Abstract
Objective:
The aim of this randomized, controlled study was to compare the sedoanalgesic effects of ketamine-dexmedetomidine and ketamine-midazolam on dressing changes of burn patients.
Materials and Methods:
Following Ethics Committee approval and informed patient consent, 90 ASA physical statuses I and II adult burn patients were included in the study. Patients were randomly divided into three groups. Ten minutes before dressing change, the dexmedetomidine group (group KD) (n = 30) received a continuous infusion of dexmedetomidine at a rate of 1 μg kg-1, the midazolam group (group KM) (n = 30) received a continuous infusion of midazolam at a rate of 0.05 mg kg-1 and the saline group (group KS) (n = 30) received a continuous infusion of saline intravenously. One minute before dressing change, each patient was administered 1 mg kg-1 ketamine intravenously. Hemodynamic variables, pain and sedation scores, the number of patients requiring additional ketamine, time to dressing change and recovery time were recorded.
Results:
Systolic blood pressure (SBP) values were significantly lower at, before and after ketamine administration; and 5, 10 and 15 minutes after the procedure in group KD in comparison with the other groups (P <0.05). There was no significant difference in pain scores among the groups during the study period. Sedation scores were significantly higher in group KD than in groups KM and KS at the end of the first hour (P <0.05). Time to dressing change and recovery time were similar in all the groups
Conclusion:
In burn patients undergoing dressing changes, although both combinations ketamine-dexmedetomidine and ketamine-midazolam offered an effective sedoanalgesia without causing any significant side effect, the former resulted in higher sedation and lower hemodynamic discrepancy.
Keywords: Burn, dexmedetomidine, dressing changes, ketamine, midazolam
Introduction
Patients treated for burn injuries commonly experience high levels of acute pain and anxiety during hospitalization, particularly as it relates to their dressing changes and other medical procedures.[1–4]
Ketamine has been widely used in burn dressing changes during excision and grafting and for sedation. Ketamine remains a relatively safe drug; however, monitoring of these patients is essential, particularly since there are reports of respiratory and cardiovascular depression.[5–9]
Benzodiazepines are commonly used in burns units.[3,9,10] It is widely understood that pain is exacerbated by anxiety. In burns, the commonly used benzodiazepine is midazolam. Patients may receive midazolam by intravenous, intranasal, rectal or oral route. Ketamine is associated with emergence phenomena. Midazolam seems to somewhat help alleviate discomfort arising from these physchological reactions.[11]
Dexmedetomidine, a selective a2-adrenergic agonist, is being studied for its potential use in anesthetic practice because of its combined analgesic, sedative, hypnotic and anxiolytic effect.[12,13] Dexmedetomidine reduces the dose requirements of opioids and anesthetic agents and attenuates the hemodynamic responses to tracheal intubation and surgical stimuli.[14] Horvath et al.[15] showed that endomorphin-1, like morphine, shows synergistic interaction with both the N methyl D aspartate(NMDA) antagonist S-ketamine and the a2-adrenoceptor agonist dexmedetomidine. The synergistic interaction between these drugs may be of therapeutic significance in the future, by allowing a decrease in the dose of either drug required to achieve an acceptable level of analgesia.
This study was designed to indicate the alternative methods of pain control and to compare the effects of ketamine, ketamine-midazolam and ketamine-dexmedetomidine on the hemodynamic variables, analgesia and sedation in burn patients undergoing dressing changes.
Materials and Methods
After receipt of Institutional Review Board approval and patients' written informed consent, 90 ASA I-III burn patients, between the ages of 19 and 65 years, scheduled for dressing changes with sedoanalgesia, were recruited. The study period was March 2006 to August 2007 (18 months). Patients were included in the study if total burn surface area (TBSA) was between 10% and 25%. Patients who were less than 18 years of age, pregnant or nursing; or had abnormal laboratory test results, hypersensitivity to opioids, significant psychiatric, cardiovascular, renal or hepatic diseases were excluded.
On arrival in the operating room of patients, routine monitors were applied for recording heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial blood pressure (MBP), peripheral oxygen saturation (SpO2), and urine output (bladder catheter). After obtaining baseline values and those 10 minutes before dressing change, patients were randomly (computer-generated random table) allocated to receive one of three study protocols.
Patients in group ketamine-dexmedetomidine (KD) (n = 30) received intravenous (IV) dexmedetomidine (1 μg kg-1) over 10 minutes, before intervention, followed by 1 mg kg-1 of IV ketamine.
Patients in group ketamine-midazolam (KM) (n = 30) received IV midazolam (0.05 mg kg-1) over 10 minutes, before intervention, followed by 1 mg kg-1 of IV ketamine.
Patients in group ketamine-saline (KS) (n = 30) received IV saline over 10 minutes, before intervention, followed by 1 mg kg-1 of IV ketamine.
The number of patients requiring additional ketamine; and the pain and sedation scores, time to dressing change and recovery time for all patients were recorded. Hemodynamic variables were also recorded at baseline (before the study drug infusion), after loading dose of study drug, before and after ketamine administration, and at 5, 10, 15, 30, 45 and 60 minutes after the procedure. It was also planned that if hypotension occurred (SBP < 80 mm Hg), the patients would be primarily treated with fluid administration (0.9% saline 10 mL kg-1h-1).
Patients were instructed on the use of Visual Analogous Scale (VAS) self-rating method. All patients used a separate 10-cm VAS device to assess the level of pain (0, no pain; 10, worst possible pain). Sedation was assessed on a five-point scale (‘0’ = no sedation—patient wide awake and alert; 4' = deep sleep, difficult to rouse). Pain and sedation were assessed by an assistant at 1, 2, 4, 6, 12 hours postoperatively.
A pain score <5 was considered adequate analgesia. As required to treat inadequate analgesia (e.g., increase in mean SBP, 25% above baseline; purposeful movements; swallowing; grimacing), the ketamine bolus (0.5-1 mg kg-1) was given as a rescue analgesic. The total dose of ketamine used for rescue analgesia was also recorded. Sedoanalgesia time was recorded for all groups. Sedoanalgesia was defined primarily as VAS <5 and sedation scores >2.
During the study period, the number of patients requiring additional ketamine; and time to dressing change and recovery time for all patients were recorded. Incidence and severity of side effects (e.g., nausea, vomiting, hemodynamic events), if any, were recorded.
Qualitative data were analyzed with pearson Chi-square test. Quantitative data, expressed as ‘mean ± standart deviation (SD)’, were analyzed by one way ANOVA test. A probability value of .05 was considered statistically significant. All analyses were done by using statistical package for social sciences (SPSS) version 10.0 (SPSS, Chicago, IL).
Results
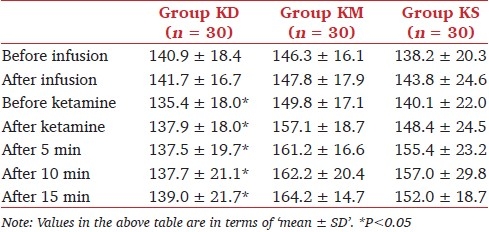
The characteristics of the 90 patients who completed the study are summarized in Table 1. Demographic characteristics (age, weight, sex), time to dressing change and recovery time were similar among the groups. SBP was significantly lower in group KD in comparison with the other groups at, before and after ketamine administration; and 5, 10 and 15 minutes after the procedure (P < .05) [Table 2]. Thereafter, there was no significant difference in SBP among the groups (data not shown in Table 2). No significant difference was found in DBP and HR among the groups.
Table 1.
Demographic characteristics of patients in the study groups
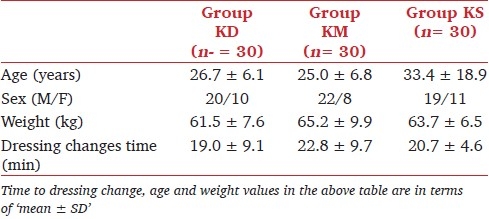
Table 2.
Systolic blood pressure in the study groups
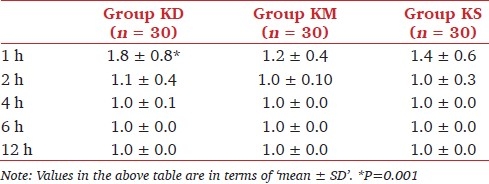
There was no statistically significant difference in pain scores among the groups during the study period [Table 3]. At the first hour, sedation scores were higher in group KD than in group KM and KS. Sedation scores are shown in Table 4. The number of patients requiring additional ketamine was similar among the groups, and there was no significant difference. Duration of sedoanalgesia was significantly longer in group KD than in group KM (P <0.05).
Table 3.
Pain scores in the study groups
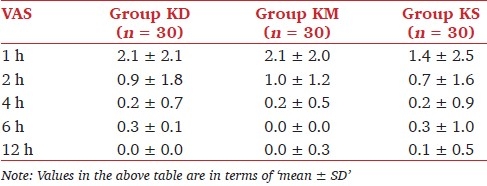
Table 4.
Sedation scores in the study groups
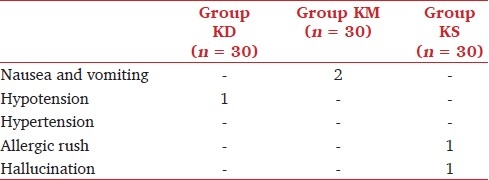
There were four adverse events in all the groups [Table 5]. A 23-year-old male patient who had received ketamine-dexmedetomidine combination experienced brief (<1 hour) episode of hypotension (SBP, 60 mm Hg), and it was treated mainly with IV fluid (0.9% saline infusion 10 mL kg-1h-1) administration. Two patients in group KM experienced nausea and vomiting. Only 1 patient among those who had received ketamine-saline combination experienced hallucination. Hypoxia and apnea were not observed in any of the study patients.
Table 5.
Side effects in the study groups
Discussion
In this study, we have demonstrated that three sedoanalgesic techniques provided effective sedation and analgesia during wound dressing changes in burn patients.
Burn care requires daily debridement, dressing changes and assessment regarding the need for skin grafting. These procedures are painful and may require an operating room environment. To increase the comfort of burn patients during dressing changes, it is necessary to give a patient tailored IV sedation with analgesic and anxiolytic drugs and to take into account the daily self-evaluation of the patient's pain.[1–4] The pain following burn injury is a complex mixture of background and incident pain with inflammatory and neuropathic components. Burn injury is among the most severe forms of trauma, and burn pain, in particular, is one of the most severe forms of acute pain, which necessitates aggressive use of opioids. Currently, narcotics such as morphine, meperidine and fentanyl are the most common forms of analgesic therapy in use for burn patients.[4] Nowadays ketamine and other analgesic drugs such as acetaminophen, NSAIDs (nonsteroidal anti-inflammatory drugs), local anesthetics, benzodiazepines, clonidine, nitrous oxide–oxygen mixtures; and psychological techniques are used.[3–11]
The effect of ketamine is thought to be the result of N-methyl-D-aspartate (NMDA) receptor antagonism, opioid l receptor agonism, and voltage-sensitive sodium channel interactions.[15,16] In humans, ketamine is agent for providing intraoperative and postoperative analgesia in burn patients. Humphries et al.[7] used oral ketamine as an analgesic and sedative for wound-care procedures in children with burns, and demonstrated improved analgesia and sedation with oral ketamine compared with commonly used narcotics and sedatives. Owens et al.[8] used IV ketamine for painful procedures in pediatric burn patients. They found that, ketamine can be safely and effectively used for bedside procedures in pediatric burn patients. During dressing changes in burn patients, the major advantage of ketamine is that it usually preserves airway patency and respiratory function. In our study, ketamine did not result in any respiratory depression or apnea during the study period.
Dexmedetomidine is a recently developed α2-agonist that shows much greater selectivity for the 2-adrenoceptor than the other widely used agonists (e.g., clonidine).[12–14] It produces dose-dependent analgesia (involving spinal and supraspinal sites) without respiratory depression.[17] The analgesic profile of dexmedetomidine has not been fully characterized in humans. In a previous study, it was reported that clonidine counterbalanced the sympathetic stimulation of ketamine by virtue of its action in reducing sympathetic outflow, and the combination of clonidine and ketamine may be useful for burn patients with hypertension or myocardial ischemia.[18] In this study, counterbalance of the sympathetic stimulation by ketamine may have been provided by dexmedetomidine.
Midazolam provides sedation anxiolysis and less respiratory depression.[9–11] Walker et al.[19] compared dexmedetomidine infusion with standard sedation regimen of opioids and benzodiazepines in pediatric burn patients. They reported that with dexmedetomidine titration, all patients were rated “adequately sedate,” even though all were sedation failures with opioids and benzodiazepines.
To our knowledge, this is the first study comparing the sedoanalgesic effects of ketamine, ketamine-dexmedetomidine combination and ketamine-midazolam combination during wound dressing changes in burn patients. Previous studies have reported attenuation of hypertension and tachycardia in response to laryngoscopy and intubation by dexmedetomidine.[20] Hemodynamic responses may be seen during dressing changes in burn patients, and they are not as frequent and profound as those seen with intubation or laryngoscopy. Hemodynamic events seen during dressing changes may be related with either plasma concentration of catecholamines or study drugs. In our study, there was no greatly significant difference between the groups in hemodynamic parameters, except that systolic blood pressure was significantly lower in the KD group than in KM and KS groups at the first hour. The changes in HR and DBP were similar for the treatment groups. Talke et al.[21] reported that dexmedetomidine (plasma concentrations in the range of 0.18 to 0.35 ng/mL) attenuates the increases in HR and plasma norepinephrine concentrations observed during emergence from anesthesia. Furthermore, it has also been reported that dexmedetomidine attenuates the hyperadrenergic state associated with ketamine. We did not measure either norepinephrine or dexmedetomidine plasma concentrations. Therefore, we failed to demonstrate any correlation between hemodynamic variables and plasma catecholamine concentrations.
The most frequently seen adverse effect of ketamine is emergence of reactions or hallucinations. Recovery agitation of ketamine has been modestly associated with decreasing age and the presence of an underlying medical condition.[22] In this study, only 1 patient among those who had received only ketamine-saline combination experienced hallucination. Owens et al.[8] reported that 2.9% of the patients who received ketamine during sedation experienced side effects such as desaturation, apnea, hypotension. Walker et al.[18] stated that no respiratory depression associated with the use of dexmedetomidine had occurred. Similarly, in a recent study, Taghinia et al.[23] reported that dexmedetomidine decreased the frequency of oxygen desaturation and reduced the amounts of narcotic and anxiolytic requirement. In this study, we did not observe any respiratory depression, hypoxia or apnea in any group. Hemodynamic variables were also similar among the groups in each study period, except SBP was significantly lower in group KD than in groups KM and KS at the first hour. The most frequently seen adverse effects of IV dexmedetomidine that have been reported are hypotension and bradycardia.[24] In this study, only a brief episode (<1 hour) of hypotension (SBP, 60 mm Hg) was observed in a 23-year-old male patient who had received ketamine-dexmedetomidine combination, and it was treated mainly with IV fluid (0.9% saline 5-10 mL kg-1h-1) administration.
Although midazolam and dexmedetomidine are known as sedative agents, sedation scores were significantly higher in group KD than in group KM following the first hour of the study period. Dexmedetomidine has been reported to be associated with a long-arousable sedation, and this could be the reason why sedation scores were significantly higher in the KD group than in KM and KS groups.[17]
Green et al.[22] reported that the incidence of emesis after ketamine administration was modestly associated with increasing age. Ünlügenç et al.[25] reported that the sedative effects of midazolam and propofol lasted for a much shorter time than the antiemetic effects of these drugs, and these drugs used in subhypnotic doses were as effective as ondansetron in treating PONV (postoperative nausea and vomiting) in patients undergoing abdominal or gynecological surgery. In this study, nausea and vomiting were observed in 2 patients in group KM. Lower incidence of emesis in KD group was thought to be associated with the use of dexmedetomidine. Taghinia et al.[23] have reported that dexmedetomidine decreased antiemetic use.
In conclusion; in burn patients undergoing dressing changes, although both combinations, viz., ketamine-dexmedetomidine and ketamine-midazolam, offered effective sedoanalgesia without causing any significant side effects, the former resulted in higher sedation and lower hemodynamic discrepancy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Choinière M, Melzack R, Rondeau J, Girard N, Paquin MJ. The pain of burns: Characteristics and correlates. J Trauma. 1989;29:1531–9. doi: 10.1097/00005373-198911000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Summer GJ, Puntillo KA, Miaskowski C, Green PG, Levine JD. Burn injury pain: The continuing challenge. J Pain. 2007;8:533–48. doi: 10.1016/j.jpain.2007.02.426. [DOI] [PubMed] [Google Scholar]
- 3.Ashburn MA. Burn pain: The management of procedure-related pain. J Burn Care Rehabil. 1995;16:365–71. doi: 10.1097/00004630-199505001-00006. [DOI] [PubMed] [Google Scholar]
- 4.Pall SK, Cortiella J, Herndon D. Adjunctive methods of pain control in burns. Burns. 1997;23:404–12. doi: 10.1016/s0305-4179(97)00029-6. [DOI] [PubMed] [Google Scholar]
- 5.Pagel PS, Kampine JP, Schmeling WT, Warltier DC. Ketamine depresses myocardial contractility as evaluated by the preload recruitable stroke work relationship in chronically instrumented dogs with autonomic nervous system blockade. Anesthesiology. 1992;76:564–72. doi: 10.1097/00000542-199204000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Greene CA, Gillette PC, Fyfe DA. Frequency of respiratory compromise after ketamine sedation for cardiac catheterization in patients less than 21 years of age. Am J Cardiol. 1991;68:1116–7. doi: 10.1016/0002-9149(91)90512-j. [DOI] [PubMed] [Google Scholar]
- 7.Humphries Y, Melson M, Gore D. Superiority of oral ketamine as an analgesic and sedative for wound care procedures in the pediatric patient with burns. J Burn Care Rehabil. 1997;18:34–6. doi: 10.1097/00004630-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Owens VF, Palmieri TL, Comroe CM, Conroy JM, Scavone JA, Greenhalgh DG. Ketamine: A safe and effective agent for painful procedures in the pediatric burn patient. J Burn Care Res. 2006;27:211–6. doi: 10.1097/01.BCR.0000204310.67594.A1. [DOI] [PubMed] [Google Scholar]
- 9.Cederholm I, Bengtsson M, Björkman S, Choonara I, Rane A. Long-term high dose morphine, ketamine, and midazolam infusion in a child with burns. Br J Clin Pharmacol. 1990;30:901–5. doi: 10.1111/j.1365-2125.1990.tb05459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinrich M, Wetzstein V, Muensterer OJ, Till H. Conscious sedation: Off-label use of rectal S(+)-ketamine and midazolam for wound dressing changes in paediatric heat injuries. Eur J Pediatr Surg. 2004;14:235–9. doi: 10.1055/s-2004-817960. [DOI] [PubMed] [Google Scholar]
- 11.Doenicke A, Kugler J, Mayer M, Angster R, Hoffmann P. Ketamine racemate or S-(+)-ketamine and midazolam.The effect on vigilance, efficacy and subjective findings. Anaesthesist. 1992;41:610–8. [PubMed] [Google Scholar]
- 12.Peden CJ, Prys-Roberts C. Dexmedetomidine-a powerful new adjunct to anaesthesia? Br J Anaesth. 1992;68:123–5. doi: 10.1093/bja/68.2.123. [DOI] [PubMed] [Google Scholar]
- 13.Dexmedetomidine: sedation, analgesia and beyond. Expert Opin dug Metab Toxicol. 2008;4:619. doi: 10.1517/17425255.4.5.619. 27. [DOI] [PubMed] [Google Scholar]
- 14.Aantaa R, Kanto J, Scheinin M, Kallio A, Scheinin H. Dexmedetomidine, an alpha 2-adrenoceptor agonist, reduces anaesthetic requirements for patients undergoing minor gynecological surgery. Anesthesiology. 1990;73:230–5. doi: 10.1097/00000542-199008000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Horvath G, Joo G, Dobos I, Klimscha W, Toth G, Benedek G. The synergistic antinociceptive interactions of endomorphin-1 with dexmedetomidine and/or S(+)-ketamine in rats. Anesth Analg. 2001;93:1018–24. doi: 10.1097/00000539-200110000-00044. [DOI] [PubMed] [Google Scholar]
- 16.Smith DJ, Bouchal RL, deSanctis CA, Monroe PJ, Amedro JB, Perrotti JM, et al. Properties of the interaction between ketamine and opiate binding sites in vivo and in vitro. Neuropharmacology. 1987;26:1253–60. doi: 10.1016/0028-3908(87)90084-0. [DOI] [PubMed] [Google Scholar]
- 17.Arain SR, Ruehlow RM, Uhrich TD, Ebert TJ. The efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgery. Anesth Analg. 2004;98:153–8. doi: 10.1213/01.ANE.0000093225.39866.75. [DOI] [PubMed] [Google Scholar]
- 18.Kariya N, Shindoh M, Nishi S, Yukioka H, Asada A. Oral clonidine for sedation and analgesia in a burn patient. J Clin Anesth. 1998;10:514–7. doi: 10.1016/s0952-8180(98)00068-3. [DOI] [PubMed] [Google Scholar]
- 19.Walker J, Maccallum M, Fischer C, Kopcha R, Saylors R, McCall J. Sedation using dexmedetomidine in pediatric burn patients. J Burn Care Res. 2006;27:206–10. doi: 10.1097/01.BCR.0000200910.76019.CF. [DOI] [PubMed] [Google Scholar]
- 20.Aho M, Lehtinen AM, Erkola O, Kallio A, Korttila K. The effect of intravenously administered dexmedetomidine on perioperative hemodynamics and isoflurane requirements in patients undergoing abdominal hysterectomy. Anesthesiology. 1991;74:997–1002. doi: 10.1097/00000542-199106000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Talke P, Chen R, Thomas B, Aggarwall A, Gottlieb A, Thorborg P, et al. The hemodynamic and adrenergic effects of perioperative dexmedetomidine infusion after vascular surgery. Anesth Analg. 2000;90:834–9. doi: 10.1097/00000539-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Green SM, Kuppermann N, Rothrock SG, Hummel CB, Ho M. Predictors of adverse events with intramuscular ketamine sedation in children. Ann Emerg Med. 2000;35:35–42. doi: 10.1016/s0196-0644(00)70102-8. [DOI] [PubMed] [Google Scholar]
- 23.Taghinia AH, Shapiro FE, Slavin SA. Dexmedetomidine in aesthetic facial surgery: Improving anesthetic safety and efficacy. Plast Reconstr Surg. 2008;121:269–76. doi: 10.1097/01.prs.0000293867.05857.90. [DOI] [PubMed] [Google Scholar]
- 24.Arain SR, Ebert TJ. The efficacy, side effects and recovery characteristics of dexmedetomidine versus propofol when used for intraoperative sedation. Anesth Analg. 2002;95:461–6. doi: 10.1097/00000539-200208000-00042. [DOI] [PubMed] [Google Scholar]
- 25.Unlugenc H, Guler T, Gunes Y, Isik G. Comparative study of the antiemetic efficacy of ondansetron, propofol and midazolam in the early postoperative period. Eur J Anaesthesiol. 2004;21:60–5. doi: 10.1017/s0265021504001103. [DOI] [PubMed] [Google Scholar]


