Abstract
MZF1 is a transcription factor belonging to the Krüppel family of zinc finger proteins, expressed in totipotent hemopoietic cells as well as in myeloid progenitors. Here we have inactivated Mzfi1 by gene targeting. Mzf1−/− mice develop lethal neoplasias characterized by the infiltration and complete disruption of the liver architecture by a monomorphic population of cells of myeloid origin reminiscent of human chloromas. Mzf1 inactivation results in a striking increase of the autonomous cell proliferation and of the ability of Mzf1−/− hemopoietic progenitors to sustain long-term hemopoiesis. These findings demonstrate that Mzf1 can act as a tumor/growth suppressor in the hemopoietic compartment.
Keywords: MZF, hematopoiesis, tumorigenesis, knockout mice, myeloid progenitors
Zinc finger proteins play a crucial role in regulating normal hemopoiesis (Shivdasani and Orkin 1996). Furthermore, several genes encoding zinc finger transcription factors are involved in chromosomal translocations associated with hemopoietic malignancies (Look 1997), and their functional deregulation or inactivation is an essential step in leukemogenesis (He et al. 2000 and references therein). Myeloid zinc finger 1 (MZF-1) is a transcription factor of the Krüppel family of zinc finger proteins originally cloned from a cDNA library from a patient with chronic myeloid leukemia (Hromas et al. 1991). Within the hemopoietic compartment MZF-1 expression is restricted to totipotent bone marrow (BM) progenitor cells and early myeloid progenitors and precursors, and is not detectable in fully differentiated blood cells (Bavisotto et al. 1991). MZF-1 contains 13 zinc finger domains divided into two groups, which can bind DNA independently of each other (Morris et al. 1994; Hromas et al. 1996). In vitro, in transient transfection experiments, MZF-1 can activate transcription in cells of hemopoietic origin, and it has also been found to repress transcription in nonhemopoietic cells (Morris et al. 1995; Hromas et al. 1996). MZF1 antisense oligonucleotides inhibit granulocyte colony-stimulating factor (G-CSF)-driven granulocyte colony formation (Bavisotto et al. 1991). However, MZF1 overexpression in embryonic stem (ES) cells also appears to interfere with the ability of these cells to undergo hemopoietic commitment as well as erythromyeloid colony formation (Perrotti et al. 1995). These data may suggest a role for MZF1 in the control of myeloid differentiation. However, the biological function of MZF1 is presently unknown, also in view of the seemingly contradictory nature of these observations.
Here we show, in vivo, in knockout (KO) mice, that Mzf1 controls the proliferative potential of hemopoietic cells acting as a growth and tumor suppressor.
Results and Discussion
Generation of Mzf1−/− mice
We characterized and ablated the murine Mzf1 gene by homologous recombination. Sequencing of the Mzf1 coding region, which is retained in a single exon, revealed an 87.5% identity and a 97.2% similarity between the human and mouse proteins. The murine Mzf1 gene was found to be expressed in BM cells, but also in adult brain, testis, keratinocytes, and thymus (not shown). Using a targeting vector for positive/negative selection in mouse ES cells, we replaced the Mzf1 coding region with the positive selectable marker (neomycin cassette), completely ablating the region encoding the Mzf1 DNA-binding domain, and obtained several Mzf1+/− ES cell clones. Mzf1−/− mutants in a pure 129Sv background were generated from 4 of 27 independently targeted ES cell clones (Fig. 1A–C; Materials and Methods). Mzf1−/− mutants were born at Mendelian frequencies and were developmentally normal (not shown). The disruption of Mzf1−/− was confirmed by RT–PCR analysis on RNA from BM cells (Fig. 1D; Materials and Methods).
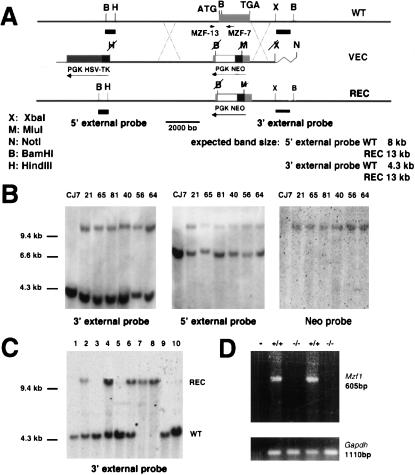
Figure 1.
Targeted disruption of Mzf1 in the mouse germ line. (A) Maps of the wild-type Mzf1 locus (WT, top), the targeting vector (VEC, middle), and the predicted targeted gene (REC, bottom). The Mzf1 genomic sequence is depicted as a thick line with a gray box representing the Mzf1 coding sequence. Sequences from the PNT plasmid (Tybulewicz et al. 1991) are shown as thin lines, with an open box representing the neomycin resistance cassette (Neo), a dark gray box representing the herpes simplex virus thymidine kinase cassette (HSV-TK), and the filled black boxes representing the PGK promoters driving the expression of the HSV-TK and Neo genes. The Neo and Mzf1 genes are transcribed in opposite directions. The Mzf1 genomic fragments used as probes for Southern blot analysis are indicated (5′ external probe and 3′ external probe). (B) BamHI; (H) HindIII; (X) XbaI restriction sites are shown. (B) Southern blot analysis of CJ7 untransfected cells and recombined clones after digestion with BamHI and hybridization with the three indicated probes. (C) Southern blot of mouse tail DNAs from one litter obtained by crossing Mzf1 heterozygous mice after digestion with BamHI. (D) RT–PCR analysis using oligonucleotides MZF-13 and MZF-7 confirming the complete inactivation of the Mzf1 locus in BM cells. Amplification with a Gapdh primer pair was used to normalize for the amount of cDNA.
Progressive accumulation of myeloid cells in the bone marrow from Mzf−/− mice
We characterized myeloid and lymphoid hemopoiesis in Mzf1−/− mice and control littermates. Analysis of peripheral blood (PB) cell populations with the use of an automated counter (Technicon H2), and by differential counts performed on Wright-Giemsa-stained smears, did not reveal significant differences between Mzf1−/− mice and wild-type sex-matched syngeneic littermates (not shown; Materials and Methods). Spleen, BM, lymph nodes, and thymus from Mzf1−/− and wild-type mice were then analyzed by differential counts and by flow cytometry with antibodies specific for hemopoietic stem cells/progenitors, myeloid cells, T- and B-lymphocytes. No significant differences were found in the lymphoid compartment (not shown; Materials and Methods). However, we observed a progressive and consistent accumulation of the mature Mac-1+ (Sca-1 and Gr-1 negative) myeloid population in the BM of Mzf1−/− mice compared to syngeneic sex, age matched wild-type controls (increase ranging between 5%, at 1 mo of age, to 10% in 10-month-old mutants; 10 mutants and 10 wild-type controls were analyzed; mean difference 8.7%; P < .001) (Fig. 2A). Thus, unexpectedly, at the steady state, Mzf1 inactivation does not impair the ability of myeloid and lymphoid cells to terminally differentiate, but affects the size of the Mac-1+ myeloid compartment in the BM.
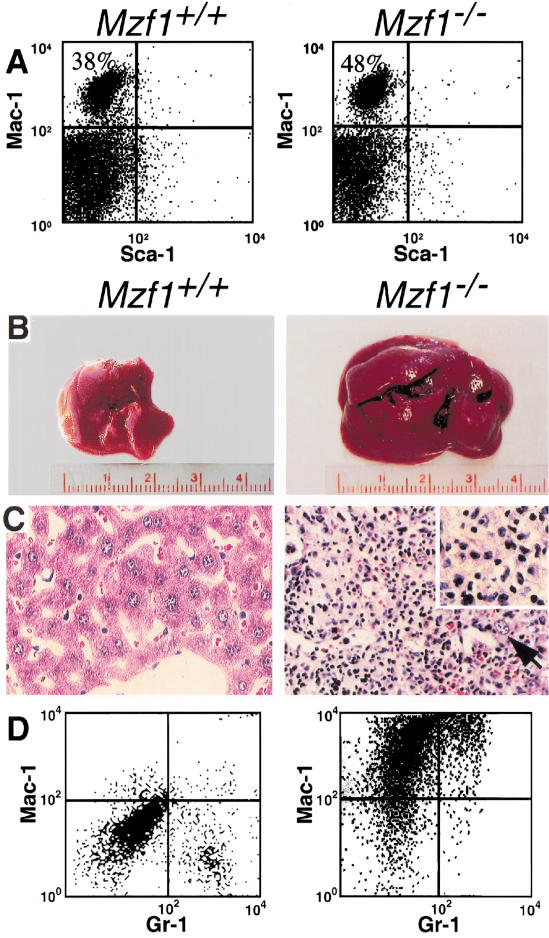
Figure 2.
Accumulation of myeloid cells and hepatic neoplasias in Mzf1−/− mice. (A) Flow cytometry analysis of BM cells in 10-month-old mice, stained with Mac-1 and Sca-1 antibodies. The genotype of the mice is indicated. At this age, Mzf1−/− mice did not show signs of overt disease. (B–D) Hepatic neoplasias in Mzf1−/− mice: wild-type (left) and diseased Mzf1−/− mice (right). (B) Macroscopic illustration of liver. The liver of Mzf1−/− mouse is markedly enlarged in view of the infiltration of the neoplastic population. (C) Histopathological analysis of liver sections stained with hematoxylin and eosin (original magnification, 400×): The liver architecture is effaced by the dramatic infiltration of a monomorphic blast population. The black arrow indicates a residual hepatocyte. (D) Flow cytometric analysis of liver cells stained with Mac-1 and Gr-1 antibody. Mac-1 positive cells (upper left quadrant) infiltrate the diseased liver from a Mzf1−/− mutant.
Mzf1−/− mice develop lethal myeloid neoplasias characterized by the infiltration and complete disruption of the liver architecture
To determine whether the inactivation of Mzf1 would result in overt disease, mice were followed throughout their life (during a 30-mo follow-up period). One group of mice was bled on a monthly basis, together with age-matched littermate controls, and sacrificed when developing manifest signs of disease (e.g., ascites; see below), or when hemoglobin levels dropped to <8 g/dL. Automated and differential counts, as well as morphological analysis of PB cells, were performed on each sample. In a second group, Mzf1−/− and wild-type controls were aged, and four from each genotype sacrificed every month for gross and microscopic examination of all organs, in particular PB, BM, spleen, liver, lymph nodes, and thymus. Cell populations in these organs were analyzed morphologically on smears, touch preparations, and cytospin preparations stained with Wright-Giemsa. By year 2, we observed extramedullary hemopoiesis in the liver or spleen in all Mzf1−/− mice analyzed (28 of 28 older than 2 yr). Extramedullary hemopoiesis affecting the spleen, but never the liver, was observed only in 5 of the 36 wild-type mice older than 2 yr (not shown). Of the Mzf1−/− mice, 30% (n = 9) developed a peculiar neoplastic disease affecting the liver and the spleen of which they succumbed between 24 to 32 mo. In these mice a monomorphic blastic cell population completely effaced the liver architecture (Fig. 2B,C). The morphological features of these cells were characteristic of hemopoietic blast with prominent nuclei. In some cases the neoplastic lesion was infiltrating the adjacent organs (i.e., the intestine). These neoplasias were highly reminiscent of solid tumor composed of myeloid cells. Surprisingly, the BM was never infiltrated by the neoplastic population (data not shown). However, the BM of Mzf1−/− mice was almost invariably hypercellular. In one case the PB was affected, but even in this case the BM was normal (blasts <10%). Four (14%) Mzf1−/− mice developed high-grade B-cell lymphomas. Of the 36 wild-type mice analyzed, four developed lymphomas (11%). None of the wild-type mice developed the liver/spleen neoplasia.
To define the histological origin and the proliferative properties of the neoplastic cell population, we performed flow cytometry and immunohistochemistry analysis. The infiltrating cells were: (1) Mac-1 positive. Mac-1 (CD11b) is expressed either in macrophages/monocytes or in a very immature myeloid precursor (Fig. 2D); (2) positive for GM-colony-stimulating factor receptor alpha (GM-CSFRα), a marker also expressed on early myeloid progenitors (Fig. 3A–C); (3) S100 negative. S100 is a marker normally expressed on mature histiocytes (not shown); (4) strongly positive for the Ki67 proliferation marker (Fig. 3D–F). Leukemic cells from Mzf1−/− mice were transplantable in 129/Sv syngeneic mice sublethally irradiated (500 Gy) (Material and Methods). Thus, Mzf1 inactivation leads to the development of lethal tumors made of highly proliferating myeloid blasts, neoplasias that are reminiscent of human chloromas (see below; Harrison 1987).
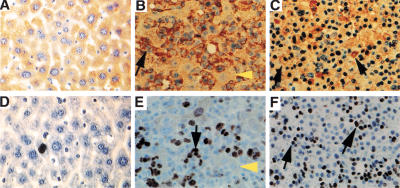
Figure 3.
Neoplasias in Mzf1−/− mice are made by highly proliferating myeloid cells. Immunohistochemical analysis of liver sections using anti-GM-CSFRα antibody (top) and anti-Ki-67 antibody (bottom). (A,D) Wild-type and (B,C,E,F) diseased Mzf1−/− mice. Black arrows indicate positively stained cells and yellow arrowheads, unstained residual hepatocytes. Original magnifications, (A,B,D,E) 400×; (C,F) 200×. Two different magnifications of the tumors are shown.
Increased number of progenitor cell-derived colonies from the bone marrow and spleen of Mzf1−/− mice
To unravel the mechanisms by which Mzf1 acts as tumor suppressor, we then assessed the cell autonomous hemopoietic potential of Mzf1−/− BM progenitors before the occurrence of the neoplasias in mice of 12–16 wk of age. To this end, we performed in vitro cultures of BM cells in standard cytokine and growth factor concentrations, from Mzf1−/− and control mice (Materials and Methods). Mzf1 inactivation resulted in a marked increase of both myeloid and erythroid colonies: colony-forming unit-granulocyte, macrophage (CFU-GM), burst-forming unit-erythroid (BFU-E), and colony-forming unit-granulocyte, erythroid, macrophage, megakaryocyte (CFU-GEMM), from both the BM and the spleen (Fig. 4). Thus, under these concentrations of cytokines, Mzf1−/− BM and spleen progenitors possess an increased clonogenic capacity.
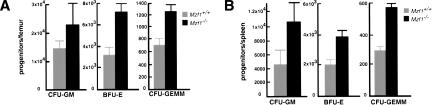
Figure 4.
Increased numbers of progenitor-derived colonies in Mzf1−/− mice. The clonogenic potential of Mzf1−/− hemopoietic progenitors was evaluated in a methylcellulose colony-forming assay by scoring CFU-GM, BFU-E, and CFU-GEMM obtained from (A) BM cells and (B) spleen cells and expressing these results per organ. The values are averages ± SE calculated from triplicates of one representative experiment carried out with five Mzf1−/− (black bars) and five syngeneic 129Sv control littermates (gray bars), plated as independent cultures.
Increased proliferation of Mzf1−/− hemopoietic progenitor cells
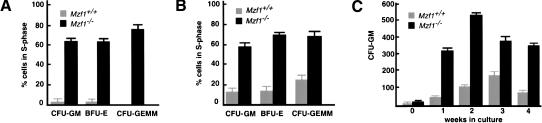
To determine whether the greater numbers of BM- and spleen-derived colonies obtained from Mzf1−/− mutants are the result of an increased proliferative potential of hemopoietic progenitor cells, we performed [3H]thymidine suicide assays. This established procedure allows the estimation of the percent of progenitors in S-phase: incorporation of [3H]thymidine into DNA takes place in actively proliferating cells resulting in cell death (Material and Methods; Maze et al. 1992). Thus, in this assay an increased proliferative potential of progenitor cells results in a reduced number of hemopoietic colonies. The BM and spleen cells from Mzf1−/− and wild-type mice were cultured in the absence or presence of high-specific activity [3H]thymidine as a pulse exposure. After removal of [3H]thymidine, cells were plated in methylcellulose, in the presence of growth factors, to trigger the formation of hemopoietic colonies. Mzf1−/− inactivation dramatically enhanced the proliferative capacity of hemopoietic progenitors and, in turn, their responsiveness to the hemopoietic growth factors present in the medium (Fig. 5A,B). As previously mentioned, in vitro, Mzf1 is known to regulate c-Myb expression at the transcription level. However, the effects on cell proliferation observed in Mzf1−/− hemopoietic cells were not caused by an aberrant c-Myb expression. In fact, the levels of c-Myb were never found increased in Mzf1−/− mutants in both Western blot and TaqMan analyses performed on total BM cells or sorted early hemopoietic progenitors (not shown; Materials and Methods).
Figure 5.
Increased cell cycling potential of hemopoietic progenitors and increased numbers of myeloid progenitor-derived colonies in long-term BM cultures from Mzf1−/− mice. (A) The proportion of hemopoietic progenitors in S-phase, evaluated with the [3H]thymidine suicide assay, and calculated as the reduction in CFU-GM, BFU-E, and CFU-GEMM formation upon treatment with [3H]thymidine as opposed to cold thymidine in BM and (B) spleen cells. The values are averages ± SE calculated from triplicates of one experiment representative of two carried out with five Mzf1−/− (black bars) and five syngeneic 129Sv control littermates (gray bars), plated as independent culture. (C) Long-term BM culture in Mzf1−/− mice. The number of CFU-GM colonies obtained from BM cells after the indicated weeks of culture is shown. The values are averages ± SE calculated from triplicates of one representative experiment carried out with BM cells from five Mzf1−/− (black bars) and five syngeneic 129Sv control littermates (gray bars), plated as independent cultures.
Increased long-term proliferative potential of Mzf1−/− hemopoietic progenitors
We then investigated whether the proliferative advantage of Mzf1−/− hemopoietic progenitors would result in an increased ability to support long-term hemopoiesis by performing long-term BM liquid cultures from Mzf1−/− mice and controls littermates (Material and Methods). From these cultures, clonogenic assays in standard cytokines conditions were carried out on a weekly basis. A much greater number of CFU-GM was produced from Mzf1−/− BM cells throughout the 4-wk culture (Fig. 5C). Therefore, Mzf1 inactivation also augments the ability of progenitor cells to support long-term hemopoiesis.
In summary, the present study leads to three major conclusions:
- 1.
Mzf1 is dispensable for myeloid/hemopoietic differentiation at the steady state. Although in vitro antisense experiments have shown that MZF1 might control myeloid hemopoietic differentiation (Bavisotto et al. 1991; Perrotti et al. 1995), the inactivation of Mzf1 in vivo in the mouse did not affect this process, at least at the steady state, but resulted instead in an accumulation of BM Mac-1+ myeloid cells. In this respect, it is also worth noting that with gene targeting, the Mzf1 region coding for the DNA-binding domain was entirely deleted, thus resulting in the complete inactivation of Mzf1, including the Mzf1 isoform termed MZF1B or Mzf2 (Murai et al. 1997, 1998; Sander et al. 2000).
- 2.
Mzf1 plays a crucial role in the negative regulation of the proliferative capacity of hemopoietic progenitors. Cells from the BM and spleen of Mzf1−/− mice yielded a greater number of colonies of all hemopoietic lineages in in vitro cultures. This may be explained either by an increased proliferation or by an increased commitment of progenitor cells to differentiate in response to the cytokines and growth factors present in the cultures. However, [3H]thymidine suicide assays show that Mzf1 inactivation results in a marked increase in the proportion of hemopoietic progenitors that are actively cycling at a given time, both in the bone marrow and in the spleen. Furthermore, this increased proliferative rate allows self-renewal of progenitors, as demonstrated by the fact that Mzf1−/− BM cells gave rise to a greater number of colonies even after long-term BM culture. In contrast, analysis of apoptosis in early hemopoietic progenitor cells as evaluated by terminal deoxynucleotide end-labeling (TUNEL) and Annexin V assays did not reveal any significant difference between Mzf1−/− and wild-type mice (data not shown; Materials and Methods).
- 3.
Mzf1 acts as growth and tumor suppressor in the myeloid hemopoietic compartment suggesting that Mzf1 inactivation or deregulated function could participate in tumorigenesis by lending a proliferative advantage to the neoplastic cells. From this point of view, it is intriguing that the human MZF1 gene is one of the most subtelomeric genes described so far located only a few kilobases from the subtelomeric repeat region of 19q, which may lead to MZF1 loss as a consequence of telomeric erosion (Hoffman et al. 1996). The incidence of myeloid neoplasia in Mzf1−/− mice is higher than the incidence of myeloid leukemia in transgenic mice harboring potent oncogenes such as the PML–RARα fusion gene of acute promyelocytic leukemia (only 20% of these transgenic mice develop leukemia after a long latency; He et al. 1997). However, the fact that Mzf1−/− mice are not born with cancer and that, on the contrary neoplasias in Mzf1−/− mice occur after a latency period strongly suggests that additional mutations are needed for full-blown transformation. The progressive expansion of the hemopoietic compartment within the BM, liver, and spleen as a result of Mzf1 inactivation would then favor the occurrence of additional transforming events. Mzf1 inactivation could dictate the peculiar hepatic and splenic localization of these neoplasias by modulating the expression of adhesion molecule that would retain the blasts within these organs. These findings prompt investigating whether MZF1 mutations or loss of heterozygosity occur in human hemopoietic tumors: myeloproliferative syndromes and myeloid leukemias are possible candidates. However, it is also worth noticing that malignant myeloid tumors localized to both soft tissues such as the liver and the bones (also known as chloromas; Harrison 1987) have been described and are, in this respect, straightforward candidate for this analysis.
Materials and methods
Generation of Mzf1−/− mice
To characterize the mouse Mzf1 genomic locus, we screened a 129Sv mouse λ-phage genomic library (Stratagene) with a human MZF1 cDNA probe encoding the acidic non-zinc finger N-terminal domain of the protein. Two overlapping clones were obtained. The coding sequence was determined by restriction enzyme mapping, DNA sequencing, and PCR. The targeting vector contains PGK-HSV-TK and PGK-NEO cassettes, the latter of which replaces, in the opposite orientation, the 1100-bp BamHI–MluI fragment of Mzf1 coding region (which codes for almost the entire DNA-binding domain of Mzf1 and its Mzf2 isoform: the first 11 zinc finger domains) and is flanked by Mzf1 genomic DNA (5′ arm, 7.0-kb HindIII–BamHI fragment, 3′ arm, 2.0-kb MluI–XbaI fragment). In addition, the presence of the PGK-NEO cassette within Mzf1 coding region introduces stop codons in all three frames, thereby interrupting the translation of the remaining two C-terminal zinc finger domains of the Mzf1 protein. The NotI linearized vector was electroporated into CJ7 ES cells and transfectants were selected in G418 (350 μg/mL) and Gancyclovir (2 μM). Resistant clones were screened by Southern blotting with the 3′ external probe using a BamHI digestion. Recombined ES cell clones were further characterized by Southern blotting with 5′ external and internal NEO probes, using a BamHI digestion. Twenty-six mutant CJ7 ES clones heterozygous for the Mzf1 deletion were obtained. Mzf1−/− mutants were generated from four independently targeted ES cell clones according to standard procedures (Barna et al. 2000). Inactivation of the gene was confirmed by RT-PCR analysis. To this end, total BM RNA from 6- to 8-week-old mice was prepared using TRIzol (GIBCO BRL). RT–PCR was performed with 2 μg of DNase I-treated RNA, by using cDNA Cycle Kit for RT–PCR (Invitrogen), according to the manufacturer's protocol. Two microliters of the synthesized cDNA was used for PCR with the following primers: sense MZF-13, 5′-TGCCTCACCAACCAGTTCCAA-3′; anti-sense MZF-7, 5′-CTGCGCACGAAGCCCTGGCCA-3′. The quality of cDNA was accessed by amplification with Gapdh control primers: sense 5′-GTGAAGGTCGGTGTGAACGGA-3′; antisense 5′-TTATTA TGGGGGTCTGGGATGGAA-3′. The specificity of amplification was evaluated by hybridizing the PCR products, upon transfer on a nylon membrane, with internal primers (not shown).
Analysis of hemopoiesis in Mzf1−/− mice
Animals were analyzed throughout life along with littermate controls at bimonthly intervals (at least four mice from each genotype per time point). Mice were bled by retroorbital venipuncture. Leukocyte, platelet, and red cell counts were performed with an automated counter (Technicon H2). Differential counts of myeloid and lymphoid subpopulations were carried out on PB smears stained with Wright-Giemsa. Differential counts of three or four smears per animal were scored for a total of at least 300 cells. Single-cell suspensions from BM, spleen, lymph nodes, liver, and thymus were analyzed on a five-color FACStar Plus flow-cytometer (Beckton Dickinson, Mountain View, CA). Fluorochrome-conjugated antibodies for flow cytometry were CD45R/B220, CD34, CD43, Ter-119, Sca-1, c-Kit, Mac-1, CD3, CD4, CD8, CD24, GR-1 (Pharmingen).
Autopsy, histopathology, and immunohistochemistry
Animals were autopsied as needed and all tissues were examined regardless of their pathological status. Normal and tumor tissue samples were fixed in 10% buffered formalin and embedded in paraffin. Sections (4–5 μm) were stained with hematoxylin and eosin according to standard protocols. Representative samples were selected for immunohistochemical analysis of GM-CSFRα and Ki-67 expression. For GM-CSFRα immunohistochemistry, liver sections from wild-type and Mzf1−/− mice were dewaxed in xylene, rehydrated through a graded series of alcohols, treated with 1% H2O2 in PBS, pretreated with 0,01 M citric acid, microwaved for 15 min, and incubated with anti-mouse GM-CSFRα antibody (Santa Cruz), overnight at 4°C. Positive signals were developed with diaminobenzidine (DAB) substrate using the avidin-biotin-peroxidase system and slides were counterstained with hematoxylin. Immunohistochemical analysis of Ki-67 (Novocastra) expression was performed on paraffin sections after dewaxing and rehydration through a graded series of alcohols, treatment with 1% H2O2, and incubated overnight at 4°C with the anti-Ki67. Positive signals were developed using DAB as mentioned above for GM-CSFRα staining. Immunohistochemical analysis was performed also for the following primary antibodies B220, CD45, TER119, CD34 (Pharmingen), CD3, and S100 (Dako), as described previously (Cordon-Cardo and Richon 1994).
In vitro BM culture
To score for BFU-E, CFU-GM, and CFU-GEMM, BM cultures from Mzf1−/− and wild-type mice were carried out by plating 5 × 104 cells in 1 mL of 1% methylcellulose Iscove's modified Dulbecco medium (IMDM) with 30% FBS, 1 U/mL recombinant human erythropoietin (EPO), 5% (v/v) pokeweed mitogen mouse spleen cell-conditioned medium (PWMSCM), 50 ng/mL recombinant murine steel factor, and 0.1 mM hemin at 5% CO2 and lowered 5% O2. Colonies were counted after 7 d of incubation. A total of 10 Mzf1−/− and 10 control wild-type syngeneic littermates, sex and age (12-week-old) matched were analyzed in two independent experiments.
Cycling status of hemopoietic progenitor cells
The proportion of each progenitor cell type in DNA synthesis (S phase of the cell cycle) was estimated by means of the high-specific-activity (20 Ci/mM) [3H]thymidine kill technique, which is based on calculation of the reduction in the number of colonies formed in vitro after pulse exposure of cells for 20 min to [3H]thymidine, as compared with control nonradioactive thymidine (Maze et al. 1992). Mzf1−/− and control wild-type syngeneic littermates sex and age (12-week-old) matched were used for this analysis.
Long-term BM culture
Cells were plated at low density, 105 per 1.5 mL of IMDM containing 20% FBS, 20 μM β-mercaptoethanol, 100 ng/mL murine Steel factor, 100 ng/mL Flt3-ligand, 50 ng/mL IL-6, and antibiotics (Pen-Strep-Ampho, BRL). Independent cultures were set up for each mouse and 1 mL of medium was removed weekly. Progenitor assays were carried out as described above, on a weekly base for 4 wk, with 104 cells taken from these cultures. Mzf1−/− and control wild-type syngeneic littermates sex and age (12–16-week-old) matched were used for this analysis.
Western blot and Real-time PCR (TaqMan)
Analysis of Myb expression was performed by Western blot on whole-cell protein extracts prepared at different time points (0, 2, 4, and 6 d) from BM cultures treated with IL3 + SC-F + GM-CSF using with the 5E anti-c-Myb specific antibody (Sleeman 1993; courtesy of Joe Lipstick, Stanford University School of Medicine, CA). TaqMan analysis of c-Myb expression was performed on total RNA extracted from sorted c-kit/FcγIII/II Receptor double positive myeloid progenitors using an ABI Prism 7700 Sequence Detection System (PE Biosystems). Sequences of primers and probe for mouse c-Myb are as follows: sense 5′-CGCCGAAGCACA AAACATC-3′; antisense 5′-GGGACGTTGACTATATTAACATGCA-3′; 6FAM-CCAGTCACGTTCCCTATCCTGTCGCA-TAMRA. Early myeloid progenitors from a total of four Mzf1−/− and four control wild-type syngeneic littermates, sex and age matched were analyzed in four independent experiments.
Apoptosis analysis by TUNEL and Annexin V
Detection analysis of apoptosis was performed by Annexin V staining according to published procedures (Rego et al. 2001) in tricolor flow cytometry on the following BM cellular populations from Mzf1−/− and control littermates: CD34+/lin− or lin+; CD34+/Sca-1+; CD34+/c-kit+. Fluorochrome-conjugated antibodies for flow cytometry were: CD34, c-kit, and Sca-1 (Pharmingen). B220, CD3, Mac-1, Ter119, and Gr-1 antibodies (Pharmingen), all PE conjugated, were pooled as “lineage positive” (lin+) to subdivide CD34+ cells in lin+ and lin− subsets. FACS-sorted populations were also collected and spun on glass slides, fixed in 4% buffered formalin and stained by in situ TUNEL assay according to published protocols (Di Cristofano et al. 2001).
Transplantation
Leukemic cells were transplanted essentially as described previously (Rego et al. 2000). Briefly, cells were collected from Mzf1−/−mice with liver neoplasia and leukemia as well as wild-type controls either by passing liver cells through a strainer to remove hepatocytes, or by flow-sorting CD45-expressing myeloid cells from the liver or the spleen. Blasts (2 × 107) were injected intraperitoneally into 129/Sv syngeneic sublethally irradiated mice (500 Gy), in triplicate, immediately after collection. The recipient mice were followed up for tumor growth on the site of injection and for leukemia development.
Acknowledgments
We thank J. Lipsick and J.F. Morris for materials. We also thank M. Barna, P. Clarck, J. Costoya-Puente, M. Jiao, S. Kalantry, L. Longo, L. Luzzatto, T. Merghoub, R. Notaro, E. M. Rego, K. Scotto, and V. Soares for assistance and advice. These studies were partially supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC) (M.G.), and by National Institutes of Health grants to H.E.B., R.H., and P.P.P. R.H. and P.P.P. are Scholars of the Leukemia and Lymphoma Society (formerly known as the Leukemia Society of America).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL p-pandolfi@ski.mskcc.org; FAX (212) 717-3374.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.902301.
References
- Barna M, Hawe N, Niswander L, Pandolfi PP. Plzf regulates limb and axial skeletal pattering. Nat Genet. 2000;25:166–172. doi: 10.1038/76014. [DOI] [PubMed] [Google Scholar]
- Bavisotto L, Kaushansky K, Lin N, Hromas R. Antisense oligonucleotides from the stage-specific myeloid zinc finger gene MZF-1 inhibit granulopoiesis in vitro. J Exp Med. 1991;174:1097–1101. doi: 10.1084/jem.174.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordon-Cardo C, Richon VM. Expression or the retinoblastoma protein is regulated in normal human tissue. Am J Pathol. 1994;144:500–510. [PMC free article] [PubMed] [Google Scholar]
- Di Cristofano A, De Acetis M, Koff A, Cordon-Cardo C, Pandolfi PP. Pten and p27KIP1 cooperate in prostate cancer tumor suppression in the mouse. Nat Genet. 2001;27:222–224. doi: 10.1038/84879. [DOI] [PubMed] [Google Scholar]
- Harrison TR. The leukemias. In: Braunwald E, Isselbacher KJ, Petersdorf RG, Wilson JD, Martin JB, Fauci AS, editors. Harrison's principles of internal medicine. 11th ed. New York: McGraw-Hill Book Company; 1987. pp. 1545–1589. [Google Scholar]
- He LZ, Tribioli C, Rivi R, Peruzzi D, Pelicci PG, Soares V, Cattoretti G, Pandolfi PP. Acute leukemia with promyelocytic features in PML/RARα transgenic mice. Proc Natl Acad Sci. 1997;94:5302–5307. doi: 10.1073/pnas.94.10.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He LZ, Bhaumik M, Tribioli C, Rego EM, Ivins S, Zelent A, Pandolfi PP. Two critical hits for promyelocytic leukemia. Mol Cell. 2000;6:1131–1141. doi: 10.1016/s1097-2765(00)00111-8. [DOI] [PubMed] [Google Scholar]
- Hoffman SM, Hromas R, Amemiya C, Mohrenweiser HW. The location of MZF-1 at telomere of human chromosome 19q makes it vulnerable to degeneration in aging cells. Leuk Res. 1996;20:281–283. doi: 10.1016/0145-2126(95)00158-1. [DOI] [PubMed] [Google Scholar]
- Hromas R, Collins SJ, Hickstein D, Raskind W, Deaven LL, O'Hara P, Hagen FS, Kaushansky K. A retinoic acid-responsive human zinc finger gene, MZF-1, preferentially expressed in myeloid cells. J Biol Chem. 1991;266:14183–14187. [PubMed] [Google Scholar]
- Hromas R, Davis B, Rauscher FJ, Klemsz M, Tenen D, Hoffman S, Xu D, Morris JF. Hematopoietic transcriptional regulation by the myeloid zinc finger gene, MZF-1. Curr Top Microbiol Immunol. 1996;211:159–164. doi: 10.1007/978-3-642-85232-9_16. [DOI] [PubMed] [Google Scholar]
- Look AT. Oncogenic transcription factors in the human acute leukemias. Science. 1997;278:1059–1064. doi: 10.1126/science.278.5340.1059. [DOI] [PubMed] [Google Scholar]
- Maze R, Sherry B, Kwon BS, Cerami A, Broxmeyer HE. Myelosuppressive effects in vivo of purified recombinant murine macrophage inflammatory protein-1 alpha. J Immunol. 1992;149:1004–1009. [PubMed] [Google Scholar]
- Morris JF, Hromas R, Rauscher FJ. Characterization of the DNA-binding properties of the myeloid zinc finger protein MZF1: Two independent DNA-binding domains recognize two DNA consensus sequences with a common G-rich core. Mol Cell Biol. 1994;14:1786–1795. doi: 10.1128/mcb.14.3.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JF, Rauscher FJ, Davis B, Klemsz M, Xu D, Tenen D, Hromas R. The myeloid zinc finger gene, MZF-1, regulates the CD34 promoter in vitro. Blood. 1995;86:3640–3647. [PubMed] [Google Scholar]
- Murai K, Murakami H, Nagata S. A novel form of the myeloid-specific zinc finger protein (MZF-2) Genes Cells. 1997;2:581–591. doi: 10.1046/j.1365-2443.1997.1430341.x. [DOI] [PubMed] [Google Scholar]
- ————— Myeloid-specific transcriptional activation by murine myeloid zinc-finger protein 2. Proc Natl Acad Sci. 1998;95:3461–3466. doi: 10.1073/pnas.95.7.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti D, Melotti P, Skorski T, Casella I, Peschle C, Calabretta B. Overexpression of the zinc finger protein MZF1 inhibits hematopoietic development from embryonic stem cells: Correlation with negative regulation of CD34 and c-myb promoter activity. Mol Cell Biol. 1995;15:6075–6087. doi: 10.1128/mcb.15.11.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rego E, He LZ, Warrell RP, Jr, Wang ZG, Pandolfi PP. Retinoic acid (RA) and As2O3 treatment in transgenic models of acute promyelocytic leukemia (APL) unravel the distinct nature of the leukemogenic process induced by the PML-RARα and PLZF-RARα oncoproteins. Proc Natl Acad Sci. 2000;97:10173–10178. doi: 10.1073/pnas.180290497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rego E, Wang Z, Peruzzi D, He LZ, Cordon-Cardo C, Pandolfi PP. Role of promyelocytic leukemia (pml) protein in tumor suppression. J Exp Med. 2001;193:521–530. doi: 10.1084/jem.193.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander TL, Haas AL, Peterson MJ, Morris JF. Identification of a novel SCAN box-related protein that interacts with MZF1B. The leucine-rich scan box mediates hetero- and homoprotein associations. J Biol Chem. 2000;275:12857–12867. doi: 10.1074/jbc.275.17.12857. [DOI] [PubMed] [Google Scholar]
- Shivdasani RA, Orkin SH. The transcriptional control of hematopoiesis. Blood. 1996;87:4025–4039. [PubMed] [Google Scholar]
- Sleeman JP. Xenopus A-myb is expressed during early spermatogenesis. Oncogene. 1993;8:1931–1941. [PubMed] [Google Scholar]
- Tybulewicz VLJ, Crawford CE, Jackson PK, Bronson RT, Mulligan RC. Neonatal lethality and lymphopenia in mice with homozygous disruption of the c-abl proto-oncogene. Cell. 1991;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]