Abstract
Blood transfusion refers to the perioperative administration of blood and blood components. Adherence to proper indications for blood component therapy is essential because of its potential adverse effects and costs of transfusion. Over the years, the significance of blood components in treating certain diseases or conditions has been recognized. In this article, the most commonly used blood components along with the new developments in component therapy have been discussed. Recommendations by different academic and clinical trials and studies have been presented for quick reference. The individual coagulation factors are discussed in brief.
Keywords: Blood transfusion, blood components, bleeding disorder, coagulopathy
History
The first documented animal-to-animal (dog) blood transfusion was performed at Oxford in 1665 by Richard Lower, followed by the first animal-to-human blood transfusion in 1667 by Jean Denis. The first human-to-human blood transfusion was performed by James Blundell in 1818. In the year 1900, the ABO blood grouping system was classified by Landsteiner and, based on this, the first pretransfusion cross-match was done by Ottenberg in 1907. The system of Rh typing was invented by Landsteiner and Wiener in the year 1940. After this, there have been major inventions in the 20th century and that made component therapy possible, e.g. invention of anticoagulant and preservative solutions, refrigeration, plastic blood bags, component administration, infectious disease testing, high-risk donor screening, etc.
Clinical Practice Guidelines
Following are the guidelines issued for blood transfusion from time to time:
1980: The National Institutes of Health
1984: The American College of Obstetricians and Gynaecologists (ACOG)
1990: The Transfusion Practices Committee of the American Association of Blood Banks (coronary artery bypass surgery)
1992: The American College of Physicians (ACP)
1994: The College of American Pathologists (CAP) - fresh frozen plasma (FFP), cryoprecipitate and platelet transfusion
1994: The American Association of Blood Banks
1996: American Society of Anaesthesiologists – Task Force on Blood Component Therapy
2006: An Updated Report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies.[1]
A variety of comprehensive intervention strategies and transfusion algorithms have been associated with reductions in inappropriate blood component therapy.[2–4] The most familiar cellular components include packed red blood cells (PRBC), washed PRBC, leukoreduced PRBC and pooled or aphaeresis platelets. Plasma products such as FFP or cryoprecipitate antihemophilic factor (CRYO), on the other hand, may not be as familiar.
Preoperative Evaluation
The ASA Task Force 2006 recommends that preoperative evaluation should include the coagulation profile of the patient, e.g. review of medical records, laboratory investigations and also looking for any family history of bleeding disorder. Preoperative patient preparation should include discontinuation or modification of anticoagulation therapy and prophylactic administration of drugs to promote coagulation and minimize blood loss thus reducing the requirement of allogenic blood transfusion.
Perioperative Transfusion
Red cell components and substitutes
Approximately 12,000,000 units of RBCs are transfused each year in the United States.[5] The objective of RBC transfusion is to improve oxygen delivery to the tissues. Following are the red cells and their different components for transfusion:
Packed red cells
The red cells from a donor unit are concentrated to a hematocrit of about 75%, and the volume is made to 200 ml. Storing red cells (just above freezing) allows survival for 42 days but, unfortunately, decreases the 2,3-DPG and ruins the platelets and neutrophils. Giving packed red cells is the fastest way to increase the oxygen-delivering capacity of the blood. A unit of whole blood or packed red cells will raise the hematocrit by 3% and the haemoglobin by 1 gm/dl.
Washed red cells
RBCs are washed with sterile saline using machines specially designed for this purpose. These washed RBCs are suspended in sterile saline, usually at hematocrits of 70–80%, with a volume of approximately 180 ml. Saline washing removes all but traces of plasma (98%), reduces the concentration of leukocytes and removes platelets and cellular debris. Saline washing may be performed at any time during the shelf-life of a unit of blood but, because washing is ordinarily performed in an “open” system, the resultant red cell component can be stored for only 24 h at 1–6°C.
Irradiated red cells
Packed red cells are gamma radiated to kill the lymphocytes. The lack of T-cells prevents donor vs. host disease. They are used for severely immunocompromised patients, lymphoma patients, stem-cell/marrow transplants and unborn children undergoing intrauterine transfusion.
Leukoreduced red cells (“filtered” and/or “washed” red cells)
These are packed red cells from which 99.9% of the white cells are filtered out (pre- or post-storage) or removed by freezing/thawing/washing. The use of “Leuko Poor filters” is becoming very popular. These reduce (but do not eliminate) the risk of cytomegalovirus (CMV), Epstein-Barr, human T-lymphotropic virus (HTLV) infections and febrile reactions.
Red Cell Substitutes
There are different types of red cell substitutes currently being studied for use in transfusion medicine. The goal is to enhance the oxygen-carrying capacity for patients suffering from acute anaemia due to blood loss. All of these have shown some promise for use in patients who refuse blood transfusion or in situations where blood is not readily available.
Haemoglobin-Based Oxygen Carriers
These are purified cell-free hemoglobins, where the globulin portion of the molecule has been modified chemically by conjugation, cross-linking or polymerization. Modification increases the oxygen-releasing ability of the haemoglobin. The bovine Hemoglobin-Based Oxygen Carriers (HbOCs) has completed phase III clinical trials and has been approved in South Africa for treatment of perioperative anaemia in adult surgical patients.[6]
Perfluorocarbon-Based Red Cell Substitutes
These consist of carbon backbones highly substituted with fluorine. They can dissolve large amounts of oxygen. The perfluorocarbons are biologically inert; however, the phospholipids are required to emulsify them. The only perfluorcarbon that remains in clinical trials is Oxygent, an emulsion of perfluoroocytl bromide and egg yolk phospholipid. Oxygent is being studied for use in perioperative period to allow more extensive hemodilution.[7]
Recommendations
A consensus conference convened by the Royal College of Physicians of Edinburgh concluded that RBC transfusion is indicated only to increase the oxygen-carrying capacity. The ASA Task Force 2006 concludes that:
A close watch on assessment of blood loss during surgery and assessment of tissue perfusion is to be maintained.
Transfusion is rarely indicated when the haemoglobin concentration is greater than 10 gm/dl, and is almost always indicated when it is less than 6 gm/dl.
For intermediate haemoglobin concentrations (6–10 gm/dl), justifying or requiring RBC transfusion should be based on the patient's risk for complications of inadequate oxygenation.
Use of a single haemoglobin “trigger” for all patients and other approaches that fail to consider all important physiologic and surgical factors affecting oxygenation are not recommended.
When appropriate, preoperative autologous blood donation, intraoperative and postoperative blood recovery, acute normovolemic hemodilution and measures to decrease blood loss (deliberate hypotension and pharmacologic agents) may be beneficial.
The indications for transfusion of autologous RBCs may be more liberal than for allogeneic RBCs because of the lower (but still significant) risks associated with the former.
Platelets
More than 7,000,000 units of platelets are transfused each year in the United States.[5] Platelets are used in the perioperative setting, when a quantitative or qualitative platelet defect is the suspected cause of bleeding. The scientific rationale rests on two principal arguments: (1) surgical patients with thrombocytopenia and (2) presence of platelet defects. The aim is thereby to reduce, minimize or prevent bleeding.
Normal values: Adult, 300,000/cumm and for full-term and pre-term newborn, 250,000/cumm.
Collection and Storage
Single donor units are prepared from individual units of whole blood, each bag containing not less than 5500/cumm platelets with 50–70 ml of residual plasma volume. Earlier, separation of platelets by centrifugation yielded a product with a shelf-life of about 2 h, and refrigeration of platelet concentrates at 1–6°C provided products with a shelf-life of 24 h. Thirty years ago, platelets were stored only at room temperature. The advent of new plastic containers allows platelets to be kept for up to 5 days before outdating. They are stored with continuous agitation to prevent clumping of cells.
Pooled Platelets
Obtaining a “pooled platelet” preparation is a two-step procedure. First, one unit of platelets is harvested from a unit of whole blood. Then, four to six of these individual units (from different donors) are “pooled” together in a single pack to be given to a thrombocytopenic patient.
Apheresis Platelets
These are collected from a single donor using the cell separator instrumentation. As blood cycles through the machine, platelets are removed and all other blood constituents are returned to the donor. The amount of platelets collected with this procedure represents the equivalent of four to six units of random donor platelets.
Dosage
One random unit of platelets will raise the platelet count in an adult by 5,000–8,000/cumm. In children, 0.1–0.2 units/kg will increase the platelet count by 30–50,000/cumm. The expected increase will be less if the patient has sepsis, splenomegaly, platelet auto- or allo antibodies or is receiving chemotherapy.
Recommendations
In 1987, the National Institutes of Health Consensus Conference on Platelet Transfusion Therapy recommended protocols for prophylactic platelet transfusion.[8] These were further revised by the ASA Task Force in 1994 and 2006, and they recommended that:
Prophylactic platelet transfusion is ineffective and rarely indicated when thrombocytopenia is due to increased platelet destruction (e.g. idiopathic thrombocytopenic purpura)
Prophylactic platelet transfusion is rarely indicated in surgical patients with thrombocytopenia due to decreased platelet production when the platelet count is greater than 1,00,000/cucc and is usually indicated when the count is below 50,000/cucc. The determination of whether patients with intermediate platelet counts (50–100,000/cucc) require therapy should be based on the risk of bleeding.
Surgical patients with microvascular bleeding usually require platelet transfusion if the platelet count is less than 50,000/cucc and rarely require therapy if it is greater than 100,000/cucc. With intermediate platelet counts (50–100,000/cucc), the determination should be based on the patient's risk for more significant bleeding, type of surgery and site of operation, e.g. closed space like brain or eye.
Platelet transfusion may be indicated despite an apparently adequate platelet count if there is known platelet dysfunction and microvascular bleeding.
Platelet Products and Substitutes
Cryopreserved platelets
Platelets suspended in dimethylsulfoxide (DMSO) at –80°C have been preserved up to 10 years. During the thawing and post-thaw processing, however, these platelets develop functional and morphologic defects. There are investigations under way to develop methods that do not require processing after freezing, and can be directly infused after thawing. Because of the complexities of storing, processing and thawing frozen platelets, the current use is limited.
Lyophilized platelets
Lyophilized platelets are created after treatment with a paraformaldehyde solution and then freeze-dried.[9] Specific advantages of this product include storage measured in years instead of days, reduced storage space and true sterility. Once rehydrated, they appear to retain structural integrity and attach only to damaged subendothelial surfaces.[10]
Infusible Platelet Membranes (IPMs)
Infusible Platelet Membranes (IPMs) are manufactured from outdated platelet units. Platelet-derived microparticles (microvesicles) are the particles that form spontaneously from a platelet during collection and processing of components. They appear to have the ability to function as a platelet. They are procoagulant-active, adhere to the vascular subendothelium and enhance platelet adhesion to form a primary hemostatic plug. One application for IPMs may be in patients who are refractory to platelet transfusions and for whom finding human leukocytic antigen (HLA)-matched platelet pheresis donors is difficult. One problem appears to be a relatively short life (less than 24 h) in vivo.[11]
Miscellaneous microspheres
There are a number of products that use formaldehyde-fixed platelets, liposomes or 10% albumin spheres, on which fibrinogen or platelet membrane glycoproteins coat. Results from some of the preclinical trials show that these products appear to be able to enhance the adhesion of platelets and formation of aggregates, but the in vivo stability remains a problem.
Plasma
FFP
Approximately 2,000,000 units of FFP are transfused each year in the United States.[5] FFP is used primarily to provide replacement coagulation factors. One ml. of FFP contains approximately one unit of coagulation factor activity.
Indications
FFP is indicated for use in bleeding patients with multiple coagulation factor deficiencies secondary to liver disease, disseminated intravascular coagulation (DIC) and the dilutional coagulopathy[12] resulting from massive blood or volume replacement. It is also indicated for patients with congenital factor deficiencies for which there is no coagulation concentrate available, such as deficiencies of Factor V or XI. FFP is also used along with plasmapheresis in the treatment of thrombotic thrombocytopenic purpura (TTP) and haemolytic uremic syndrome. FFP should not be used as a source of protein for nutritionally deficient patients or as a volume expander.
Collection, Storage and Transfusion
Frozen plasma (FP) is prepared from whole blood by separating and freezing the plasma (200–250 ml) within 6 h of donation. It may be stored for up to 1 year at –18°C or lower. Under these conditions, the loss of labile Factors V and VIII is <30%. The FP should be thawed between 30 and 37°C with constant agitation. After thawing, it may be refrigerated, but should be used within 24 h to obtain adequate coagulation levels of Factors V and VIII, which start to diminish after 6 h. Only ABO-compatible plasma should be transfused through a standard 170-μm blood filter. Four to five platelet concentrates, one unit single-donor aphaeresis platelets or one unit fresh whole blood provide a quantity of coagulation factors similar to that contained in one unit FFP.
FFP should be given in doses calculated to achieve a minimum of 30% of plasma factor concentration (usually achieved with the administration of 10–15 ml/kg FFP), except for urgent reversal of warfarin anticoagulation, for which 5–8 ml/kg FFP will usually suffice. Whenever depletion of coagulation factors is considered to be clinically important, 800–2,000 ml (four to eight packs of FP) in a 70-kg adult for each blood volume lost should be given over 90–120 min. Slower rates of infusion or smaller volumes of FFP are probably ineffective.[13]
Recommendations: Latest recommendations for use of FFP are:
Urgent reversal of warfarin therapy
Correction of known coagulation factor deficiencies for which specific concentrates are unavailable
Correction of microvascular bleeding in the presence of elevated (>1.5-times normal) prothrombin time (PT) or partial thromboplastin time (PTT)
Correction of microvascular bleeding secondary to coagulation factor deficiency in patients transfused with more than one blood volume and when PT and PTT cannot be obtained in a timely fashion
FFP should be given in doses calculated to achieve a minimum of 30% of plasma factor concentration
FFP is contraindicated for augmentation of plasma volume or albumin concentration
FFP should not be used to reconstitute packed RBCs
FFP should not be used as a source of proteins or routinely after cardiopulmonary bypass
Cryoprecipitate
Almost 1,000,000 units of cryoprecipitate are transfused each year in the United States.[5] Cryoprecipitate from one donor usually contains 100 antihemophilic units (AHU) and 250 mg of fibrinogen; it is thawed at 37°C and administered through a standard blood filter.
Indications
Cryoprecipitate, which contains factor VIII, fibrinogen, fibronectin, von Willebrand's factor (vWF) and factor XIII, is used for the correction of inherited and acquired coagulopathies. Its use in the operative setting is based on the assumptions that: (i) patients with these coagulation factor deficiencies are at increased risk of hemorrhagic complications and (ii) replacement of coagulation factors is effective in decreasing these risks. One unit of cryoprecipitate per 10 kg body weight raises the plasma fibrinogen concentration by approximately 50 mg/dl in the absence of continued consumption or massive bleeding.
Recommendations
Earlier, cryoprecipitate transfusions were recommended in bleeding patients with hypofibrinogenemia, von Willebrand's disease and patients with haemophilia A (when factor VIII concentrate is not available). Recommendations for the use of Cryoprecipitate are:
Prophylaxis in non bleeding perioperative or peripartum patients with congenital fibrinogen deficiencies or von Willebrand's disease unresponsive to 1-desamino-8-D-arginine vasopressin (DDAVP).
Bleeding patients with von Willebrand's disease
Correction of microvascular bleeding in massively transfused patients with fibrinogen concentrations less than 80–100 mg/dl (or when fibrinogen concentrations cannot be measured in a timely fashion)
Cryoprecipitate Reduced Plasma
Cryoprecipitate Reduced Plasma (CRP) is the plasma remaining once the cryoprecipitate has been prepared. Preparation of the cryoprecipitate removes much of the fibrinogen, Factor VIII and vWF from the plasma. However, the vWF enzyme remains. This eliminates a source of vWF for multimer formation and provides the patient with the deficient enzyme to degrade the existing multimers. Therapeutic plasma exchange (TPE) is the only disease in which the component is used as therapeutic treatment.
Intravenous Immunoglobulin
The fractionation of immunoglobulins was successfully performed in the 1940s. During the 1980s and 1990s, changes in manufacturing allowed fractionation of the product into IgG portions that could be solubilized and used intravenously. Intravenous immunoglobulin is made from large pools of donor plasma (hundreds to thousands of donors). This polyclonal preparation contains 90-98% IgG and small amounts of IgA and IgM.[14]
The FDA has approved the use of immunoglobulins for the treatment of more than 30 disease conditions, including primary immune deficiency, B-cell chronic lymphocytic leukemia, idiopathic thrombocytopenia purpura, pediatric human immunodeficiency virus infection, Kawasaki syndrome and neuroimmunologic diseases such as Guillain-Barre syndrome and selected obstetric conditions.
Fibrin Glue
Fibrin glue has two main components, i.e. fibrinogen and thrombin. The thrombin converts the fibrinogen to fibrin by enzymatic action. The concentrated thrombin solution produces a fibrin clot in about 10s. Both the extrinsic and the intrinsic mechanisms of blood coagulation are bypassed with the use of fibrin glue.
Blood Component Transfusion in Pediatric Patients
In contrast to the large numbers of RBC transfusions given to neonates, relatively few other blood components are transfused. It has been shown that, in general for the pediatric population, the primary use of FFP is in the treatment of coagulation disorders. The sole purpose of treating hypovolemia with plasma is not recommended. The same is true for transfusion of RBCs to improve hematocrit. Platelet transfusion practices have also been shown to be variable, with the most controversial issue being the platelet level at which prophylactic transfusion should be given to sick premature infants. The efficacy of granulocyte transfusions in the septic and neutropenic neonates is still not fully established.
In certain chronic anaemia of childhood, there are clinical settings in which RBC transfusions are required to suppress the endogenous hemoglobin production. Children with sickle cell anaemia who have suffered a cerebrovascular accident or major splenic sequestration and who are not candidates for splenectomy may require a chronic RBC transfusion in order to suppress the production of sickle cell haemoglobin (HbS). Children with thalassemia syndromes are given routine RBC transfusions to prevent tissue hypoxia and suppress endogenous erythropoiesis in order to support more normal growth and development.
Monitoring and Treatment of the Adverse Effects of Transfusions
Periodically, check for signs and symptoms of bacterial contamination, transfusion related acute lung injury (TRALI), and haemolytic transfusion reactions, including urticaria, hypotension, tachycardia, increased peak airway pressure, hyperthermia, decreased urine output, hemoglobinuria and microvascular bleeding. Before instituting therapy for transfusion reactions, stop the blood transfusion and order appropriate diagnostic testing.
Current Research and Trials
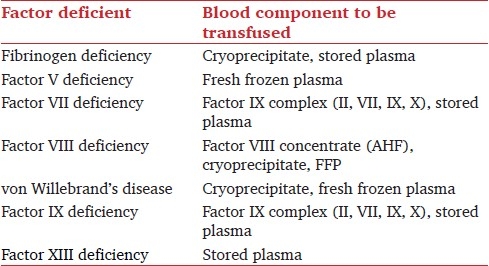
The role of the routine blood bank in providing therapeutic products has changed dramatically within the past few years. More emphasis is being placed on new and improved blood components and alternate uses for products. Donor lymphocyte infusion is a new use for the lymphocyte fraction obtained from apheresis. Blood substitutes are coming up and several sources of haemoglobin-based oxygen carriers are currently in Phase III clinical trials. A promising immunotherapeutic approach to cancer therapy is dendritic cell vaccines. A number of dendritic cell vaccines are in Phase I and II clinical trials. FFP and cryosupernatent (the supernatant left after the cryoprecipitate is made) are being used in TPE. Intravenous immunoglobulin has been used for over 10 years. Cryopreservation and lyophilization of platelets and platelet substitutes are all being studied as remedies for the short shelf-life of the platelet components. To establish a stronger scientific basis for transfusion practice, future research is essential to provide meaningful evidence and development of algorithms framing the indications and effectiveness of blood components and haemoglobin-based oxygen carriers or other synthetic blood substitutes to reduce transfusion requirements. Table 1 shows quick reference of individual blood component in particular clotting factor deficiency.
Table 1.
Quick reference for blood component transfusion
Conclusion
Inappropriate blood component transfusion use has been decreased by audits, discussions with ordering physicians, ward rounds, computer-based decision-support systems and comprehensive educational outreach programs. The lack of data from prospective, randomized studies with adequate sample size, control groups, clinical outcome measurements and other features of well-designed clinical effectiveness research impedes the development of evidence-based clinical practice guidelines for blood component therapy. As our understanding of how the immune system functions and as technology has progressed, specialized components or manufactured products such as blood substitutes have been advanced as remedies to some of the complications with component transfusion. Most importantly, transfusion decisions should be based on sound physiologic principles and a comprehensive assessment of the patient's risk factors.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Practice Guidelines for Perioperative Blood Transfusion and Adjuvant Therapies: An Updated Report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 2.Stehling L, Luban NL, Anderson KC, Sayers MH, Long A, Attar S, et al. Guidelines for blood utilization review. Transfusion. 1994;34:438–48. doi: 10.1046/j.1537-2995.1994.34594249058.x. [DOI] [PubMed] [Google Scholar]
- 3.Despotis GJ, Grishaber JE, Goodnough LT. The effect of an intraoperative treatment algorithm on physicians′ transfusion practice in cardiac surgery. Transfusion. 1994;34:290–6. doi: 10.1046/j.1537-2995.1994.34494233575.x. [DOI] [PubMed] [Google Scholar]
- 4.Paone G, Spencer T, Silverman NA. Blood conservation in coronary artery surgery. Surgery. 1994;116:672–8. [PubMed] [Google Scholar]
- 5.Wallace EL, Surgenor DM, Hao HS, An J, Chapman RH, Churchill WH. Collection and transfusion of blood and blood components in the United States, 1989. Transfusion. 1993;33:139–44. doi: 10.1046/j.1537-2995.1993.33293158046.x. [DOI] [PubMed] [Google Scholar]
- 6.Standl T. Haemoglobin-based erythrocyte transfusion substitutes. Expert Opin Biol Ther. 2001;1:831–43. doi: 10.1517/14712598.1.5.831. [DOI] [PubMed] [Google Scholar]
- 7.Water JA, Trouwborst A, Spense RK. A pilot study of the effects of perflubron emulsion, AF0104, on mixed venous oxygen tension in anesthetized surgical patients. Anesth Analg. 1996;82:103–7. doi: 10.1097/00000539-199601000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Consensus conference. Platelet transfusion therapy. JAMA. 1987;257:1777–80. [PubMed] [Google Scholar]
- 9.Bode AP. Preclinical testing of lyophilized platelets as a product for transfusion medicine. Transfus Sci. 1995;16:183–5. doi: 10.1016/0955-3886(95)97401-k. [DOI] [PubMed] [Google Scholar]
- 10.Bode AP, Read MS. Lyophilized platelets: Continued development. Transfus Sci. 2000;22:99–105. doi: 10.1016/s0955-3886(00)00028-x. [DOI] [PubMed] [Google Scholar]
- 11.Chao FC, Kim BK, Houranieh AM, Liang FH, Konrad MW, Swisher SN, et al. Infusible platelet membrane microvesicles: A potential transfusion substitute for platelets. Transfusion. 1996;36:536–42. doi: 10.1046/j.1537-2995.1996.36696269513.x. [DOI] [PubMed] [Google Scholar]
- 12.Hehne HJ, Nyman HB, Burri M, Wolfe G. Management of bleeding disorders in traumatic-haemorrhagic shock states with deep frozen fresh plasma. Eur J Intensive Care Med. 1979;2:157–61. doi: 10.1007/BF00624608. [DOI] [PubMed] [Google Scholar]
- 13.College of American Pathologists: Practice parameter for the use of fresh-frozen plasma, cryoprecipitate, and platelets. JAMA. 1994;271:777–81. [PubMed] [Google Scholar]
- 14.Swenson MR. Autoimmunity and immunotherapy. J IV Nuts. 2000;23:SB–13. [Google Scholar]


