclosing all associated cracks was drawn, and its radius, developed from the center of the indentation, was taken to determine the crack length value 34. A t-test data analysis (α=0.05; SigmaPlot 11.0, Ashburn, VA, US) was performed to determine whether fracture toughness was significantly different between the over-pressed and hand-veneered porcelain.
3. Results
3.1. Failure analysis
In all FDPs, buccal chipping of the veneering porcelain was the dominant failure mode under fatigue testing. Fractographic analysis of the chipped areas demonstrated that cracks developed from the loading area and stopped in the buccal-apical zone as indicated by the arrest lines (Fig. 4a,b).
Figure 4.
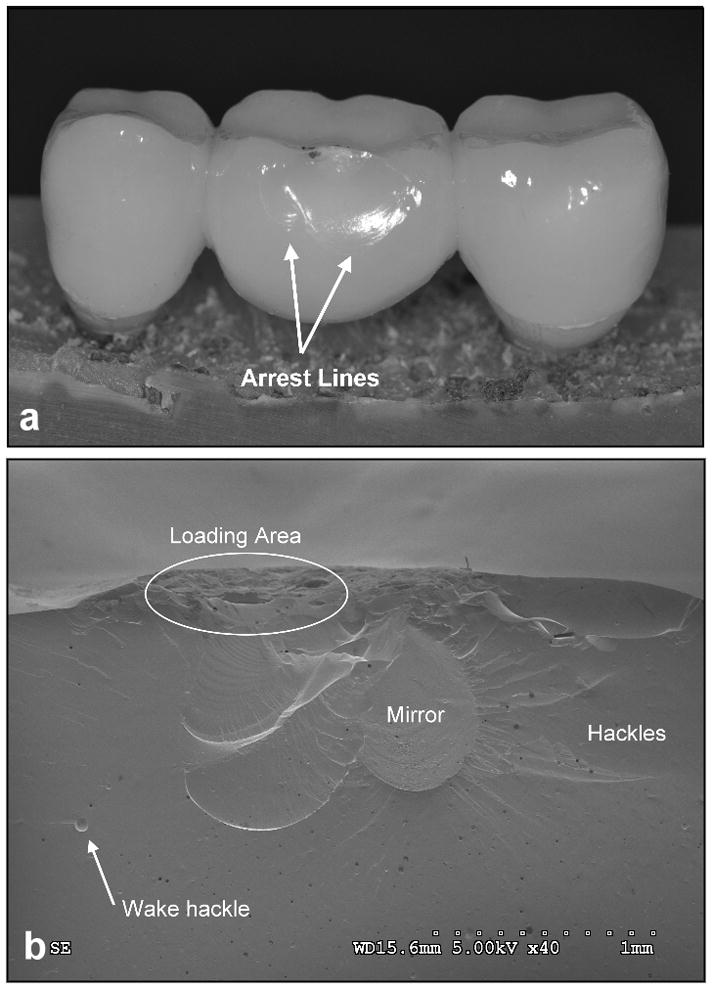
Buccal chipping of the porcelain in the loading area of an over-pressed FDP:
a) Overview of buccal porcelain chipping arresting in the coronal area of the FDP pontic. b) Fractographic features: Mirror with hackles shows chipping origin in the loading area of the FDP pontic and development towards the coronal region (SEM).
Close examination of the as-fractured as well as sectioned (along the mesial-distal plane) hand-veneered FDPs revealed the following fatigue damage modes. Out of the fourteen specimens six failed by buccal chipping of the porcelain only. For the remaining eight specimens, buccal chipping was associated with fracture in the connector/pontic areas. Connector/pontic cracks always initiated at the gingival aspect of the connector, but propagated in a curvature toward the occlusal loading region of the pontic in six FDPs (Fig. 5a) while extended upward toward the occlusal area of the connector in the other two. Three of the connector/pontic cracked FDPs also exhibited a fracture in one of the zirconium-oxide abutments (Fig. 6). In two of the three specimens, the premolar abutment fractured in combination with pontic failure involving the molar connector. In the other specimen, the molar abutment fractured along with the pontic failure of the premolar connector (Fig. 6). It is important to note that most of the connector/pontic fractured FDPs showed a very thin layer of veneering porcelain (≤0.1 mm) at the gingival aspect of the framework (Fig. 6c). In addition, out of the six FDPs with solely buccal chipping, the cross-sections revealed subcritical crack growth in the framework connector area and including the molar zirconium-oxide abutment for one sample. Five of the six specimens showed crack propagation in the occlusal veneering porcelain layer extending towards the abutment crowns (Fig. 7).
Figure 5.
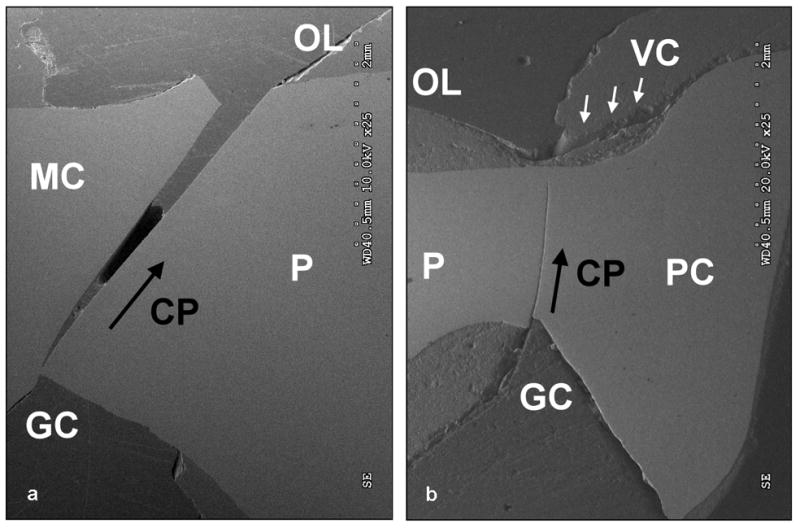
Connector/pontic crack initiated at the gingival aspect of the connector in combination with chipping of the veneer (VC) (SEM): a) Fracture propagation (CP) from the gingival aspect (Gingival Connector = GC) of the molar connector (MC) towards the occlusal loading region (OL) of the pontic (P) of a FDP sectioned along the mesial-distal plane. b) Crack propagation (CP) from the gingival aspect (GC) towards the occlusal loading area (OL) of the premolar connector (PC) in the pontic (P) of a FDP sectioned along the mesial-distal plane.
Figure 6.
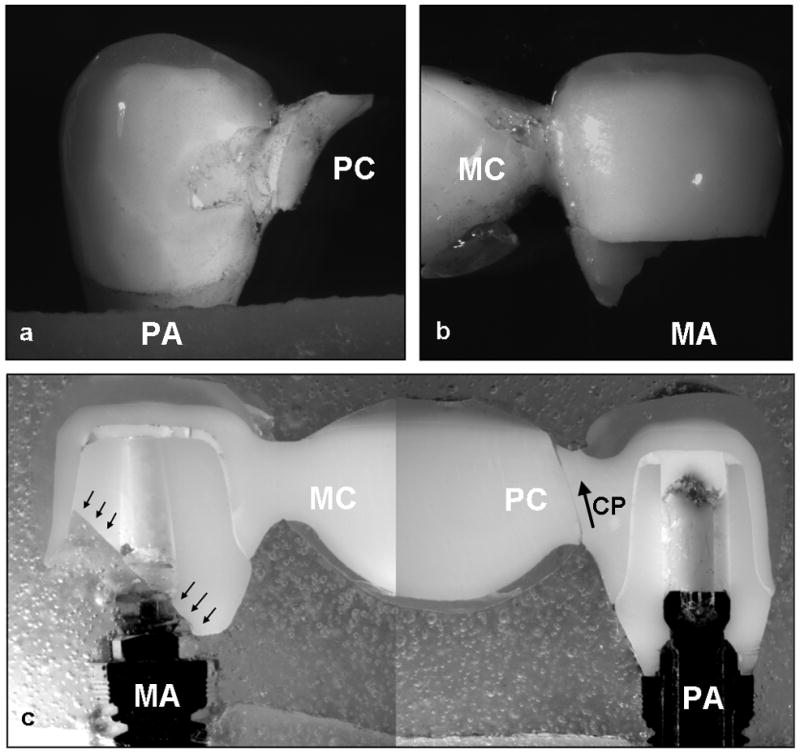
Exemplary failure modes of a hand-veneered FDP: a) Fracture of the pontic from the gingival aspect of the premolar connector (PC) relative to the premolar abutment (PA) towards the loading area of the pontic. b) Fracture of the molar abutment (MA) relative to the molar connector (MC) (optical image). c) Overview of the FDP sectioned in the mesial-distal plane showing the crack propagation (CP) relative to the premolar connector (optical image) and the fracture of the molar abutment (black arrows).
Figure 7.
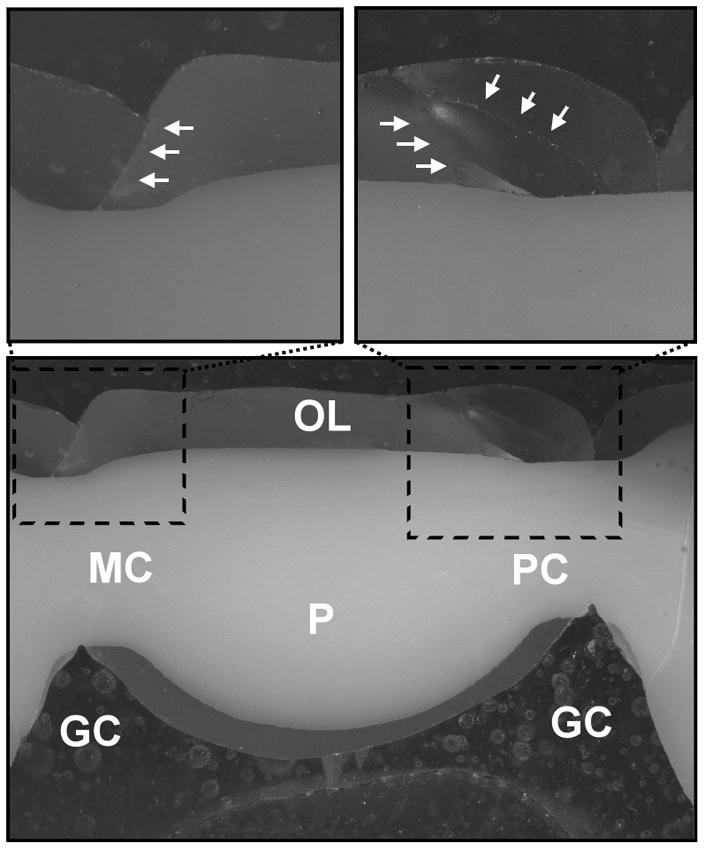
Hand-veneered FDP sectioned in the mesial-distal plane: a) Chipping of the porcelain in the occlusal loading area (OL) of the pontic (P). No cracks were found in the gingival aspect (GC) of the molar (MC) and premolar connectors (PC). b) and c) Cracks in the veneering porcelain (white arrows) did not propagate into the zirconium-oxide core but towards the abutment crowns (optical image).
Out of the fourteen over-pressed FDPs, eight failed only by buccal chipping of the porcelain veneer (Fig. 4). Out of these eight specimens, four showed no further internal damage; one revealed a crack propagation along the molar connector towards the occlusal surface (Fig. 5b). In this case, only a thin veneer layer was found in the gingival area of the connector. The remaining three FDPs demonstrated crack development in one or both of the zirconium-oxide abutments. Three other over-pressed FDPs showed fractures of one or both zirconium-oxide implant abutments in combination with buccal chipping; no framework fracture was observed in these specimens. Another FDP demonstrated premolar abutment fracture combined with framework failure in the molar connector area. The remaining two over-pressed FDPs failed by combined chipping of the veneer and framework fracture initiated at the connector area.
3.2. Weibull-reliability analysis
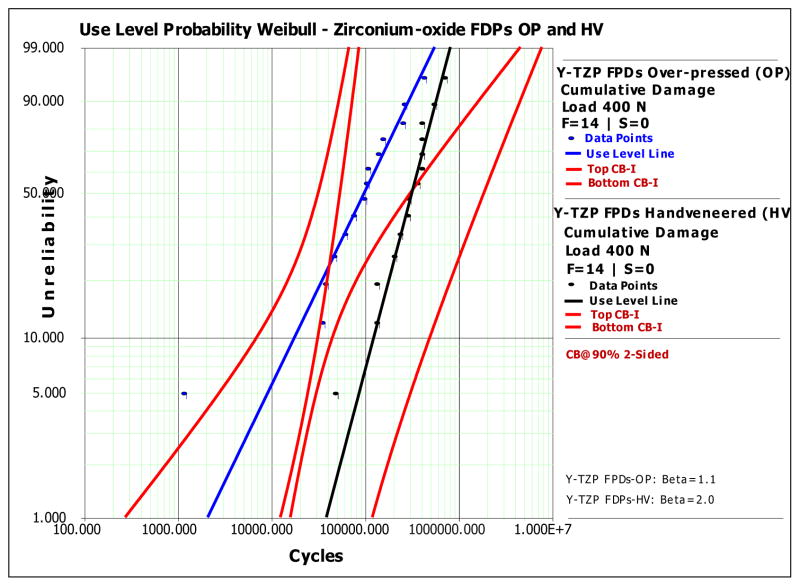
A master Weibull probability curve was calculated for both zirconium-oxide FDPs groups using the SSALT data of all failures 32. Reliability calculations (2-sided at 90.0% confidence bounds) for completion of a mission of 50,000 cycles and 100,000 cycles at 400 N load (Fig. 8) resulted in 0.95 (0.99-0.76) and 0.84 (0.98-0.24) reliability for the hand-veneered FDPs and 0.72 (0.88-0.42) and 0.50 (0.82-0.09) reliability for over-pressed FDPs, respectively. The calculations demonstrated that the probability of failure by veneering porcelain chipping was higher for over-pressed then hand-veneered FDPs.
Figure 8.
Weibull-reliability calculations of SSALT data: Weibull probability graph of probability of failure i.e. unreliability (percentage) vs. number of cycles (time) of hand-veneered (HV) (n=14) vs. over-pressed (OP) (n=14) zirconium-oxide (Y-TZP) FDPs (load level 400N) at 2-sided 90% confidence bounds.
When profile I specimens were statistically analyzed, failure occurred at significantly lower loads for the over-pressed FDPs (696 ± 149 N, mean ± SD) compared to the hand-veneered FDPs (882 ± 61 N) (t-test; p=0.01). Further, a significant difference was found in the number of cycles to failure (t-test; p=0.01) between the two groups, being 78,000 ± 14,000 (mean ± SD) for the over-pressed and 95,000 ± 9,000 for the hand-veneered FDPs. The minimum failure load at which buccal chipping of the porcelain occurred was 500 N for the over-pressed specimens, and 800 N for the hand-veneered samples. Complete fracture of the pontic associated with buccal chipping of porcelain occurred in one over-pressed sample at 850 N; a similar failure mode was present in three hand-veneered FDPs at 875 ± 43 N.
3.3. Micro-indentation
The veneering porcelain of the hand-veneered specimens showed significantly higher fracture toughness (0.95 ± 0.13 MPa·m1/2) than their over-pressed counterparts (0.53 ± 0.12 MPa·m1/2) (p<0.001) (Fig. 9a,b ) as measured by the indentation crack length method.
Figure 9.
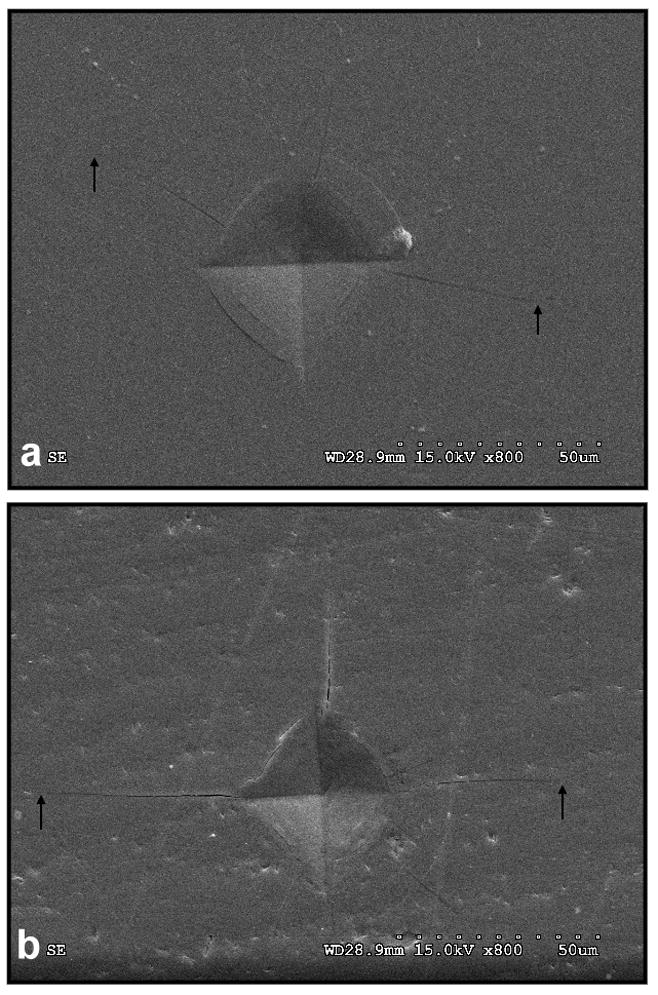
Micro-indentations (SEM): a) Crack propagation in an exemplary micro-indentation performed on a hand-veneered FDP. b) Crack propagation in a micro-indentation performed on an over-pressed FDP.
4. Discussion
In the present study, failure modes and reliability of implant-supported three-unit Y-TZP FDPs using two different veneering methods, over-pressing and hand-veneering, were investigated. Both groups demonstrated chipping of the veneering porcelain in the occlusal aspect of the pontic as a dominant failure mode. Porcelain chipping occurred at much lower loads and less number of cycles for the over-pressed FDPs than the hand-veneered restorations. However, in both systems, connector/pontic failure and abutments fracture of the zirconium-oxide framework were associated with a higher load and larger number of cycles compared to those for veneer chipping. From a clinical prospective, chipping of the veneering porcelain is the dominant failure mode 9, 14; yet fracture of the zirconium-oxide framework has been reported in some cases 36.
The earlier porcelain chipping of the over-pressed specimens might have been a consequence of the one time application of the veneering porcelain in the over-pressing technique. The thermal contraction coefficient (TCC) (~9.5 μm/(m·K)) of the NobelRondo Press* was lower than that of the zirconium-oxide framework* (~10.8 μm/(m·K) (*Nobel Biocare, Gothenburg, Sweden) 37–39. The over-pressed porcelain had to cool down as a complete layer, by which higher residual stresses in the veneering porcelain might have been generated between the surface layer and the zirconium-oxide interface. While for the hand-veneering method, the veneering porcelain (NobelRondo*, similar TCC (~9.5 μm/(m·K)) was applied in multiple layers with various firing cycles which allow for small shrinkage volumes for each layer, leading to lower residual stresses in the porcelain 40.
The various fabrication temperatures and pressures involved in the two veneering techniques would result in different mechanical properties of the porcelain. The micro-indentation results showed that hand-veneered porcelain is significantly tougher than the over-pressed veneer. Note that the fracture toughness measured here is actually an effective toughness, representing combined effects from the material intrinsic toughness, residual stresses, etc. Higher fracture toughness would be more effective to suppress a propagating crack, leading to a better resistance to fatigue fracture in the hand-veneered porcelain relative to over-pressed veneer. Based on in-house evaluations and manufacturer’s information, the composition of both veneering porcelains appears very similar, leucite-free, feldspathic based homogeneous glass. No differences in density/porosity were found.
Fracture of the pontic in the loading area and through one of the two connectors (gingival aspect), a characteristic failure mode of our hand-veneered FDPs, was also found in a previous study on hand-veneered zirconium-oxide specimens 41. Previous works have shown that the gingival aspect of the connectors of FDPs is a zone of high tensile stress 3, 42. Therefore, total fracture might have initiated in that region, propagating toward the loading area of the porcelain in the pontic, and leading to total rupture of FDPs in addition to the buccal chipping of the veneer. The authors expect the gingival aspect of the connectors to be a zone of high stress also for the over-pressed FDPs; however, only three samples showed pontic fracture. This is because the buccal chipping of the over-pressed veneer occurred earlier, before the connector/pontic rupture could take place.
A critical role on the initiation of cracks in the connectors’ gingival region of hand-veneered FDPs might have been played by the core/porcelain thickness ratio, shown to affect the strength of bilayered structures 43–45. Cracks/fractures in the core might have easily developed from the connectors (gingival aspect) in the zirconium-oxide framework, due to a thin layer of the porcelain veneer 46, 47. Therefore, special attention should be given to the design of FDPs, by focusing on the veneer/core thickness ratio and geometry.
To better simulate the clinical environment, the mechanical testing configuration developed in the present study involved rotational loading in the occlusal area of the pontic. This type of configuration was realized by loading the buccal area of the pontic with an inclination of 30 degrees, so that rotational bending of the specimens was involved during mechanical testing. This complex loading geometry in combination with the variability of stress distribution due to the irregular shape of FDPs 48, might be the cause of the various fracture patterns observed in our specimens. As a result, cracks developed either along the connectors towards the occlusal surface or oriented towards the loading area of the pontic. Also, fracture of the abutment, developed in the opposite side of the connector/pontic fracture, might have been a consequence of the rotational loading. The current accelerated lifetime tests were developed using the SSALT method, where unusually high loads were applied. This type of fatigue testing is different from the clinical loading situation, where mainly lower loads and long durations are involved. However, the SSALT method has the advantage of applying high loads within a short time span so that failure is accelerated and specimens can be analyzed in a reasonable time period. In contrast to single load-to-failure tests, the SSALT method allows for cyclic loading, accumulation of subsurface damage and slow crack growths in ceramic materials under wet conditions 49–51. The standardized SSALT method provides a powerful mechanical testing method to screen various FDP systems. The knowledge obtained from the current study provides a useful guideline for the design of better performing FDPs.
5. Conclusions
The present study demonstrates that both over-pressed and hand-veneered three-unit zirconium-oxide FDPs resist chewing loads values 30, 52, 53, suggesting these restorations to be suitable for posterior clinical application. However, the over-pressed specimens are more susceptible to chipping of the veneer in the pontic area, suggesting that the hand-veneered FDPs are more promising with less chance of porcelain fracture.
Acknowledgments
The authors sincerely appreciate the help of Drs Bongok Kim, Jose Perez, Felix Haenssler and Marinée Cabrera, New York University, in specimen preparation, mechanical testing and SEM analysis. Optical microscopy and SEM imaging were kindly advised by Dr. Timothy Bromage in agreement with NIH/NIDCR funding. We are grateful to Ernst Hegenbarth, Zen Line Dental, Bruchköbel, Germany, for his strong commitment in fabricating the oxide-ceramic FDPs. We appreciate the support of Jeppe Magnusson and Fredrik Adilstam (Nobel Biocare, Gothenburg, Sweden) and Steffen Assmann (Wieland Dental Ceramics GmbH, Rosbach, Germany). We thank Dr. Mark Wolff for his assistance in micro-indentation testing. We appreciate Dr. Carlos Fernandes efforts for his schematic drawing. The study was partly funded by Nobel Biocare and received partial support from grants of the United States National Institute of Dental and Craniofacial Research (PO1 DE016755 and 1R01 DE017925) as well as the National Science Foundation (CMMI-0758530).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holm C, Tidehag P, Tillberg A, Molin M. Longevity and quality of FPDs: a retrospective study of restorations 30, 20, and 10 years after insertion. International Journal of Prosthodontics. 2003;16:283–9. [PubMed] [Google Scholar]
- 2.Leempoel PJ, Kayser AF, Van Rossum GM, De Haan AF. The survival rate of bridges. A study of 1674 bridges in 40 Dutch general practices. Journal of Oral Rehabilitation. 1995;22:327–30. doi: 10.1111/j.1365-2842.1995.tb00780.x. [DOI] [PubMed] [Google Scholar]
- 3.Kelly JR. Dental ceramics: what is this stuff anyway? Journal of the American Dental Association. 2008;139:4S–7S. doi: 10.14219/jada.archive.2008.0359. [DOI] [PubMed] [Google Scholar]
- 4.Kawai K, Urano M. Adherence of plaque components to different restorative materials. Operative Dentistry. 2001;26:396–400. [PubMed] [Google Scholar]
- 5.Harder S, Wolfart S, Eschbach S, Kern M. Eight-year outcome of posterior inlay-retained all-ceramic fixed dental prostheses. Journal of Dentistry. 2010;38:875–81. doi: 10.1016/j.jdent.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999;20:1–25. doi: 10.1016/s0142-9612(98)00010-6. [DOI] [PubMed] [Google Scholar]
- 7.Hannink R, Kelly P, Muddle B. Transformation toughening in zirconia-containing ceramics. Journal of the American Ceramics Society. 2000;83:461–87. [Google Scholar]
- 8.Garvie R, Hannink R, Pascoe R. Ceramic Steel? Nature (London) 1975;258:703–4. [Google Scholar]
- 9.Sailer I, Feher A, Filser F, Gauckler LJ, Luthy H, Hammerle CH. Five-year clinical results of zirconia frameworks for posterior fixed partial dentures. International Journal of Prosthodontics. 2007;20:383–8. [PubMed] [Google Scholar]
- 10.Raigrodski AJ, Chiche GJ, Potiket N, Hochstedler JL, Mohamed SE, Billiot S, et al. The efficacy of posterior three-unit zirconium-oxide-based ceramic fixed partial dental prostheses: a prospective clinical pilot study. Journal of Prosthetic Dentistry. 2006;96:237–44. doi: 10.1016/j.prosdent.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Christel P, Meunier A, Heller M, Torre JP, Peille CN. Mechanical properties and short-term in-vivo evaluation of yttrium-oxide-partially-stabilized zirconia. Journal of Biomedical Materials Research. 1989;23:45–61. doi: 10.1002/jbm.820230105. [DOI] [PubMed] [Google Scholar]
- 12.Strub JR, Rekow ED, Witkowski S. Computer-aided design and fabrication of dental restorations: current systems and future possibilities. Journal of the American Dental Association. 2006;137:1289–96. doi: 10.14219/jada.archive.2006.0389. [DOI] [PubMed] [Google Scholar]
- 13.Sturzenegger B, Feher A, Luthy H, Schumacher M, Loeffel O, Filser F, et al. Clinical study of zirconium oxide bridges in the posterior segments fabricated with the DCM system. Schweizische Monatsschrift Zahnmedizin. 2000;110:131–9. [PubMed] [Google Scholar]
- 14.Vult von Steyern P, Carlson P, Nilner K. All-ceramic fixed partial dentures designed according to the DC-Zirkon technique. A 2-year clinical study. Journal of Oral Rehabilitation. 2005;32:180–7. doi: 10.1111/j.1365-2842.2004.01437.x. [DOI] [PubMed] [Google Scholar]
- 15.Baldissara P, Llukacej A, Ciocca L, Valandro FL, Scotti R. Translucency of zirconia copings made with different CAD/CAM systems. Journal of Prosthetic Dentistry. 2010;104:6–12. doi: 10.1016/S0022-3913(10)60086-8. [DOI] [PubMed] [Google Scholar]
- 16.Ntala P, Chen X, Niggli J, Cattell M. Development and testing of multi-phase glazes for adhesive bonding to zirconia substrates. Journal of Dentistry. 2010;38:773–81. doi: 10.1016/j.jdent.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Giannetopoulos S, van Noort R, Tsitrou E. Evaluation of the marginal integrity of ceramic copings with different marginal angles using two different CAD/CAM systems. Journal of Dentistry. 2010;38:980–6. doi: 10.1016/j.jdent.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Guazzato M, Albakry M, Ringer SP, Swain MV. Strength, fracture toughness and microstructure of a selection of all-ceramic materials. Part I. Pressable and alumina glass-infiltrated ceramics. Dental Materials. 2004;20:441–8. doi: 10.1016/j.dental.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Rues S, Kroger E, Muller D, Schmitter M. Effect of firing protocols on cohesive failure of all-ceramic crowns. Journal of Dentistry. 2010;38:987–94. doi: 10.1016/j.jdent.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Bonfante EA, Coelho PG, Guess PC, Thompson VP, Silva NR. Fatigue and damage accumulation of veneer porcelain pressed on Y-TZP. Journal of Dentistry. 2010;38:318–24. doi: 10.1016/j.jdent.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Stappert CF, Kim B, Perez J, Rekow E, Thompson V. Reliability of implant-supported zirconia three-unit fixed-partial-dentures using different veneering porcelains. Journal of Dental Research. 2008;87:1229. [Google Scholar]
- 22.Sowka M, Rekow ED, Thompson VP, Stappert CF. Damage modes and fatigue analysis of Y-TZP three-unit fixed-partial dentures. Journal of Dental Research. 2007;86:888. [Google Scholar]
- 23.Beuer F, Schweiger J, Eichberger M, Kappert HF, Gernet W, Edelhoff D. High-strength CAD/CAM-fabricated veneering material sintered to zirconia copings--a new fabrication mode for all-ceramic restorations. Dental Materials. 2009;25:121–8. doi: 10.1016/j.dental.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Lorenzoni FC, Martins LM, Silva NR, Coelho PG, Guess PC, Bonfante EA, et al. Fatigue life and failure modes of crowns systems with a modified framework design. Journal of Dentistry. 2010;38:626–34. doi: 10.1016/j.jdent.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Silva NR, de Souza GM, Coelho PG, Stappert CF, Clark EA, Rekow ED, et al. Effect of water storage time and composite cement thickness on fatigue of a glass-ceramic trilayer system. J Biomed Mater Res B Appl Biomater. 2008;84:117–23. doi: 10.1002/jbm.b.30851. [DOI] [PubMed] [Google Scholar]
- 26.Bhowmick S, Melendez-Martinez JJ, Hermann I, Zhang Y, Lawn BR. Role of indenter material and size in veneer failure of brittle layer structures. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2007;82:253–9. doi: 10.1002/jbm.b.30728. [DOI] [PubMed] [Google Scholar]
- 27.Yildirim M, Fischer H, Marx R, Edelhoff D. In vivo fracture resistance of implant-supported all-ceramic restorations. Journal of Prosthetic Dentistry. 2003;90:325–31. doi: 10.1016/s0022-3913(03)00514-6. [DOI] [PubMed] [Google Scholar]
- 28.Nelson W. Accelerated testing: statistical models, test plans, and data analysis. New York: Wiley; 1990. pp. 493–520. [Google Scholar]
- 29.Coelho PG, Silva NR, Bonfante EA, Guess PC, Rekow ED, Thompson VP. Fatigue testing of two porcelain-zirconia all-ceramic crown systems. Dental Materials. 2009;25:1122–7. doi: 10.1016/j.dental.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Anderson DJ, Picton DC. Masticatory stresses in normal and modified occlusion. Journal of Dental Research. 1958;37:312–7. doi: 10.1177/00220345580370021701. [DOI] [PubMed] [Google Scholar]
- 31.DeLong R, Douglas WH. Development of an artificial oral environment for the testing of dental restoratives: bi-axial force and movement control. Journal of Dental Research. 1983;62:32–6. doi: 10.1177/00220345830620010801. [DOI] [PubMed] [Google Scholar]
- 32.Abernathy RB. The New Weibull Handbook. North Palm Beach: 2000. [Google Scholar]
- 33.Xu HH, Smith DT, Jahanmir S, Romberg E, Kelly JR, Thompson VP, et al. Indentation damage and mechanical properties of human enamel and dentin. Journal of Dental Research. 1998;77:472–80. doi: 10.1177/00220345980770030601. [DOI] [PubMed] [Google Scholar]
- 34.Baldassarri M, Margolis HC, Beniash E. Compositional determinants of mechanical properties of enamel. Journal of Dental Research. 2008;87:645–9. doi: 10.1177/154405910808700711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anstis GR, Chantikul P, Lawn BR, Marshall DB. A critical evaluation of indentation techniques for measuring fracture toughness; I. Direct crack measurements. Journal of the American Ceramics Society. 1981;64:533–38. [Google Scholar]
- 36.Taskonak B, Yan J, Mecholsky JJ, Jr, Sertgoz A, Kocak A. Fractographic analyses of zirconia-based fixed partial dentures. Dental Materials. 2008;24:1077–82. doi: 10.1016/j.dental.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Fischer J, Stawarczyk B, Trottmann A, Hammerle C. Impact of thermal properties of veneering ceramics on the fracture load of layered Ce-TZP/A nanocomposite frameworks. Dental Materials. 2009;25:326–30. doi: 10.1016/j.dental.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Aboushelib MN, de Jager N, Kleverlaan CJ, Feilzer AJ. Microtensile bond strength of different components of core veneered all-ceramic restorations. Dental Materials. 2005;21:984–91. doi: 10.1016/j.dental.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 39.Aboushelib MN, Kleverlaan CJ, Feilzer AJ. Microtensile bond strength of different components of core veneered all-ceramic restorations. Part II: Zirconia veneering ceramics. Dental Materials. 2006;22:857–63. doi: 10.1016/j.dental.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 40.Isgro’ G, Kleverlann C, Wang H, Feilzer AJ. The influence of multiple firing on thermal contraction of ceramic materials used for the fabrication of layered all-ceramic dental restorations. Dental Materials. 2005;21:557–64. doi: 10.1016/j.dental.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Sundh A, Molin M, Sjogren G. Fracture resistance of yttrium oxide partially-stabilized zirconia all-ceramic bridges after veneering and mechanical fatigue testing. Dental Materials. 2005;21:476–82. doi: 10.1016/j.dental.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 42.Filser F, Kocher P, Weibel F, Luthy H, Scharer P, Gauckler LJ. Reliability and strength of all-ceramic dental restorations fabricated by direct ceramic machining (DCM) International Journal of Computerized Dentistry. 2001;4:89–106. [PubMed] [Google Scholar]
- 43.Wakabayashi N, Anusavice KJ. Crack initiation modes in bilayered alumina/porcelain disks as a function of core/veneer thickness ratio and supporting substrate stiffness. Journal of Dental Research. 2000;79:1398–404. doi: 10.1177/00220345000790060801. [DOI] [PubMed] [Google Scholar]
- 44.Kelly JR, Tesk JA, Sorensen JA. Failure of all-ceramic fixed partial dentures in vitro and in vivo: analysis and modeling. Journal of Dental Research. 1995;74:1253–8. doi: 10.1177/00220345950740060301. [DOI] [PubMed] [Google Scholar]
- 45.Guazzato M, Proos K, Quach L, Swain MV. Strength, reliability and mode of fracture of bilayered porcelain/zirconia (Y-TZP) dental ceramics. Biomaterials. 2004;25:5045–52. doi: 10.1016/j.biomaterials.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 46.Kim J, Bhowmick S, Hermann I, Lawn B. Transverse fracture of brittle bilayers: relevance to failure of all-ceramic dental crowns. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2006;79:58–65. doi: 10.1002/jbm.b.30511. [DOI] [PubMed] [Google Scholar]
- 47.Studart AR, Filser F, Kocher P, Luthy H, Gauckler LJ. Cyclic fatigue in water of veneer-framework composites for all-ceramic dental bridges. Dental Materials. 2007;23:177. doi: 10.1016/j.dental.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 48.Oh WS, Anusavice KJ. Effect of connector design on the fracture resistance of all-ceramic fixed partial dentures. Journal of Prosthetic Dentistry. 2002;87:536–42. doi: 10.1067/mpr.2002.123850. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Pajares A, Lawn BR. Fatigue and damage tolerance of Y-TZP ceramics in layered biomechanical systems. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2004;71:166–71. doi: 10.1002/jbm.b.30083. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Lawn B. Long-term strength of ceramics for biomedical applications. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2004;69:166–72. doi: 10.1002/jbm.b.20039. [DOI] [PubMed] [Google Scholar]
- 51.Kelly JR. Clinically relevant approach to failure testing of all-ceramic restorations. Journal of Prosthetic Dentistry. 1999;81:652–61. doi: 10.1016/s0022-3913(99)70103-4. [DOI] [PubMed] [Google Scholar]
- 52.Bates JF, Stafford GD, Harrison A. Masticatory function-A review of the literature. II. Speed of movement of the mandible, rate of chewing and forces developed in chewing. Journal of Oral Rehabilitation. 1975;2:349–61. doi: 10.1111/j.1365-2842.1975.tb01535.x. [DOI] [PubMed] [Google Scholar]
- 53.Gibbs C, Mahan P, Mauderli A, Lundeen H, Walsh E. Limits of human bite strength. Journal of Prosthetic Dentistry. 1986;56:226–29. doi: 10.1016/0022-3913(86)90480-4. [DOI] [PubMed] [Google Scholar]