Abstract
Background:
Ideally, the intensity of postoperative pain should be predicted so as to customize analgesia. The objective of this study was to investigate whether preoperative electrical and pressure pain assessment can predict post-caesarean section pain and analgesic requirement.
Materials and Methods:
A total of 65 subjects scheduled for elective caesarean section, who gave written informed consent, were studied. Preoperatively, PainMatcher® was used to evaluate electrical pain threshold, while manual PainTest™ FPN 100 Algometer and digital PainTest™ FPX 25 Algometer determined pressure pain threshold and tolerance. Postoperatively, numerical rating scales were used to assess pain at regular time intervals. Patients received intramuscular pethidine (100mg, 6 hourly), rectal diclofenac (100mg, 12 hourly), and oral paracetamol (1g, p.r.n.) for pain relief. Statistical analysis was conducted using PASW Statistics 18 software.
Results:
Preoperative electrical pain threshold correlated significantly with post-caesarean pain scores at 6 and 24 hours (r = –0.26, P < 0.02; r = –0.23, P < 0.04, respectively), and with the quantity of paracetamol consumed by the patient within 48 hours of surgery (r = –0.33, P < 0.005). Preoperative pressure pain tolerance measured by PainTest™ FPX 25 Algometer was significantly correlated with pain scores 6 hours postsurgery (r = –0.21, P < 0.05). Pain scores 6 hours post-caesarean section correlated significantly with anesthesia—general or spinal (F = 4.22, v1 = 1, v2 = 63, P < 0.05).
Conclusions:
The predictive methods proposed may aid in identifying patients at greater risk for postoperative pain. Electrical pain threshold could be useful in personalizing the postoperative analgesic protocol.
Keywords: Analgesia, caesarean section, general anesthesia, postoperative pain, pain threshold, spinal anesthesia
Introduction
Post-caesarean section pain complicates the postoperative recovery in women. Individualizing pain management in the postoperative period can be problematic. It is becoming increasingly clear that neurological pathways associated with pain perception are not only determined by anatomical structures but are also influenced by genetic implications. This suggests that it is unsustainable to suppose that all individuals will perceive pain in the same way. Pain should be treated according to what the patient feels and ideally, the occurrence of pain should be predicted so as to tailor management to the patient's needs.
The results of a study by Eisenach et al.[1] demonstrate that acute pain following delivery imparts a significant risk for persistent pain and depression. Such an observation outlines a need to more carefully address pain management in the days following childbirth. Pain prediction could be the way forward.
Several studies have focused on preoperative factors that may predict the level of postoperative pain.[2–8] Preoperative assessment of pain induced by heat has been shown to predict the level of postoperative pain.[2] Clinical application of the pressure pain model has also been validated for evaluating pain sensitivity.[3,9] Burning injury and pressure algometry are complex and time-consuming. An alternative method, Pain Matcher® (Cefar Medical AB, Lund, Sweden), has been designed for clinical evaluation of pain, through electrical pain assessment, and it has proved powerful in test-retest situations. Results suggest that it may be of use in assessing acute pre- and postoperative pain.[5,6,10]
Identification of predictors could be important in the provision of customized postsurgery analgesia to offer adequate pain relief while minimizing the occurrence of side effects. Improvement in postoperative analgesia may not only increase patient satisfaction but may also diminish the duration of hospital stay and reduce the risk of complications.[4] The aim of the present study was to determine factors that predict pain and analgesic drug use following caesarean section, with particular focus on the predictive power of preoperative pain threshold and tolerance assessments.
Materials and Methods
Two pilot studies were conducted to develop the study design. The first study involved the use of the McGill pain questionnaire for the reporting of pain. The latter was replaced by numerical rating scales (NRS) in the second study.
Study population
With approval from the University of Malta Research Ethics Committee, written informed consent was obtained from healthy women at 36+ weeks' gestation, who were scheduled for elective lower segment caesarean section. Patients having obstetric complications, artificial pacemakers, intracardiac defibrillators, or other implanted electrical devices were excluded. Patients who received opioids through patient-controlled analgesia (PCA) for postoperative pain-relief were not included in the study, because in such cases, the temporal relationship between the administration of analgesics and pain reporting on NRS could not be sustained. In the 10-month timeframe allocated for the study, 65 eligible females accepted to participate.
Experimental preoperative pain assessment
Preoperative pain assessment was performed on the evening prior to the caesarean section by the following three tests: Electrical pain assessment using PainMatcher® (Cefar Medical AB, Lund, Sweden; Figure 1), and pressure pain assessment using PainTest™ FPN 100 Algometer and PainTest™ FPX 25 Algometer (Wagner Instruments, Greenwich, USA; Figures Figure 2 and 3, respectively). Before undertaking the tests, the women received an explanation of the difference between the two facets of pain; namely, threshold (the point at which a sensation becomes painful) and tolerance (the point at which a sensation can no longer be endured).
Figure 1.

PainMatcher® (Cefar Medical AB, Lund, Sweden)
Figure 2.
PainTest™ FPN 100 Algometer (Wagner Instruments, Greenwich, USA)
Figure 3.

PainTest™ FPX 25 Algometer (Wagner Instruments, Greenwich, USA)
Assessment of pressure pain threshold and tolerance
Two algometers, namely PainTest™ FPN 100 Algometer (manual) and PainTest™ FPX 25 Algometer (digital), were used, consecutively, to determine the pain threshold and pain tolerance pressure. A probe with a surface area of 1cm2 was applied to the pulp of the middle finger of the right hand, and the pressure was increased. Patients were asked to notify the investigator when they started to feel pain (pain threshold) and when they could no longer bear the pain (pain tolerance). The pressure at each point was recorded.
Assessment of electrical pain threshold
PainMatcher® generates a current which is dispersed with a monophasic rectangular pulse of 15mA and 10Hz. Gradual widening of pulse duration increases output intensity, in steps of 4μs, to a maximum of 396μs. Thus, there are 99 steps directly related to the output, resulting in a numerical cut-off range of 0 to 99.[11] The patients grasped the electrodes on the left side of PainMatcher® between the thumb and index finger of their right hand, with a pincer grip. The pain stimulation was led by the women themselves, who could start and stop the flow of current, by squeezing and releasing the electrodes, respectively. Each participant was asked to release the unit when the stimulation became painful. The procedure was repeated three times and the mean was taken as the pain threshold. Electrical pain tolerance was not measured to limit patient discomfort arising from the numerous preoperative tests.
Caesarean section and postoperative pain assessment
On patient arrival at the operating theatre, standard monitoring was begun. Before the procedure, patients received sodium citrate 30ml, as well as metoclopramide 10mg and ranitidine 50mg intramuscularly. Caesarean sections were performed under spinal anesthesia (10mg 0.5% bupivacaine and 25μg fentanyl, total of 2.5ml administered intrathecally, under aseptic technique using a 25/27-G pencil-point spinal needle) or general anesthesia (rapid sequence induction - sodium thiopentone 5mgkg-1 and suxamethonium 100mg, followed by atracurium 25mg). General anesthesia was maintained with sevoflurane 2-3% and nitrous oxide 60% in oxygen, to give a minimum alveolar concentration of 1.0. The patient was ventilated through a closed circle system with a volume-controlled mode of ventilation. Fentanyl 100μg was administered intravenously once cord was clamped. This was followed by 10 units oxytocin and an antibiotic (co-amoxiclav 1.2g IV, unless allergic). The uterus was either exteriorized for repair, or repaired in-situ. Muscle paralysis was reversed with atropine 1.2mg plus neostigmine 2.5mg. Stat doses of paracetamol 1g and diclofenac 100mg were administered per rectum. Following the surgical procedure, the patients were transferred to an obstetric ward. This was set as time zero.
Post-caesarean section, patients were given pethidine (100mg, every 6 hours, intramuscularly) and diclofenac (100mg, every 12 hours, rectally). Oral analgesia with paracetamol was provided at the patient's request, allowing patients to get a maximum oral dose of 1g every 6 hours. At six hours post-caesarean section, a NRS with values from 0to 10 (10 being more severe) was used to assess the degree of postoperative pain at rest. Pain assessment using the NRS was repeated at 12, 24, and 48 hours postoperatively, immediately prior to the administration of analgesics.
Statistical analysis
Statistical analysis was conducted using PASW Statistics 18 software (SPSS Inc., Chicago, IL). Descriptive statistics (mean, standard deviation, and range) were calculated for all variables. Data distribution was evaluated for normality by the Kolmogorov-Smirnov test. Parametric Pearson correlation and One-Way ANOVA were used to analyze the relationship between predictive factors and outcome variables. The predictive value of the explanatory variables was assessed through a general linear model. Separate analyses were performed for the following five dependent variables: Pain score at 6, 12, 24, and 48 hour, and paracetamol consumption. A range of possible predictors was introduced in each model, namely: Parity, site of uterine repair, breastfeeding, anesthesia, age, and previous sections as factors, and PainMatcher® threshold, PainTest™ FPX 25 threshold, PainTest™ FPX 25 tolerance, PainTest™ FPN 100 threshold, and PainTest™ FPN 100 tolerance as covariates. Predictors were in turn excluded from the model fit on the basis of the significance of their contribution in explaining variation. For all analyses, the level of significance was set at 0.05.
Results
A total of 65 healthy women at a mean gestation ± SD of 37.9 ± 0.6 weeks were enrolled in the study. The parturients had a mean age of 29.9 ± 5.4 years, and a median parity of 1 (range: 0-4). All subjects had approximately an equivalent size surgical wound at the same site. Data were found to be normally distributed, though there was considerable interindividual variability in the results of all preoperative pain tests and the range of postoperative pain scores and analgesic use.
Preoperative assessment
The mean preoperative pressure pain threshold and pain tolerance ± SD, measured by PainTest™ FPX 25, were 3207 ± 774mmHg and 4546 ± 1044mmHg, respectively, while when measured by PainTest™ FPN 100, these were 3257 ± 688mmHg and 4546 ± 908mmHg, respectively. The mean electrical pain threshold (0-99) ± SD, as measured by PainMatcher®, was 10 ± 5. The pain threshold/tolerance values, measured by the three different devices, were all correlated to one another; for all correlations, the correlation coefficient was below 0.8, and thus the possibility of collinearity when performing regression analysis was low.
Post-caesarean section pain assessment and analgesic requirement
Descriptive statistics [Table 1] reveal that mean NRS pain scores (0-10) were 5.82, 6.18, 4.83, and 3.60 for post-caesarean section pain at 6, 12, 24, and 48 hours, respectively. The mean consumption of paracetamol in the first 48 hours postoperatively was 3195.38mg. Table 2 illustrates the distribution of patients according to pain scores reported on the respective NRS, and the dose of paracetamol consumed.
Table 1.
Descriptive statistics
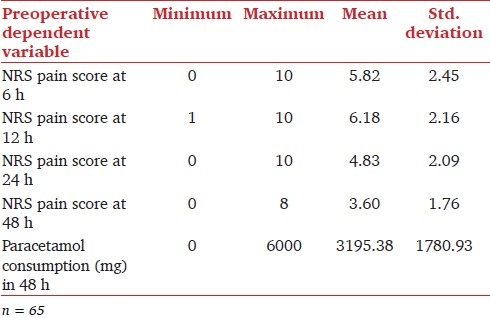
Table 2.
Distribution of patients according to NRS pain scores and paracetamol consumption
Relationship between preoperative pain assessments and post-caesarean section pain scores
Preoperative pain threshold, measured by PainMatcher®, correlated significantly with post-caesarean pain scores (NRS) at 6 and 24 hours (r = –0.26, P<0.02 and r = –0.23, P<0.04, respectively), as presented in Figure 4. Preoperative pressure pain tolerance, measured by PainTest™ FPX 25, was significantly correlated with pain scores (NRS) at 6 hours postsurgery (r = -0.21, P<0.05). Interestingly, pain scores at 6 hours post-caesarean section also correlated significantly with the categorical variable, anesthesia--general or spinal (F = 4.22, v1 = 1, v2 = 63, P<0.05). There was no significant correlation between preoperative pain assessment using PainTest™ FPN 100 and postoperative pain scores.
Figure 4.
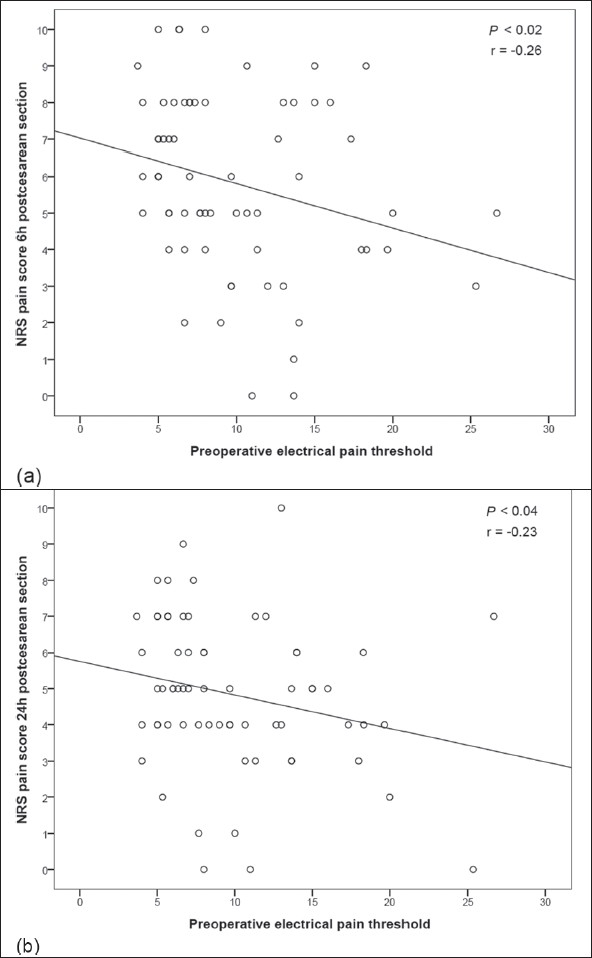
Correlation between preoperative pain threshold, measured by PainMatcher®, and NRS pain score at (a) 6, and (b) 24 h post-caesarean section
Relationship between preoperative pain assessments and post-caesarean section analgesic consumption
Preoperative pain threshold, measured by PainMatcher®, was significantly correlated with the quantity of paracetamol consumed by the patient within 48 hours postoperatively (r = –0.33, P<0.005; Figure 5). No other significant correlation was found between preoperative experimental pain assessment and post-caesarean section analgesic consumption.
Figure 5.
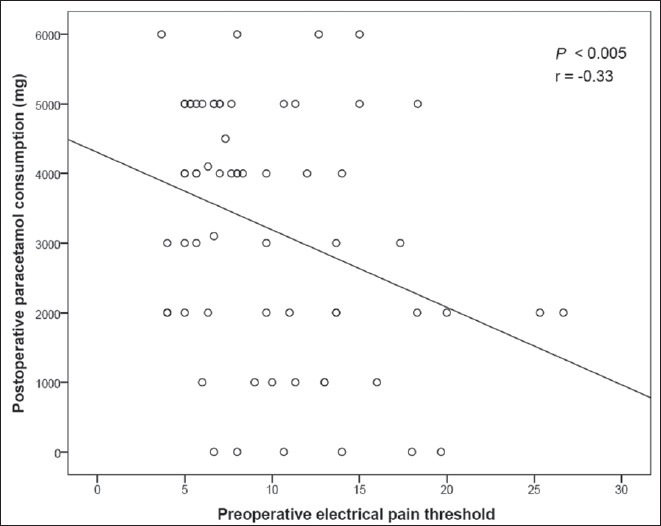
Correlation between preoperative pain threshold, measured by PainMatcher®, and paracetamol consumption in the first 48 h following surgery
Predictive model
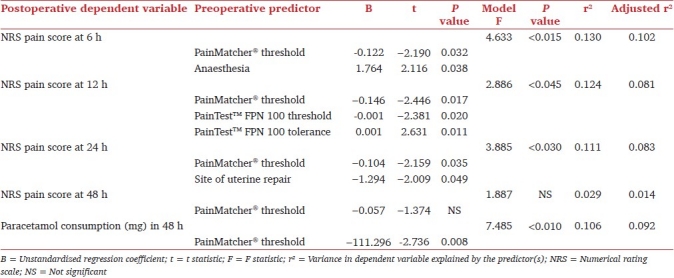
Linear regression analysis has shown that pain at 6 hours post-caesarean section can be estimated using electrical pain threshold measured by PainMatcher®, together with the type of anesthesia. Parameter estimates indicate that for every one unit increment in the electrical pain threshold, the pain score at 6 hours decreases by 0.12. Furthermore, this same pain score is on average 1.76 higher in patients on whom the caesarean section was performed under general anesthesia, compared with those who were administered spinal anesthesia.
Three predictive factors (electrical pain threshold determined by PainMatcher®, and pressure pain threshold, and tolerance, quantified using PainTest™ FPN 100) provided the parsimonious linear regression model for pain at 12 hours postsurgery. Site of uterine repair (in-situ or repair after exteriorization) and electrical pain threshold fitted the best predictive model for pain at 24 hours following caesarean section.
Electrical pain threshold, evaluated by PainMatcher®, alone, provided the most statistically significant fit for postoperative paracetamol consumption within 48 hours of surgery. Parameter estimates demonstrate that the latter decreases by 111.30mg for every unit increment in electrical pain threshold. Predictive model with regression analysis for pain and analgesic outcomes is shown in Table 3.
Table 3.
Linear regression model for predicting postoperative pain and paracetamol consumption
Discussion
A number of individuals are highly sensitive to pain, while others appear to be somewhat insensitive. Pain models are valuable since they generate a painful stimulus under controlled and standardized conditions. This allows for an essentially unbiased assessment of an exceptionally subjective experience.[3] Measurements of threshold and tolerance to thermal, mechanical, and electrical stimuli reflect the state of the peripheral and central nervous system responsible for pain processing. Contrary to standard diagnostic tools, quantitative sensory testing enables the assessment of specific components of the nociceptive system.[12]
The current study demonstrates that preoperative pressure and electrical pain assessment may both have a potential in predicting pain following a caesarean section. These results are consistent with what was reported by Hsu et al.,[3] who found a significant correlation between assessment of preoperative pressure pain tolerance and the level of postoperative pain, and Nielsen et al.,[6] whose study concluded that electrical pain threshold before caesarean sections can predict the intensity of postoperative pain.
The NRS was chosen as the most appropriate pain reporting system in this scenario, based on the results of our pilot studies. When the use of the McGill pain questionnaire was attempted, Cronbach's Alpha proved to be relatively low for most measures. This could be attributed to the patients' low level of understanding of the terms in the questionnaire and the respondents' difficulty in assessing specific aspects of their pain. In our study, no correlations were identified between preoperative assessment and pain scores reported at 12 and 48 hours postsurgery. It is worth noting that most caesarean sections were performed in the morning and thus, 12 hours postoperatively, the patient was likely to be asleep. This could have resulted in inconsistent reporting. Furthermore, 48 hours postoperatively, pain scores were appreciably lower. Lack of correlation at this stage may indicate that patients discriminated less firmly between the low values present (for example when selecting a 3 rather than a 4 on the NRS). Furthermore, different results were obtained with the two algometers used. FPX 25 is a digital algometer, as opposed to the manual FPN 100 algometer. The discrepancy in their results may indicate a lower degree of accuracy in using the manual instrument. Apart from being more time consuming, manual analysis allows for more measurement errors.
As opposed to previous studies in the field, this study applied both electrical and pressure pain assessment to the same patient population. Results obtained with electrical pain assessment seem to be more significant. Pressure and heat stimulation primarily activate the mechanoreceptive and the thermoreceptive nociceptors, respectively. Electrical stimulation, on the other hand, activates nerve fibers, and hence bypasses the receptors. This may minimize the confounding influence of nociceptor activation, thereby rendering the results more consistent.[13] In turn, these results indicate that post-caesarean section pain may be prominently determined by central mechanisms rather than peripheral mechanisms.
The consumption of paracetamol in the first 48 hours following surgery correlated significantly with the electrical pain threshold measured preoperatively by PainMatcher®. Paracetamol was the only analgesic in the protocol available to the patient on request. Thus, electrical pain threshold may be useful in personalizing the protocol with respect to paracetamol use. It would be worthy to perform these assessments on patients being administered PCA, to determine whether prediction is significant in this scenario. We are presently conducting a study to ascertain whether the preoperative assessments discussed can predict opioid requirements. The design is similar; post-caesarean section, women are no longer receiving pethidine by intermittent intramuscular injections, but are administering morphine through intravenous PCA.
Prior knowledge of the approximate analgesic dose necessary for the treatment of postoperative pain could allow the individualization of prescriptions. Predicting the dose of opioids, and analgesics with opioid sparing-effects, could result in improvements in pain relief and reduction in complications arising from excessive analgesic consumption.[7] Such complications include opioid-related side effects (viz., nausea, vomiting, urinary retention, ileus, constipation, sedation, and respiratory depression) and nonopioid side effects (viz., hepatic and renal toxicity, confusion, and dizziness), which may be aggravated when administered after surgery, as part of a multimodal regimen.
To investigate the relation between preoperative pain assessment, and postoperative pain and analgesic consumption, it is necessary to control for other issues that may influence the results. In this regard, our study was designed to include participants of the same gender, undergoing the same type of surgery. Additionally, several factors that could impact on postoperative pain, such as type of anesthesia, site of uterine repair, age, parity, previous caesarean sections, and whether mother breastfeeds the newborn, were all included in the regression model to evaluate whether they explained any of the variance in the outcome. The present study has in effect demonstrated that the site of uterine repair and the type of anesthesia used during surgery may influence postoperative pain intensity. Findings show that general anesthesia results in increased pain 6 hours post-caesarean section. Patients who are administered general anesthesia often report higher pain scores in the immediate postoperative period, whereas after regional anesthesia, there is a delay between the end of the surgical procedure and the occurrence of pain.[8] Wang et al.[14] concluded that postoperative pain after lower abdominal surgery can be significantly decreased if surgery is performed with the use of spinal anesthesia. Furthermore, general rather than spinal anesthesia is associated with a higher incidence of persistent, chronic pain following delivery.[15]
Some of the preoperative predictors found to be significantly correlated to postoperative outcomes in this study were then excluded from the predictive models due to lack of significance. A lone predictor could be a very important contributor in explaining variation in the responses, but would be rendered irrelevant in the presence of other predictors. The suitability of a predictor in a model fit often depends on what other predictors are included with it. Consequently, a combination of predictors may generate a model with substantial improvement over single variables in predicting post-caesarean section pain and analgesic requirement.
This study focused on elective caesarean sections. It is possible that patients who undergo emergency caesarean sections have less knowledge and time for psychological preparation preoperatively, resulting in increased postoperative pain and analgesic requirement.[16] Pre-existing pain, anxiety, and other psychological factors may also be taken into account since they are known to play a significant role in postobstetric surgery outcomes.[17,18] Whether these models apply to other surgical patients remains to be investigated. Different types of surgical procedures have their own unique postoperative pain characteristics and clinical consequences. Therefore, a procedure-specific approach may be recommended.[19]
Such predictive methods may not only prove useful in the allocation of additional resources to patients at potential risk for postoperative hyperalgesia, but may also serve as a screening tool in pharmacological trials of novel analgesics by decreasing the number of patients required to determine efficacy.[7] If algesimetry, the experimental triggering and quantitative recording of pain sensations, was to become a principal tool in clinical pharmacology, it would be necessary to take different dosages and kinetics into consideration, and devise tests that are sensitive to the effects of standard analgesics.[20]
In conclusion, we have demonstrated that simple sensory tests which determine pain threshold and tolerance provide an insight of the postoperative outcome; preoperative pressure pain assessment may act as a predictor of postoperative pain, and preoperative electrical pain assessment can predict postoperative pain and analgesic requirement, in women undergoing elective lower segment caesarean sections. A significant part of variability cannot be explained by the models here proposed, and thus, the implications of this study need to be evaluated with prudence. Since all patients received pethidine based on a standardized dosage regimen, intrinsic pain sensitivity may not be the only explanatory variable of variation in pain scores and paracetamol consumption. Opioid sensitivity, which has been associated with a genetic cause, may have a role in the development of pain outcomes. A standardized acute postoperative pain model evidenced that the OPRM 118 genotype, specifically the single nucleotide polymorphism A118G of the mu-opioid-receptor gene, influences the perception of pain and the consequent use of analgesia.[21] More vigorous studies with robust statistics are needed to further explore this field of interest.
Acknowledgments
Liberato Camilleri, PhD, Lecturer, Department of Statistics and Operations Research, Faculty of Science, University of Malta. Daniel Farrugia, MD, DEAA, EDIC, Consultant Anesthetist, Mentorship Programme Coordinator Anesthetic Department, Mater Dei Hospital, Msida, Malta.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Eisenach JC, Pan PH, Smiley R, Lavand'homme P, Landau R, Houle TT. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140:87–94. doi: 10.1016/j.pain.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Granot M, Lowenstein L, Yarnitsky D, Tamir A, Zimmer E. Post-caesarean section pain prediction by preoperative experimental pain assessment. Anesthesiology. 2003;98:1422–6. doi: 10.1097/00000542-200306000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Hsu Y, Somma J, Hung Y, Tsai P, Yang C, Chen C. Predicting postoperative pain by preoperative pressure pain assessment. Anesthesiology. 2005;103:613–8. doi: 10.1097/00000542-200509000-00026. [DOI] [PubMed] [Google Scholar]
- 4.Pan PH, Coghill R, Houle TT, Seid MH, Lindel WM, Parker RL, et al. Multifactorial preoperative predictors for post-caesarean section pain and analgesic requirement. Anesthesiology. 2006;104:417–25. doi: 10.1097/00000542-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Stener-Victorin E, Kowalski J, Lundeberg T. A new highly reliable instrument for the assessment of pre- and postoperative gynaecological pain. Anesth Analg. 2002;95:151–7. doi: 10.1097/00000539-200207000-00027. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen PR, Norgaard L, Rasmussen LS, Kehlet H. Prediction of postoperative pain by an electrical pain stimulus. Acta Anaesth Scand. 2007;51:582–6. doi: 10.1111/j.1399-6576.2007.01271.x. [DOI] [PubMed] [Google Scholar]
- 7.Werner MU, Duun P, Kehlet H. Prediction of postoperative pain by preoperative nociceptive responses to heat stimulation. Anesthesiology. 2004;100:115–9. doi: 10.1097/00000542-200401000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Aubrun F, Valade N, Coriat P, Riou B. Predictive factors of severe postoperative pain in the post-anaesthesia care unit. Anesth Analg. 2008;106:1535–41. doi: 10.1213/ane.0b013e318168b2ce. [DOI] [PubMed] [Google Scholar]
- 9.Kinser AM, Sands WA, Stone MH. Reliability and validity of a pressure algometer. J Strength Cond Res. 2009;23:312–4. doi: 10.1519/jsc.0b013e31818f051c. [DOI] [PubMed] [Google Scholar]
- 10.Bunketorp Käll L, Kowalski J, Stener-Victorin E. Assessing pain perception using the PainMatcher® in patients with whiplash-associated disorders. J Rehabil Med. 2008;40:171–7. doi: 10.2340/16501977-0163. [DOI] [PubMed] [Google Scholar]
- 11.Dahlin L, Lund I, Lundeberg T, Molander C. Vibratory stimulation increase the electro-cutaneous sensory detection and pain thresholds in women but not in men. [Last cited on 2011 Mar 2];BMC Complement Altern Med. 2006 6:20. doi: 10.1186/1472-6882-6-20. Available from: http://www.biomedcentral.com/content/pdf/1472-6882-6-20.pdf . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stojanovic M. Quantitative sensory testing in pain states. Curr Pain Headache Rep. 1998;2:201–5. [Google Scholar]
- 13.Eichenberger U, Neff F, Sveticic G, Björgo S, Petersen-Felix S, Arendt-Nielsen L, et al. Chronic Phantom Limb Pain: The Effects of Calcitonin, Ketamine, and Their Combination on Pain and Sensory Thresholds. Anesth Analg. 2008;106:1265–73. doi: 10.1213/ane.0b013e3181685014. [DOI] [PubMed] [Google Scholar]
- 14.Wang JJ, Ho ST, Liu HS, Tzeng JI, Tze TS, Liaw WJ. The effect of spinal versus general anaesthesia on postoperative pain and analgesic requirements in patients undergoing lower abdominal surgery. Reg Anesth. 1996;21:281–6. [PubMed] [Google Scholar]
- 15.Nikolajsen L, Sørensen HC, Jensen TS, Kehlet H. Chronic pain following caesarean section. Acta Anaesthesiol Scand. 2004;48:111–6. doi: 10.1111/j.1399-6576.2004.00271.x. [DOI] [PubMed] [Google Scholar]
- 16.Ip HY, Abrishami A, Peng PW, Wong J, Chung F. Predictors of postoperative pain and analgesic consumption: A qualitative systematic review. Anesthesiology. 2009;111:657–77. doi: 10.1097/ALN.0b013e3181aae87a. [DOI] [PubMed] [Google Scholar]
- 17.Hobson JA, Slade P, Wrench IJ, Power L. Preoperative anxiety and postoperative satisfaction in women undergoing elective caesarean section. Int J Obstet Anesth. 2006;15:18–23. doi: 10.1016/j.ijoa.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Bindra T, Anand LK, Gombar KK, Goel P. Effect of preoperative anxiolysis on postoperative pain in patients undergoing total abdominal hysterectomy under general anaesthesia: A randomized double-blind placebo controlled study. J Anaesth Clin Pharmacol. 2010;26:172–6. [Google Scholar]
- 19.White P, Kehlet H. Improving postoperative pain management: What are the unresolved issues? Anesthesiology. 2010;112:220–5. doi: 10.1097/ALN.0b013e3181c6316e. [DOI] [PubMed] [Google Scholar]
- 20.Handwerker HO. Assessment of experimentally induced pain: Old and new methods. Am J Med. 1983;75:15–8. doi: 10.1016/0002-9343(83)90227-9. [DOI] [PubMed] [Google Scholar]
- 21.Tan E, Lim E, Teo Y, Lim Y, Law H, Sia AT. Ethnicity and OPRM variant independently predict pain perception and patient-controlled analgesia usage for postoperative pain. [Last cited on 2011 Mar 2];Mol Pain. 2009 5:32. doi: 10.1186/1744-8069-5-32. Available from: http://www.molecularpain.com/content/pdf/1744-8069-5-32.pdf . [DOI] [PMC free article] [PubMed] [Google Scholar]


