Abstract
To monitor noninvasively potentially therapeutic adenoviruses for cancer, we have developed a methodology based on the sodium iodide symporter (NIS). Men with clinically localized prostate cancer were administered an intraprostatic injection of a replication-competent adenovirus, Ad5-yCD/utTKSR39rep-hNIS, armed with two suicide genes and the NIS gene. NIS gene expression (GE) was imaged noninvasively by uptake of Na99mTcO4 in infected cells using single photon emission–computed tomography (SPECT). The investigational therapy was safe with 98% of the adverse events being grade 1 or 2. GE was detected in the prostate in seven of nine (78%) patients at 1 × 1012 virus particles (vp) but not at 1 × 1011 vp. Volume and total amount of GE was quantified by SPECT. Following injection of 1 × 1012 vp in 1 cm3, GE volume (GEV) increased to a mean of 6.6 cm3, representing, on average, 18% of the total prostate volume. GEV and intensity peaked 1–2 days after the adenovirus injection and was detectable in the prostate up to 7 days. Whole-body imaging demonstrated intraprostatic gene expression, and there was no evidence of extraprostatic dissemination of the adenovirus by SPECT imaging. The results demonstrate that noninvasive imaging of adenovirus-mediated gene therapy in humans is feasible and safe.
INTRODUCTION
Adenovirus-mediated gene therapy is an investigational approach that has been evaluated in hundreds of clinical trials targeting single-gene disorders to complex diseases such as cancer. Although no products have been approved by the US Food and Drug Administration to date, the approach, overall, has demonstrated to be safe and encouraging signs of efficacy are beginning to emerge. A limitation of this technology is that it has been difficult to monitor the activity of gene therapy agents following administration to patients. As a result, important questions regarding the local and distant spread of the injected vector, and the persistence of gene expression in the target tissue, remain largely unanswered. If adenovirus-mediated gene therapy is to become a viable therapeutic option in the clinic, methods to monitor these agents while in the patient need to be developed.
We and others have been developing technology that would allow for noninvasive monitoring of gene therapy products using nuclear imaging.1–21 Although the feasibility of several approaches has been established in preclinical models, to our knowledge, there have been only two published reports of imaging virus-mediated gene expression in humans and only one was successful.22,23 In the successful case, a replication-defective adenovirus expressing the herpes simplex virus-1 thymidine kinase (HSV-1 TK) gene was injected intratumorally into patients with hepatocellular carcinoma.22 One advantage of using HSV-1 TK as a reporter gene is that a number of radioactive substrates are available that can be detected by positron emission tomography (PET) or single photon emission–computed tomography (SPECT).16,24,25 Following administration of [18F]-penciclovir, HSV-1 TK expression was detected in liver by PET at adenovirus doses ≥1 × 1012 virus particles (vp). By contrast, attempts to detect HSV-1 TK activity by [123I]-FIAU/SPECT were unsuccessful in glioblastoma patients injected with 106 plaque-forming units (pfu) of a replication-competent mutant of HSV-1.23 It is likely that the failure to detect HSV-1 TK expression was attributable to the relatively low viral dose rather than the limited sensitivity of the [123I]-FIAU/SPECT imaging technology. Although the safety and feasibility of using HSV-1 TK as a reporter gene in humans has been established, additional studies are needed to demonstrate the true potential of this imaging technology.
Another reporter gene system that is being developed is based on the human sodium iodide symporter (hNIS). hNIS catalyzes the uptake of anions such as iodide and technetium, and as with HSV-1 TK, there are a number of hNIS substrates available that can be detected by PET (124I) or SPECT (123I, 125I, 99mTcO4–). We and others demonstrated previously in preclinical models that hNIS reporter gene expression can be detected by PET or SPECT following administration of adenoviruses at doses commonly used in human gene therapy trials.6,8,9,13–15,18,20,21 Moreover, we developed methods that would allow for quantification of the volume and magnitude of gene expression following intratumoral administration.26
Based on the successful application of this technology in preclinical models, we conducted a phase I trial in prostate cancer to evaluate its safety and feasibility in humans. Using an oncolytic adenovirus (Ad5-yCD/mutTKSR39rep-hNIS) armed with two potentially therapeutic suicide genes and the hNIS reporter gene, we demonstrate here that gene expression can be measured noninvasively in the human prostate by SPECT following administration of sodium pertechnetate (Na99mTcO4). Moreover, we were able to estimate the volume, total amount, and persistence of gene expression in the prostate. The knowledge generated is being used to optimize the application of adenovirus-mediated suicide gene therapy in several randomized, controlled trials designed to test for efficacy.
RESULTS
Study design and patient baseline characteristics
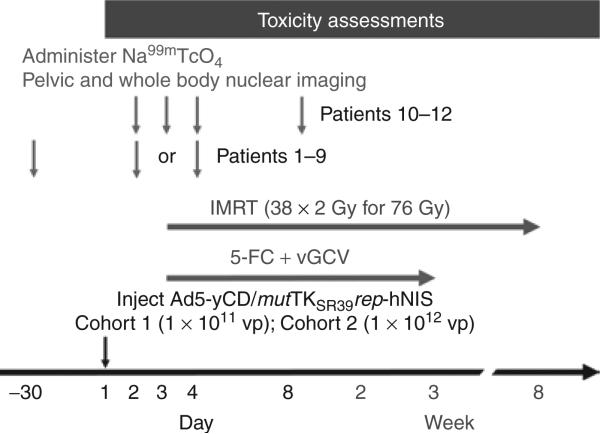
Patients received an intraprostatic injection of the Ad5-yCD/mut-TKSR39rep-hNIS adenovirus on day 1 at a dose of 1 × 1011 vp (cohort 1) or 1 × 1012 vp (cohort 2) (Figure 1). Two days later, patients were administered 5-fluorocytosine + valganciclovir prodrug therapy for 3 weeks (weekdays only) concomitant with a standard course (38 × 2 Gy for 76 Gy) of intensity-modulated radiation therapy (IMRT). Patients in cohort 1 (patients 1–3) and the first six patients in cohort 2 (patients 4–9) underwent two nuclear imaging sessions, one at baseline before the adenovirus injection and one after the adenovirus injection on day 2 or 4. The last three patients in cohort 2 (patients 10–12) underwent multiple imaging sessions, all after the adenovirus injection, to examine the kinetics and persistence of reporter gene expression. These patients did not undergo a baseline scan because (i) no 99mTcO4– uptake was detected in the prostate at baseline in the first nine patients, and (ii) to comply with Food and Drug Administration guidelines regarding the maximum allowable radiation dose delivered to tissues in human research studies [Code of Federal Regulations, 21 CFR 361.1].
Figure 1. Treatment schema.
Patients received an intraprostatic injection of the Ad5-yCD/mutTKSR39rep-hNIS adenovirus on day 1 at a dose of 1 × 1011 virus particles (vp) (cohort 1) or 1 × 1012 vp (cohort 2). Two days later (day 3), patients were administered 5-fluorocytosine (5-FC) + valganciclovir (vGCV) prodrug therapy for 3 weeks (weekdays only) concomitant with a standard course (38 × 2 Gy for 76 Gy) of intensity-modulated radiation therapy (IMRT). Patients 1–9 underwent two nuclear imaging sessions. The first (baseline scan) was within 30 days before the adenovirus injection. The second was following the adenovirus injection on day 2 or 4. Patients 10–12 underwent multiple imaging sessions on days 2, 3, 4 and 8. Na99mTcO4 was not administered, and scheduled imaging sessions were not performed, if no gene expression was detected in the prostate in the previous scan. Toxicity assessments were taken once a week through day 90. hNIS, human sodium iodide symporter.
A total of 12 patients in two cohorts were treated (Table 1). The median follow-up is 12 months (range 3–22 months). The clinical stage was predominately T1c, the mean Gleason score was 7, and the mean prostate-specific antigen (PSA) was 6.9 ng/ml (range 2.5–14.3 ng/ml). In cohort 1 (1 × 1011 vp), adenovirus was deposited in all six sextants. However, the adenovirus dose distribution was skewed toward those sextants known to contain high-grade (Gleason ≥7) cancer. In cohort 2 (1 × 1012 vp), the adenovirus was injected only into those sextants known to contain high-grade cancer with the expectation that the cancer of lower Gleason grade (≤6) would be sterilized by the IMRT.
Table 1.
Patient baseline characteristics and adenovirus injection
| Cohort | Patient | Age (years) | stage | Gleason | Pre-T PSAa (ng/ml) | Cancer locationb | Ad dose (vp) | Sextants injected |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 64 | T1c | 7 (3 + 4) | 11.1 | RA3,4 | 1 × 1011 | 6 sextants—33% RA, 20% RB, 13% RM, 11% each (–) sextant |
| 2 | 78 | T1c | 8 (4 + 4) | 4.8 / 0.2 | LA3, RM3, RA4 | 1 × 1011 | 6 sextants—33% RA, 20% RM, 17% LA, 13% RB, 8% each (–) sextant | |
| 3c | 60 | T1c | 6 (3 + 3) | 4.8 | LB3, LM3, RB3, RM3 | 1 × 1011 | 6 sextants—20% each (+) sextant, 10% each (–) sextant | |
| 2 | 4 | 67 | T1c | 7 (4 + 3) | 5.9 | RA4,3 | 1 × 1012 | 1 sextant—100% RA |
| 5 | 61 | T1c | 7 (3 + 4) | 14.3 / 8.2 | RB3,4 | 1 × 1012 | 2 sextants—50% LB, 50% RB | |
| 6 | 66 | T1c | 7 (3 + 4) | 5.0 | LB3, RB3,4, RM3,4, RA3 | 1 × 1012 | 2 sextants—67% RB, 33% RM | |
| 7 | 64 | T1c | 7 (3 + 4) | 2.5 | LB3, RM3, RA3,4 | 1 × 1012 | 1 sextant—100% RA | |
| 8 | 73 | T1c | 7 (3 + 4) | 6.2 | LM3, LA3,4 | 1 × 1012 | 2 sextants—20% LM, 80% LA | |
| 9 | 70 | T1c | 7 (4 + 3) | 10.8 | LM4,3, LA4,3, RA3 | 1 × 1012 | 2 sextants—40% LM, 60% LA | |
| 10 | 76 | T2a | 7 (3 + 4) | 3.6 | LB3, LM3, RM3,4, RA3,4 | 1 × 1012 | 2 sextants—50% RM, 50% RA | |
| 11 | 63 | T1c | 7 (4 + 3) | 8.1 | RB4,3 | 1 × 1012 | 1 sextant—100% RB | |
| 12 | 67 | T1c | 8 (4 + 4) | 6.2 | LB4,3, LM4 | 1 × 1012 | 2 sextants—40% LB, 60% LM |
Abbreviations: LA, left apex; LB, left base; LM, left midgland; PSA, prostate-specific antigen; RA, right apex; RB, right base; RM, right midgland.
Pre-treatment (Pre-T) PSA before the start of androgen suppression therapy (first number) and before the gene therapy (second number).
The subscripts indicate the Gleason grade present in that sextant. Six to twelve biopsy cores were obtained.
Patient 3 was eligible owing to his pre-treatment PSA velocity of 3.6 ng/ml/year.
Toxicities
Ninety-eight percent of the adverse events regardless of their attribution were mild (grade 1) to moderate (grade 2). There were no dose-limiting toxicities or serious adverse events. Treatment-related adverse events could be attributed to the adenovirus (transaminitis, flu-like symptoms), prodrug therapy (hematological events), or radiation therapy (gastrointestinal and genitouri-nary events, fatigue) (Table 2). Both the incidence and severity of these events mimicked those observed in our three previous gene therapy trials that also used replication-competent adenoviruses.27–29 There were no adverse events related to the nuclear imaging. We conclude that combining adenovirus-mediated gene therapy with nuclear imaging is safe.
Table 2.
Summary of treatment-related adverse events
| Likely attribution Adverse event | Gradea |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Total | |
| Adenoviral therapy | |||||
| Transaminitis | 3 (25) | 0 | 0 | 0 | 3 (25) |
| Flu-like symptomsb | 4 (33) | 0 | 0 | 0 | 4 (33) |
| Prodrug therapy | |||||
| Anemia | 3 (25) | 0 | 0 | 0 | 3 (25) |
| Leukopenia | 5 (42) | 3 (25) | 0 | 0 | 8 (67) |
| Lymphopenia | 2 (17) | 8 (67) | 2 (17) | 0 | 12 (100) |
| Neutropenia | 2 (17) | 1 (8) | 0 | 0 | 3 (25) |
| Thrombocytopenia | 2 (17) | 0 | 0 | 0 | 2 (17) |
| Radiation therapy | |||||
| Gastrointestinalc | 8 (67) | 1 (8) | 0 | 0 | 9 (75) |
| Genitourinaryd | 5 (42) | 4 (33) | 1 (8) | 0 | 10 (83) |
| Fatigue | 3 (25) | 0 | 0 | 0 | 3 (25) |
Number of patients with percent of total in parentheses.
Includes cough, fever, rigors/chills.
Gastrointestinal includes diarrhea, nausea, rectal pain/discomfort, vomiting, nonspecified.
Genitourinary includes dysuria, nocturia, urinary frequency/urgency, urinary incontinence, urinary retention.
Adenovirus-mediated gene expression in the prostate
A major objective of this study was to assess the feasibility of measuring adenovirus-mediated gene expression in the human prostate noninvasively using nuclear imaging. At various days after the adenovirus injection, patients were administered Na99mTcO4 and underwent nuclear imaging 2 hours later (Table 3). At 1 × 1011 vp (cohort 1), no reporter gene expression (i.e., 99mTcO4– uptake) was detected in the prostate 3 days after the adenovirus injection (day 4) (not shown). However, in these three patients, the adenovirus was deposited in all six sextants with 3.3 × 1010 vp being the greatest single deposit made. Based on our preclinical studies in the dog20, this dose per deposit is at the threshold for detection (1–3 × 1010 vp) using the hNIS/SPECT imaging technology used here. It is also possible that reporter gene expression peaked at an earlier time point (i.e., days 2 or 3) in these patients and was therefore missed (see below).
Table 3.
Summary of nuclear imaging results
| Prostate |
Extra-prostatic |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cohort | Patient | Imaging day | GE detecteda | GEVb (cm3) | % Total | GEV:backgroundc | Total GEd | GE detected |
| 1 | 1 | 4 | N | ND | ND | ND | N | |
| 2 | 4 | N | ND | ND | ND | N | ||
| 3 | 4 | N | ND | ND | ND | N | ||
| 2 | 4 | 4 | Y | 7.8 | 17.3 | 3.3 | 25.8 | N |
| 5 | 4 | N | ND | ND | ND | N | ||
| 6 | 4 | Y | 6.6 | 26.7 | 1.5 | 9.8 | N | |
| 7 | 4 | N | ND | ND | ND | N | ||
| 8 | 2 | Y | 6.8 | 19.8 | 2.1 | 14.3 | N | |
| 9 | 2 | Y | 8.3 | 18.1 | 1.9 | 15.8 | N | |
| 10 | 2, 3, 4, 8 | Y, Y, Y, Y | 7.0 | 22.6 | 3.8 | 26.6 | N | |
| 11 | 2, 3, 4 | Y, Y, N | 1.4 | 4.3 | 1.7 | 2.4 | N | |
| 12 | 2, 3, 4, 8 | Y, Y, Y, N | 8.1 | 15.4 | 1.8 | 14.9 | N | |
| Mean | 6.6 | 17.7 | 2.3 | 15.7 | ||||
Gene expression (GE) detected by nuclear imaging. Y, yes; N, no.
Gene expression volume (GEV) detected in the prostate at maximum. The % total is the GEV divided by the total prostate volume as determined by transrectal ultrasound.
Ratio of mean pixel intensity in region of interest (ROI) containing gene expression to mean pixel intensity of ROI in the uninjected prostate (background).
Total GE is the GEV multiplied by the GEV:background ratio. The units are arbitrary.
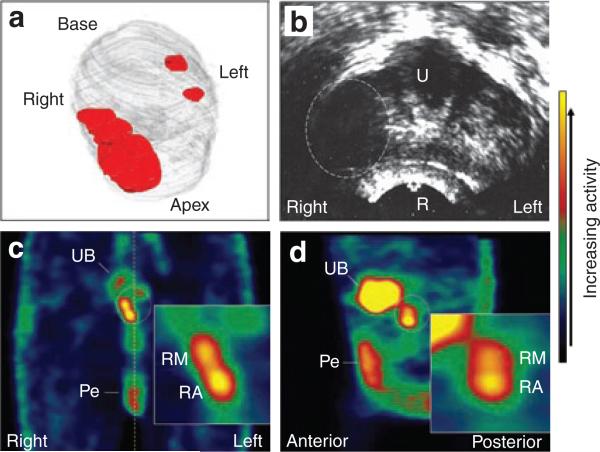
By contrast, at 1 × 1012 vp (cohort 2), reporter gene expression was detected in the prostate in 7 of 9 (78%) patients (Table 3). An example is shown in Figure 2. In patient 10, the bulk of the cancer (Gleason 7) was located in the right midgland and apex regions, and only a few malignant glands of lower Gleason grade were identified in the left base and midgland sextants (Figure 2a). The palpable tumor on the right side of the prostate was readily identified as a hypoechoic area by transrectal ultrasound (Figure 2b). Therefore, two 0.5-ml deposits of 5.0 × 1011 vp each (1 × 1012 vp total) were injected into the right midgland and apex regions where the bulk of the cancer resided. One (day 2), two (day 3), three (day 4), and seven (day 8) days after the adenovirus injection, the patient was administered Na99mTcO4 and underwent nuclear imaging. On day 3 when reporter gene expression peaked, two distinct, but overlapping, regions of 99mTcO4– uptake were detected in the right midgland and apex regions where the adenovirus was injected (Figures 2c and d). By contrast, no reporter gene expression was detected in the noninjected sextants. These results demonstrate that the sensitivity of the hNIS/SPECT technology is at least 5.0 × 1011 vp/adenovirus deposit.
Figure 2. Patient 10 treatment planning and imaging results.
(a) Three-dimensional reconstruction of the prostate (gray) with approximate location of the cancer (red) based on 12-core biopsy. The bulk of the cancer with Gleason 7 pattern resided in the right midgland and apex regions, and only a few malignant glands of Gleason 6 were noted on the left side. (b) A single, transverse transrectal ultrasound image of the midgland/apex region acquired immediately before adenovirus injection. The bulky tumor on the right side appears as a hypoechoic region (dashed oval). The urethra (U), rectum (R), right and left sides of the patient are indicated. (c,d), Coronal and sagittal single photon emission–computed tomography images, respectively, of the pelvic region 2 days following the adenovirus injection. The color bar on the right indicates the relative activity. The location of the prostate is indicated by the dotted oval. Activity in the urinary bladder (UB) and penis (Pe) is indicated. The activity in the penis is due to blood flow through that organ and is seen in the baseline scans. The dotted yellow line in c indicates the midline. The right, left, anterior and posterior sides of the patient are indicated. The inserts show gene expression in the prostate at higher magnification. RM, right midgland; RA, right apex.
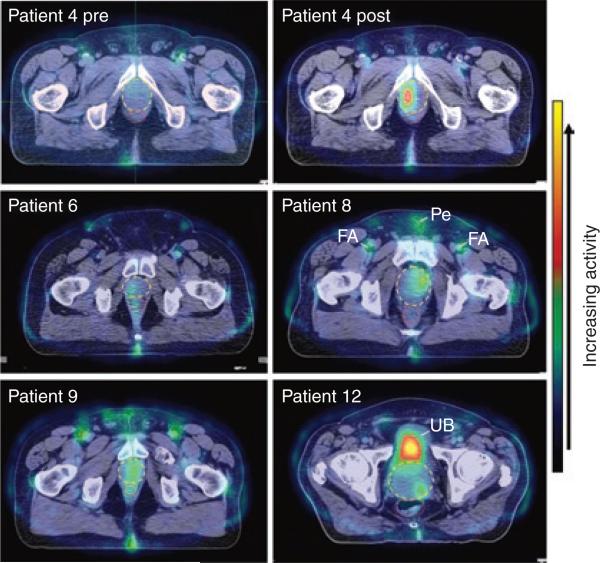
Reporter gene expression was detected in the prostate in six other patients injected with 1 × 1012 vp. As expected, the level of gene expression varied from patient to patient (Figure 3). The results of patient 4 are shown next to his baseline scan. In all cases, reporter gene expression was detected only in the sextants that were injected with adenovirus, and no 99mTcO4– uptake was detected in the prostate at baseline.
Figure 3. Gene expression in the prostate for patients 4, 6, 8, 9 and 12.
Shown are fused computerized tomography/single photon emission–computed tomography images of a single transverse section at the maximum intensity of gene expression. For patient 4, the pretreatment (pre) and post-treatment (post) scans of the same transverse section are shown for comparison. The prostate and rectum are indicated by yellow dashed and red dotted ovals, respectively. Activity in the femoral arteries (FA) and penis (Pe) can be seen in some patients owing to blood flow through those structures. The activity seen at the posterior of all patients is due to leakage of radioactive urine during the imaging session. In patients 4, 6, 8, and 9, activity in the urinary bladder (UB) is not seen because the transverse section shown is well below the bladder. In patient 12, the maximum level of gene expression was found in the left base region and, therefore, the high activity in the UB is seen. The color bar on the right indicates the relative activity.
Quantitation of gene expression amount and volume
A second objective was to demonstrate that we could quantify the volume and total amount of reporter gene expression in the prostate. The first measurement (volume) is used to estimate the amount of adenovirus spread after injecting a fixed volume and to estimate the total amount of gene expression in the prostate. The second measurement (total amount) will be used in this and future trials to examine the possible relationship between the amount of gene expression in the prostate and established measures of clinical outcome (e.g., 2-year biopsy status, time to PSA failure, survival).
Results of the nuclear imaging studies are summarized in Table 3. Following injection of 1 × 1012 vp delivered in 1 cm3, gene expression volume (GEV) increased to a mean of 6.6 cm3 (range 1.4–8.3 cm3). When excluding the one outlier (patient 11), the GEV was relatively consistent from patient to patient and varied over only a 1.5-fold range. The mean pixel intensity of the volume containing gene expression was, on average, 2.3 times that measured in the uninjected prostate. The total amount of gene expression, which is the product of GEV and mean pixel intensity after correcting for the prostate background, varied over a 11-fold range when considering all positive patients, and over a 2.7-fold range when excluding the one outlier (patient 11).
Kinetics and persistence of gene expression in the prostate
A third objective was to examine the kinetics and persistence of reporter gene expression in the prostate. To accomplish this, patients 10–12 were administered Na99mTcO4 one (day 2), two (day 3), three (day 4), and seven (day 8) days after the adenovirus injection and underwent nuclear imaging on each day. Na99mTcO4 was not administered, and scheduled imaging sessions were not performed, if no gene expression was detected in the prostate in the previous scan.
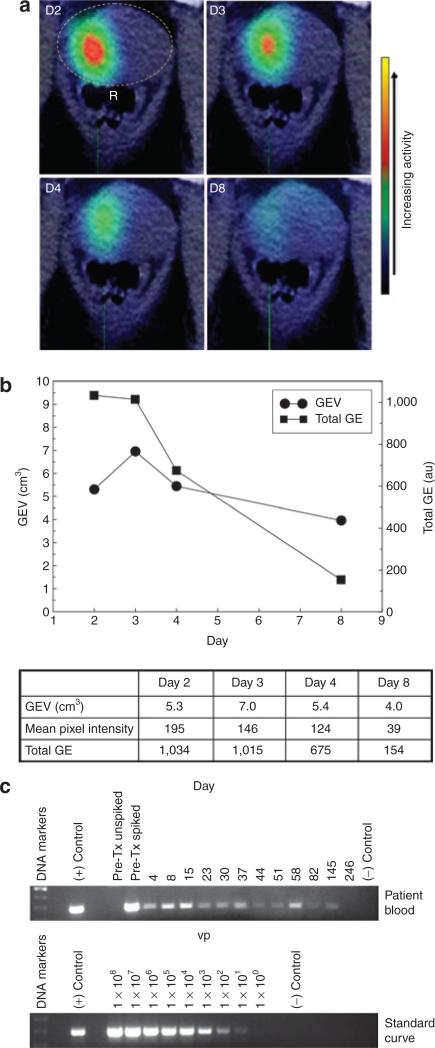
In patient 10, reporter gene expression was detected in the prostate on all days in which imaging was performed (Figure 4a). A plot of the volume (GEV) and total amount of gene expression showed that both peaked 1 or 2 days after the adenovirus injection and then declined thereafter (Figure 4b). One week after the adenovirus injection (day 8), the amount of gene expression in the prostate was ~15% that on days 2 and 3. By contrast, PCR of adenoviral DNA in blood demonstrated that the adenovirus persisted in patient 10 for at least 145 days (~5 months) (Figure 4c). Reporter gene expression was also detected on multiple days in patients 11 and 12 (Table 3). As observed in patient 10, gene expression peaked one or two days after the adenovirus injection and was undetectable by day 4 (patient 11) and day 8 (patient 12).
Figure 4. Kinetics and persistence of gene expression (GE) in the prostate in patient 10.
The Ad5-yCD/mutTKSR39rep-hNIS adenovirus was injected on day 1 at 1 × 1012 virus particles (vp). a, Fused computerized tomography/single photon emission–computed tomography images of a single transverse section at the maximum intensity of GE 1 (D2), 2 (D3), 3 (D4), and 7 (D8) days after adenovirus injection. In the day 2 panel, the prostate is indicated by a dashed yellow oval and the rectum is labeled R. The color bar on the right indicates the relative activity. (b) The volume (circles) and total amount (squares) of GE on each day of imaging are plotted. Actual values are shown in the table along with the mean pixel intensity. (c) PCR of Ad5-yCD/mutTKSR39rep-hNIS viral DNA in blood. Pre-Tx unspiked, pretreatment blood with no added adenovirus. Pre-Tx spiked, pretreatment blood spiked with 1 × 104 vp of Ad5-yCD/mutTKSR39rep-hNIS. The day of each blood draw is indicated above the lanes with day 1 being the day of the adenovirus injection. (+) Control, purified Ad5-yCD/mutTKSR39rep-hNIS viral DNA. (–) Control, no DNA. The PCR signal reflects the amount of adenoviral DNA present in 20 μl of blood. To calculate the total adenoviral load, use the standard curve to estimate the viral particles and multiply by 250,000 (50 to account for volume of blood used in the PCR assay and 5,000 to account for the average human blood volume). At its peak (day 15), the viral load was ~2.5 × 108 vp, or ~0.025% of the injected adenovirus dose. The number above each lane in the standard curve indicates the amount of adenoviral DNA in vp. The sensitivity of the PCR assay is at least 10 vp. hNIS, human sodium iodide symporter.
Whole-body imaging
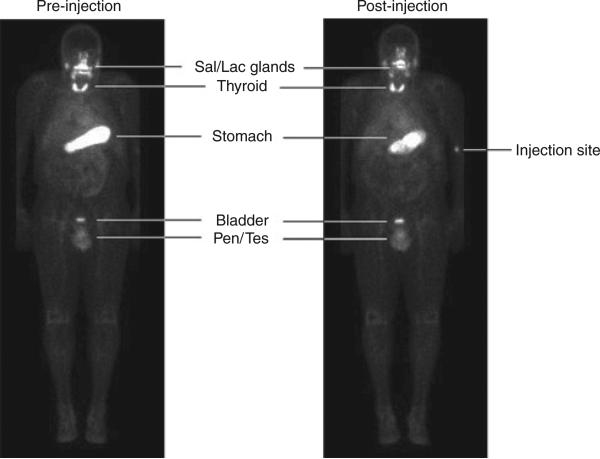
Nine patients were imaged before the adenovirus injection to establish a baseline level of 99mTcO4 uptake in the prostate and to examine the expression of reporter gene in extraprostatic tissues. At baseline, several organs including salivary and lachrymal glands, thyroid, and stomach demonstrated 99mTcO4– uptake owing to endogenous hNIS expression (Figure 5). The high activity observed in urinary bladder, penis, and testicles is attributable to Na99mTcO4 in urine or blood, not hNIS expression. Following the adenovirus injection, no 99mTcO4– uptake was detected in any extraprostatic tissue other than those tissues that demonstrated activity at baseline.
Figure 5. Whole-body imaging.
Shown are whole-body planar scans before (preinjection) and 3 days after (post-injection) the adenovirus injection for patient 7. Activity in the salivary glands (Sal), lachrymal glands (Lac), thyroid gland, and stomach is due to endogenous human sodium iodide symporter expression. The activity observed in the penis (Pen) and testicles (Tes) is due to blood flow through those structures. Na99mTcO4 is secreted through the urinary tract, accounting for the activity in the urinary bladder. The intravenous 99mTcO4 injection site can be seen in the post-injection scan.
DISCUSSION
This phase I study had multiple objectives. The first was to determine the safety of combining adenovirus-mediated gene therapy with nuclear imaging. The combined treatment was associated with low morbidity and found to be safe. A second objective was to determine the feasibility of measuring adenovirus-mediated gene expression in the human prostate gland noninvasively using nuclear imaging. The likelihood of success was uncertain owing to the close proximity of the urinary bladder to the prostate. Because many radioactive tracers, including Na99mTcO4, are excreted through the urinary tract, it was possible that the intense signal observed in the urinary bladder would mask any reporter gene expression in the prostate. We demonstrate that at adenovirus doses ≥5 × 1011 vp/deposit, hNIS reporter gene expression can be detected in the prostate by SPECT. To our knowledge, this is the first demonstration of imaging adenovirus-mediated gene expression in the human prostate. The sensitivity we observed here agrees well with a previous study in hepatocellular carcinoma in which adenovirus-mediated HSV-1 TK gene expression was detected by PET at doses ≥1 × 1012 vp.22 Based on our preclinical studies in dog,20 and the robust signals observed in patient 10 who received two deposits of 5 × 1011 vp, we believe the sensitivity of the hNIS/SPECT technology is likely to be greater (probably ≥1 × 1011 vp/deposit). However, it is important to point out that a replication-competent adenovirus was used here, and it is likely that higher gene expression was achieved relative to what would be possible with a replication-defective adenovirus. Nevertheless, it is clear that both the hNIS/SPECT and HSV-1 TK/PET imaging technologies can be used to monitor adenovirus-mediated gene expression at adenovirus doses (~1012 vp) that are known to be safe in humans.
Another objective was to estimate the volume and total amount of gene expression in the prostate. Details regarding the methodology, which was developed in preclinical models, have been described previously.20,26 We demonstrate that following injection of 1 × 1012 vp in a volume of 1 cm3 (1 ml), GEV increased to a mean of 6.6 cm3 representing, on average, ~18% of the total prostate volume. However, owing to the limited resolution of SPECT (~8 mm), the GEVs reported here are only estimates, and the true GEV, which can only be determined with greater resolving power,20,26 is likely to be lower. Because most of the increase in GEV (relative to the injected volume) occurred 1 day after the adenovirus injection and before the completion of one viral replication cycle (≥1.5 days), it is likely that most of the observed increase in GEV is attributable to spread at the time of injection. This spread is likely to occur by dilution of the injectate by tissue fluids. Any additional increase in GEV beyond that could be attributable to viral replication, for the initial infection volume is probably fixed shortly after deposition of the virus. Our estimates of the GEV suggest that it would require at least six 1-cm3 (1 ml) adenovirus deposits, or about one deposit per sextant, to cover the average human prostate (~40 cm3) with therapeutic gene expression. This is achievable, for it has been demonstrated that up to 8 ml (80 × 0.1 ml deposits, total dose of 1 × 1013 vp) of a replication-competent adenovirus can be injected into the human prostate safely.30 Thus, for a patient who has disease throughout the prostate (e.g., 10 of 12 cores positive for cancer), a reasonable treatment plan might be to inject two 0.5–0.75-ml deposits (1.0–1.5 ml) into each of the four larger sextants (right and left base and midgland sextants), and one 0.5–0.75-ml deposit into each of the two smaller apex sextants, for a total volume of 5.0–7.5 ml. By contrast, when treating a patient with more focal disease, it might make sense to skew the adenovirus dose distribution toward those regions known to contain the bulk, or high grade, cancer. The rationale behind this strategy is to concentrate the gene therapy in those regions of high (or high grade) tumor burden where standard cancer treatments such as radiation therapy are more likely to fail. As with IMRT, each gene therapy treatment plan should be tailored to achieve the maximal possible benefit to the patient.
In addition to measuring the GEV, we were able to estimate the total amount of gene expression in the prostate. We found that the total amount of gene expression varied over an 11-fold range when considering all positive patients, and over a 2.7-fold range when excluding the one outlier (patient 11). Although this measurement, by itself, has little value, it will allow us to correlate the amount of gene expression in the prostate with established measures of clinical outcome (e.g., 2-year biopsy status, time to PSA failure, survival). Such correlations could help establish cause and effect, as well as determine the minimum threshold of gene expression in the prostate required to achieve an improvement in clinical outcome.
We also obtained preliminary information regarding the kinetics and persistence of adenovirus-mediated gene expression in the human prostate. This information can be used to determine the optimal duration of prodrug administration, and to develop treatment plans that call for repeated adenovirus dosing. We demonstrate that adenovirus-mediated gene expression in the prostate peaks 1–2 days after administration of the virus, and that it can persist in the prostate for at least 7 days. The rapid decline in gene expression is likely attributable to death of infected cells due to the cytolytic actions of the replication-competent adenovirus, as well as the immune response to adenovirus-infected cells.31–33 However, it is likely that gene expression persists in the prostate for a longer period, and that the hNIS/SPECT imaging is detecting only the “tip of the iceberg.” This possibility is supported by our previous observations that gene expression can be detected in the prostate by biopsy 3 weeks after the adenovirus injection.28 Moreover, PCR of adenoviral DNA in blood showed that the adenovirus can persist in patients for up to ~5 months. We speculate that the protracted persistence of adenoviral DNA in blood may reflect a low level of viral replication (infection and re-infection) that is below the threshold of detection by SPECT. Although the source of the adenoviral DNA detected in blood is unknown, the results of our first gene therapy trial without radiation therapy may shed light on this issue.27 Patients who received the gene therapy and responded to the treatment exhibited PSA control as long as adenoviral DNA was detected in blood. Once the adenovirus was cleared, all patients exhibited PSA relapse. These observations suggest that the adenoviral DNA detected in blood likely reflects active adenovirus back in the prostate. If the source of the adenoviral DNA was an extraprostatic tissue, e.g., liver, we would not expect to see such a tight association between adenovirus persistence and PSA control. Taken together, the data indicate that the adenovirus can persist in the prostate for a prolonged period (up to 5 months), although high levels of gene expression are short lived (<1 week). In the near future, the hNIS/SPECT imaging technology will be used to measure the magnitude and persistence of gene expression after repeated adenovirus injections.
We are pleased to find that no adenovirus-mediated gene expression was detected in extraprostatic tissues, particularly liver, in the 12 patients treated. However, this conclusion must be tempered owing to the relatively low sensitivity of the hNIS/SPECT imaging technology. It is unlikely that we would be able to detect any extraprostatic gene expression unless the amount was significant (≥1011 vp, or 10% of the injected dose) and the infection was focal. In 55 prostate cancer patients treated to date, 33% have developed transaminitis with the vast majority (89%) being short-lived (~1 week) grade 1 events. These events are likely attributable to dissemination of a small amount of the injected adenovirus to liver, which cannot be detected by hNIS/SPECT imaging. Nevertheless, they are minor, transient, and produce no noticeable symptoms to the patient. A strength of using Ad5-yCD/mutTKSR39rep-hNIS for biodistribution studies is that the hNIS reporter gene is under the control of the ubiquitous cytomegalovirus promoter. Therefore, hNIS can be expressed in any tissue, allowing for accurate assessment of its biodistribution provided the level of gene expression is above the threshold for detection. This is not possible with adenoviruses in which the reporter gene is under the control of prostate-specific promoters.34 In this case, reporter gene expression can only be detected in the prostate, which might lead to incorrect conclusions regarding its dissemination to critical organs.
Another advantage of using Ad5-yCD/mutTKSR39rep-hNIS for imaging studies is that multiple imaging modalities can be used. For example, in tissues that are not amenable to hNIS/SPECT imaging due to endogenous hNIS activity (e.g., stomach, thyroid) or 99mTcO4– uptake is masked by nearby activity (e.g., stomach masking pancreas), Ad5-yCD/mutTKSR39rep-hNIS could still be imaged using the HSV-1 TK/PET reporter system.35 Unfortunately, the normal brain does not take up pertechnetate or any HSV-1 TK substrate. Therefore, it is unclear whether these reporter systems could be used in studies of glioblastoma. Nevertheless, because Ad5-yCD/mutTKSR39rep-hNIS contains three genes that can be detected noninvasively (cytosine deaminase by magnetic resonance spectroscopy, HSV-1 TK by SPECT and PET, hNIS by SPECT and PET), it can be used to monitor adenovirus-mediated suicide gene therapy in multiple cancer sites using multiple imaging modalities.
In conclusion, the results described here shed light on a number of issues regarding adenovirus-mediated gene therapy. Now that the feasibility and limitations of this technology have been established in humans, we believe the knowledge generated here, and in future studies, has the potential to improve the safety and efficacy of human gene therapy.
MATERIALS AND METHODS
Study design
The primary objective of this phase I study was to determine the safety and feasibility of monitoring adenovirus-mediated gene expression in humans using nuclear imaging. All patients were treated in the Department of Radiation Oncology at the Henry Ford Hospital. The study called for two cohorts of three to six patients with clinically localized prostate cancer to receive a single intraprostatic injection (cohort 1–1011 vp; cohort 2–1012 vp) of the Ad5-yCD/mutTKSR39rep-hNIS adenovirus followed by 3 weeks of 5-fluorocytosine and valganciclovir prodrug therapy and concomitant 76-Gy IMRT. Within 30 days before the adenovirus injection, patients 1–9 were administered 16-mCi Na99mTcO4 and underwent whole-body and pelvic planar scans using gamma camera scintigraphy, and pelvic SPECT, to establish a baseline. At various times following the adenovirus injection (see figure legends), patients were administered 16 mCi Na99mTcO4 and underwent whole-body and pelvic planar scans to examine for Ad5-yCD/mut TKSR39rep-hNIS-mediated 99mTcO4– uptake in body tissues. If specific 99mTcO4– uptake was detected in the prostate, SPECT of the pelvic region was performed to obtain a three-dimensional tomographical image of the prostate to estimate the volume and total amount of gene expression.
The primary end point was toxicity up to and including Day 90. Secondary end points included (i) feasibility of using hNIS as a reporter gene to monitor adenoviral gene therapy vectors noninvasively in vivo, (ii) volume of reporter gene expression in the prostate, (iii) kinetics and persistence of reporter gene expression in the prostate, and (iii) whole-body 99mTcO4– uptake before and after the adenovirus injection. The protocol received all required regulatory approvals before initiation and was conducted under BB-IND 12786 (RAC protocol 0501-690). Good clinical practices were used throughout.
Patient selection
Patients were required to have histologically confirmed, localized adenocarcinoma of the prostate, clinical stages T1/T2, Gleason score ≥7 or serum PSA > 10 ng/ml and ≤50 ng/ml or serum PSA velocity >2 ng/ml/year. Patients were required to have adequate baseline organ function, as assessed by the following laboratory values, before initiating the protocol: (i) adequate renal function with serum creatinine ≤1.5 mg/dl or creatinine clearance ≥45 ml/min/m2; (ii) platelet count >100,000/mm3; (iii) absolute neutrophil count >1,000/mm3; (iv) hemoglobin >10.0 g/ml; (v) normal partial thromboplastin time and prothrombin time; and (vi) bilirubin <1.5 mg/dl, and serum glutamic oxaloacetic transaminase and serum glutamic pyruvic transaminase <2.5 times the upper limit of normal. Patients had to have the ability to give informed consent and express a willingness to meet all of the expected requirements of the protocol for the duration of the study. Patients with any one of the following conditions were excluded from the study: (i) radiological evidence of metastatic disease, (ii) prior pelvic radiation therapy or chemotherapy, (iii) active acute infection, (iv) on immunosuppressive therapy including systemic corticosteroids, or (v) prior history of liver disease including hepatitis. Patients receiving neoadjuvant androgen-suppression therapy for their prostate cancer were eligible. All patients met the eligibility requirements of the protocol and signed the informed consent document. There were no protocol violations.
Pretreatment planning and injection of Ad5-yCD/mutTKSR39rep-hNIS adenovirus
Pretreatment planning included transrectal ultrasound–guided needle biopsy of the prostate and computerized tomography (CT) simulation. Six to fourteen biopsy cores were taken to map the location of the cancer within the prostate. Three-dimensional CT reconstructions of the prostate were generated for IMRT treatment planning.
Injection of the Ad5-yCD/mutTKSR39rep-hNIS adenovirus was performed on an outpatient basis on day 1. Thirty minutes before the injection, the adenovirus was diluted to the proper concentration with sterile saline. The final injection volume was 3 ml (cohort 1) and 1 ml (cohort 2). With patients in the lateral decubitus position, the adenovirus was injected under transrectal ultrasound–guidance using a 20-gauge needle as indicated in Table 1. One to six individual deposits were made depending on the location and extent of the cancer.
Administration of prodrugs
Prodrugs were administered on an outpatient basis. 5-Fluorocytosine (Ancobon; Roche Laboratories, Basel, Switzerland) was administered orally beginning on day 3 and continued for 3 weeks (weekdays only). A total of 150 mg/kg/day was given in four equally divided doses. valganciclovir (Valcyte; Roche Laboratories) was administered orally beginning on day 3 and continued for 3 weeks (weekdays only). A total of 1,800 mg/day was given in two equally divided doses every 12 h. The research nurse assigned to the trial counted the pills periodically to monitor patient compliance.
Administration of IMRT
All patients received 76-Gy (38 fractions × 2 Gy) IMRT using state-of-the-art treatment techniques, including three-dimensional CT simulation, three-dimensional treatment planning, and Varian 2100C/D linear accelerator with multileaf collimator beam shaping device. The initial clinical target volume was the prostate and entire seminal vesicles, which was treated to 50 Gy in 2-Gy fractions. This was followed by a cone-down to the prostate and proximal seminal vesicles for an additional 26 Gy in 2-Gy fractions. In each case, the planning-target volume was conformed to the clinical target volume plus 0.6–1.0-cm margin to account for organ motion. Daily image-guided radiation therapy was performed using ultrasound. IMRT began on day 3 concomitant with the initiation of the prodrug therapy.
Patient safety monitoring
The following evaluations were conducted once a week following the adenovirus injection through day 90: (i) physical examination, (ii) blood chemistries and complete blood counts, (iii) serum PSA, (iv) presence of Ad5-yCD/mutTKSR39rep-hNIS viral DNA in blood. The primary end point was toxicity up to and including day 90. Toxicities were graded using the National Cancer Institute's Common Toxicity Criteria. The study was monitored by an internal Data and Safety Monitoring Board.
Nuclear image acquisition and processing
Scans were conducted with a Siemens E CAM dual head gamma camera (Siemens Medical Solutions USA, Hoffman Estates, IL). Two hours before imaging, patients were administered intravenously a mean dose of 16 mCi (range 14.3–17.4 mCi) sodium pertechnetate (Na99mTcO4) in 0.5 ml saline. Image acquisition consisted of three separate scans. A whole-body scan (15 cm/min scan rate; 189 cm scan length) was acquired first. The second scan consisted of a 10-minute static acquisition of the pelvic region. A SPECT scan was acquired last, in which a total of 120 (60/head) 30-second views were acquired using a 128 × 128 matrix and a zoom of 1. To reduce the activity in the urinary bladder, patients were asked to void immediately before the whole-body scan and again before the SPECT acquisition. Total imaging time was ~90 minutes. All acquired images were analyzed using commercially available software (MIM version 3.5; MIMVista, Cleveland, OH). CT treatment simulations obtained for IMRT treatment plans were used to generate the CT/SPECT fusion images. CT and SPECT image registration was conducted using automated and manual registration functions of the image processing software.
Quantitative measurements of reporter gene expression were obtained by identifying regions of interest (ROIs) that included, at a minimum, gene expression in the regions of the prostate that were injected with adenovirus. ROIs of similar size were identified in the uninjected side of the prostate to determine the background level of activity. ROI contours were drawn automatically using a tissue segmentation threshold function of the MIM software. GEV and mean pixel intensity of each ROI were determined and used to calculate the total amount of gene expression in the prostate.
Technetium uptake is transient and its energy emission is relatively low; hence, the total dose delivered to thyroid is minimal. Based on the results from preclinical models, the calculated dose delivered to the thyroid when exposed to internal radiation from systemically injected technetium is ~1.4 rem (36). Thus, there was no need to block the thyroid before radiotracer administration.
PCR of Ad5-yCD/mutTKSR39rep-hNIS adenoviral DNA in blood
Blood was obtained before the adenovirus injection and at least once a week after for semi-quantitative determination of adenoviral DNA in blood. DNA from blood was purified on Qiagen columns using procedures recommended by the manufacturer. DNA from 20 μl of blood was used as template in the PCR assays. The 5′ primer hybridizes to the linker between the yCD and mutant HSV-1 TK gene, and the 3′ primer hybridizes to the mutant HSV-1 TK gene. The PCR product is 388 bp in length and specific for the yCD/mutTKSR39 fusion gene contained in Ad5-yCD/mutTKSR39rep-hNIS. To generate a standard curve, 2.5 × 109 vp of Ad5-yCD/mutTKSR39rep-hNIS was added to 0.5 ml of human volunteer blood making a concentration of 5.0 × 109 vp/ml. Serial tenfold dilutions were prepared down to 50 vp/ml using human volunteer blood as the diluent.
Manufacturing Ad5-yCD/mutTKSR39rep-hNIS adenovirus
The replication-competent Ad5-yCD/mutTKSR39rep-hNIS adenovirus has been described previously.20 Clinical-grade (GMP) adenovirus was manufactured at the Baylor College of Medicine Vector Production Facility (Houston, TX). The adenovirus was supplied as a sterile, clear, frozen liquid in vials containing 1.25 ml at a concentration of 1.0 × 1012 vp/ml. The vp-to-pfu ratio of the undiluted final product was 15. The potency of the clinical lot was re-evaluated every 6 months and there was no diminution of potency over the phase 1 study period.
REFERENCES
- 1.Tjuvajev JG, Finn R, Watanabe K, Joshi R, Oku T, Kennedy J, et al. Noninvasive imaging of herpes virus thymidine kinase gene transfer and expression: a potential method for monitoring clinical gene therapy. Cancer Res. 1996;56:4087–4095. [PubMed] [Google Scholar]
- 2.Shimura H, Haraguchi K, Miyazaki A, Endo T, Onaya T. Iodide uptake and experimental 131I therapy in transplanted undifferentiated thyroid cancer cells expressing the Na/I symporter gene. Endocrinology. 1997;138:4493–4496. doi: 10.1210/endo.138.10.5571. [DOI] [PubMed] [Google Scholar]
- 3.Tjuvajev JG, Chen SH, Joshi A, Joshi R, Guo ZS, Balatoni J, et al. Imaging adenoviral-mediated herpes virus thymidine kinase gene transfer and expression in vivo. Cancer Res. 1999;59:5186–5193. [PubMed] [Google Scholar]
- 4.Gambhir SS, Barrio JR, Phelps ME, Iyer M, Namavari M, Satyamurthy N, et al. Imaging adenoviral-directed reporter gene expression in living animals with positron emission tomography. Proc Natl Acad Sci USA. 1999;96:2333–2338. doi: 10.1073/pnas.96.5.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacLaren DC, Gambhir SS, Satyamurthy N, Barrio JR, Sharfstein S, Toyokuni T, et al. Repetitive, non-invasive imaging of the dopamine D2 receptor as a reporter gene in living animals. Gene Ther. 1999;6:785–791. doi: 10.1038/sj.gt.3300877. [DOI] [PubMed] [Google Scholar]
- 6.Mandell R, Mandell L, Link C. Radioisotope concentrator gene therapy using the sodium/iodide symporter gene. Cancer Res. 1999;59:661–698. [PubMed] [Google Scholar]
- 7.Herschman HR, MacLaren DC, Iyer M, Namavari M, Bobinski K, Green LA, et al. Seeing is believing: non-invasive, quantitative and repetitive imaging of reporter gene expression in living animals, using positron emission tomography. J Neurosci Res. 2000;59:699–705. doi: 10.1002/(SICI)1097-4547(20000315)59:6<699::AID-JNR1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Boland A, Ricard M, Opolon P, Bidart JM, Yeh P, Filetti S, et al. Adenovirus-mediated transfer of the thyroid sodium/iodide symporter gene into tumors for a targeted radiotherapy. Cancer Res. 2000;60:3484–3492. [PubMed] [Google Scholar]
- 9.Spitzweg C, O'Connor M, Bergert E, Tindall D, Young C, Morris J. Treatment of prostate cancer by radioiodine therapy after tissue-specific expression of the sodium iodide symporter. Cancer Res. 2000;60:6526–6530. [PubMed] [Google Scholar]
- 10.Yu Y, Annala AJ, Barrio JR, Toyokuni T, Satyamurthy N, Namavari M, et al. Quantification of target gene expression by imaging reporter gene expression in living animals. Nat Med. 2000;6:933–937. doi: 10.1038/78704. [DOI] [PubMed] [Google Scholar]
- 11.Gambhir SS, Bauer E, Black ME, Liang Q, Kokoris MS, Barrio JR, et al. A mutant herpes simplex virus type 1 thymidine kinase reporter gene shows improved sensitivity for imaging reporter gene expression with positron emission tomography. Proc Natl Acad Sci USA. 2000;97:2785–2790. doi: 10.1073/pnas.97.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs A, Tjuvajev JG, Dubrovin M, Akhurst T, Balatoni J, Beattie B, et al. Positron emission tomography-based imaging of transgene expression mediated by replication-conditional, oncolytic herpes simplex virus type 1 mutant vectors in vivo. Cancer Res. 2001;61:2983–2995. [PubMed] [Google Scholar]
- 13.Haberkorn U, Henze M, Altmann A, Jiang S, Morr I, Mahmut M, et al. Transfer of human NaI symporter gene enhances iodide uptake in hepatoma cells. J Nucl Med. 2001;42:317–325. [PubMed] [Google Scholar]
- 14.Groot-Wassink T, Aboagye EO, Glaser M, Lemoine NR, Vassaux G. Adenovirus biodistribution and noninvasive imaging of gene expression in vivo by positron emission tomography using human sodium/iodide symporter as reporter gene. Hum Gene Ther. 2002;13:1723–1735. doi: 10.1089/104303402760293565. [DOI] [PubMed] [Google Scholar]
- 15.Sharma V, Luker GD, Piwnica-Worms D. Molecular imaging of gene expression and protein function in vivo with PET and SPECT. J Magn Reson Imaging. 2002;16:336–351. doi: 10.1002/jmri.10182. [DOI] [PubMed] [Google Scholar]
- 16.Tjuvajev JG, Doubrovin M, Akhurst T, Cai S, Balatoni J, Alauddin MM, et al. Comparison of radiolabeled nucleoside probes (FIAU, FHBG, and FHPG) for PET imaging of HSV1-tk gene expression. J Nucl Med. 2002;43:1072–1083. [PubMed] [Google Scholar]
- 17.Liang Q, Nguyen K, Satyamurthy N, Barrio JR, Phelps ME, Gambhir SS, et al. Monitoring adenoviral DNA delivery, using a mutant herpes simplex virus type 1 thymidine kinase gene as a PET reporter gene. Gene Ther. 2002;9:1659–1666. doi: 10.1038/sj.gt.3301899. [DOI] [PubMed] [Google Scholar]
- 18.Blankenberg FG, Strauss HW. Nuclear medicine applications in molecular imaging. J Magn Reson Imaging. 2002;16:352–361. doi: 10.1002/jmri.10171. [DOI] [PubMed] [Google Scholar]
- 19.Richard JC, Factor P, Welch LC, Schuster DP. Imaging the spatial distribution of transgene expression in the lungs with positron emission tomography. Gene Ther. 2003;10:2074–2080. doi: 10.1038/sj.gt.3302117. [DOI] [PubMed] [Google Scholar]
- 20.Barton KN, Tyson D, Stricker H, Lew YS, Heisey G, Koul S, et al. GENIS: gene expression of sodium iodide symporter for non-invasive imaging of gene therapy vectors and quantification of gene expression in vivo. Mol Ther. 2003;8:508–518. doi: 10.1016/s1525-0016(03)00153-9. [DOI] [PubMed] [Google Scholar]
- 21.Kristian Räty J, Liimatainen T, Unelma Kaikkonen M, Gröhn O, Airenne KJ, Ylä-Herttuala S. Non-invasive imaging in gene therapy. Mol Ther. 2007;15:1579–1586. doi: 10.1038/sj.mt.6300233. [DOI] [PubMed] [Google Scholar]
- 22.Peñuelas I, Mazzolini G, Boán JF, Sangro B, Martí-Climent J, Ruiz M, et al. Positron emission tomography imaging of adenoviral-mediated transgene expression in liver cancer patients. Gastroenterology. 2005;128:1787–1795. doi: 10.1053/j.gastro.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Dempsey MF, Wyper D, Owens J, Pimlott S, Papanastassiou V, Patterson J, et al. Assessment of 123I-FIAU imaging of herpes simplex viral gene expression in the treatment of glioma. Nucl Med Com. 2006;27:611–617. doi: 10.1097/00006231-200608000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Min JJ, Iyer M, Gambhir SS. Comparison of [18F]FHBG and [14C]FIAU for imaging of HSV1-tk reporter gene expression: adenoviral infection vs stable transfection. Eur J Nucl Med Mol Imaging. 2003;30:1547–1560. doi: 10.1007/s00259-003-1238-6. [DOI] [PubMed] [Google Scholar]
- 25.Buchmann I, Riedmüller K, Hoffner S, Mack U, Aulmann S, Haberkorn U. Comparison of 99mtechnetium-pertechnetate and 123iodide SPECT with FDG-PET in patients suspicious for breast cancer. Cancer Biother Radiopharm. 2007;22:779–789. doi: 10.1089/cbr.2007.358. [DOI] [PubMed] [Google Scholar]
- 26.Barton KN, Xia X, Yan H, Stricker H, Heisey G, Yin FFTN, et al. A quantitative method for measuring gene expression magnitude and volume delivered by gene therapy vectors. Mol Ther. 2004;9:625–631. doi: 10.1016/j.ymthe.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Freytag SO, Khil M, Stricker H, Peabody J, Menon M, DePeralta-Venturina M, et al. Phase I study of replication-competent adenovirus-mediated double suicide gene therapy for the treatment of locally recurrent prostate cancer. Cancer Res. 2002;62:4968–4976. [PubMed] [Google Scholar]
- 28.Freytag SO, Stricker H, Pegg J, Paielli D, Pradhan DG, Peabody J, et al. Phase I study of replication-competent adenovirus-mediated double-suicide gene therapy in combination with conventional-dose three-dimensional conformal radiation therapy for the treatment of newly diagnosed, intermediate- to high-risk prostate cancer. Cancer Res. 2003;63:7497–7506. [PubMed] [Google Scholar]
- 29.Freytag SO, Movsas B, Aref I, Stricker H, Peabody J, Pegg J, et al. Phase I trial of replication-competent adenovirus-mediated suicide gene therapy combined with IMRT for prostate cancer. Mol Ther. 2007;15:1016–1023. doi: 10.1038/mt.sj.6300120. [DOI] [PubMed] [Google Scholar]
- 30.DeWeese T, van der Poel H, Li S, Mikhak B, Drew R, Goemann M, et al. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001;61:7464–7472. [PubMed] [Google Scholar]
- 31.Stein CS, Pemberton JL, van Rooijen N, Davidson BL. Effects of macrophage depletion and anti-CD40 ligand on transgene expression and redosing with recombinant adenovirus. Gene Ther. 1998;5:431–439. doi: 10.1038/sj.gt.3300616. [DOI] [PubMed] [Google Scholar]
- 32.Lamfers ML, Fulci G, Gianni D, Tang Y, Kurozumi K, Kaur B, et al. Cyclophosphamide increases transgene expression mediated by an oncolytic adenovirus in glioma-bearing mice monitored by bioluminescence imaging. Mol Ther. 2006;14:779–788. doi: 10.1016/j.ymthe.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Vries W, Haasnoot J, van der Velden J, van Montfort T, Zorgdrager F, Paxton W, et al. Increased virus replication in mammalian cells by blocking intracellular innate defense responses. Gene Ther. 2008;15:545–552. doi: 10.1038/gt.2008.12. [DOI] [PubMed] [Google Scholar]
- 34.Sato M, Figueiredo ML, Burton JB, Johnson M, Chen M, Powell R, et al. Configurations of a two-tiered amplified gene expression system in adenoviral vectors designed to improve the specificity of in vivo prostate cancer imaging. Gene Ther. 2008;15:583–593. doi: 10.1038/gt.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freytag SO, Barton KN, Brown SL, Narra V, Zhang Y, Tyson D, et al. Replication-competent adenovirus-mediated suicide gene therapy with radiation in a preclinical model of pancreatic cancer. Mol Ther. 2007;15:1600–1606. doi: 10.1038/sj.mt.6300212. [DOI] [PubMed] [Google Scholar]
- 36.Siddiqui F, Barton K, Stricker H, Steyn P, LaRue S, Karvelis KC, et al. Dosimetric considerations for using sodium iodide symporter reporter gene imaging in human gene therapy trials of prostate cancer. Hum Gene Ther. 2007;18:306–317. doi: 10.1089/hum.2006.131. [DOI] [PubMed] [Google Scholar]