Abstract
Objective:
This study examined the relationship between postmortem precuneus cholinergic enzyme activity, Pittsburgh compound B (PiB) binding, and soluble amyloid-β concentration in mild cognitive impairment (MCI) and Alzheimer disease (AD).
Methods:
Choline acetyltransferase (ChAT) activity, [3H]PiB binding, and soluble amyloid-β1–42 (Aβ42) concentration were quantified in precuneus tissue samples harvested postmortem from subjects with no cognitive impairment (NCI), MCI, and mild AD and correlated with their last antemortem Mini-Mental State Examination (MMSE) score and postmortem pathologic evaluation according to the National Institute on Aging–Reagan criteria, recommendations of the Consortium to Establish a Registry for Alzheimer's Disease, and Braak stage.
Results:
Precuneus ChAT activity was lower in AD than in NCI and was comparable between MCI and NCI. Precuneus [3H]PiB binding and soluble Aβ42 levels were elevated in MCI and significantly higher in AD than in NCI. Across all case subjects, reduced ChAT activity was associated with increased [3H]PiB binding, increased soluble Aβ42, lower MMSE score, presence of the APOE*4 allele, and more advanced AD pathology.
Conclusions:
Despite accumulating amyloid burden, cholinergic enzyme activity is stable in the precuneus during prodromal AD. A decline in precuneus ChAT activity occurs only in clinical AD, when PiB binding and soluble Aβ42 levels are substantially elevated compared with those in MCI. Anti-amyloid interventions in MCI case subjects with a positive PiB PET scan may aid in reducing cholinergic deficits and cognitive decline later in the disease process.
Loss of cortical cholinergic innervation contributes to cognitive dysfunction in Alzheimer disease (AD); however, the relationship between the cholinergic deficit and amyloid burden, a pathologic hallmark of AD, is unclear. Cholinergic agonists modulate metabolic processing of the amyloid-β (Aβ) precursor protein in vitro, and cholinergic activity impairment increases fibrillar Aβ levels in transgenic AD mice.1,2 PET using the amyloid-binding agent Pittsburgh compound B (PiB) facilitates in vivo detection of fibrillar Aβ deposits3; however, the status of the cholinergic system in brain areas prone to amyloid deposition remains unknown. To address this issue, we quantified choline acetyltransferase (ChAT) activity, [3H]PiB binding to insoluble Aβ, and soluble amyloid-β1–42 (Aβ42) peptide, the initial Aβ form in amyloid plaques,4 in precuneus (mesial Brodmann area 7) tissue harvested at autopsy from subjects with antemortem clinical diagnoses of no cognitive impairment (NCI), mild cognitive impairment (MCI), or mild AD (mAD). The precuneus exhibits high levels of PiB PET retention and may be selectively vulnerable to structural and functional alterations in preclinical and early AD.5–8 Neuroimaging studies implicate the precuneus in episodic memory retrieval and as a component of the brain's default mode network,9,10 which is disrupted in the presence of amyloid pathology.11,12 The parietal cortex receives cholinergic innervation from select subfields of the nucleus basalis,13 which is important in cognitive function. Whether amyloid deposition marked by in vivo PiB PET retention in the precuneus affects cholinergic enzyme activity has important implications for timely therapeutic intervention during the earliest phase of AD.
METHODS
Standard protocol approvals, registrations, and patient consents.
The study was approved by Rush University Medical Center and University of Kentucky institutional review boards and the University of Pittsburgh's Committee for Oversight of Research and Clinical Training Involving the Dead. Written informed consent for research and autopsy was obtained for all subjects in the study.
Subjects.
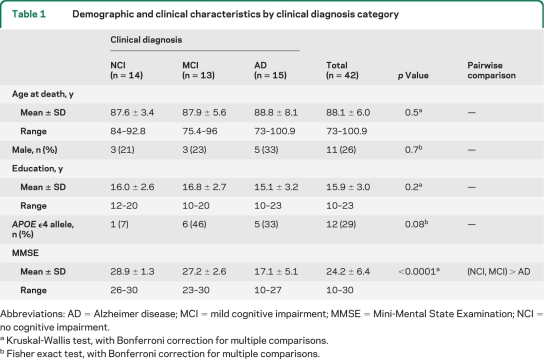
Forty-two cases matching our inclusion criteria were randomly chosen from brain banks of the Rush Religious Orders Study, a longitudinal clinicopathologic study of aging and AD in retired Catholic clergy,14 the University of Kentucky's community-dwelling cohort,15 and the University of Pittsburgh Alzheimer Disease Research Center.16 Sample size was limited by availability of frozen and fixed precuneus tissue. On the basis of the last antemortem clinical diagnosis, cases were assigned to 3 clinical groups (NCI, n = 14; MCI, n = 13; and AD, n = 15) matched by age, gender, years of education, and postmortem interval. The AD case subjects had mild to moderate disease severity (Mini-Mental State Examination [MMSE] score 10–27). The diagnosis of AD was made using standard diagnostic criteria.17 MCI was defined as impairment on neuropsychologic testing, but without diagnosis of dementia by the examining neurologist,14 criteria similar to those used at other centers.18,19 Among the cases of MCI, 9 were amnestic MCI (aMCI). Consensus conferences of neurologists and neuropsychologists reviewed clinical data, medical records, and interviews with family members and assigned a final clinical diagnosis. Neuropathologic diagnosis was based on the National Institute on Aging (NIA)–Reagan criteria,20 recommendations of the Consortium to Establish a Registry for Alzheimer's Disease (CERAD),21 and Braak staging of neurofibrillary tangles.22 Patients with pathology other than AD (e.g., stroke or Parkinson disease) were excluded from the study. All cases were deidentified and randomly assigned a unique identifier throughout the study. Investigators were blinded to case demographics and diagnosis.
Tissue samples.
Precuneus gray matter was harvested at autopsy and frozen (−80°C), homogenized on ice in phosphate-buffered saline (PBS; 150 mg/mL), and divided into 2 aliquots. One was prepared for ChAT enzyme activity assay, and the second was rehomogenized in tissue homogenization buffer (250 mM sucrose, 20 mM Tris base, and protease inhibitors [Sigma 8340 protease inhibitor cocktail; 10 μL/mL buffer]) and divided into 2 aliquots; one was prepared for Aβ ELISA, and the second was diluted to 10 mg tissue/mL with potassium PBS (pH 7.4) for the [3H]PiB binding assay.
Quantitative neuropathologic analyses.
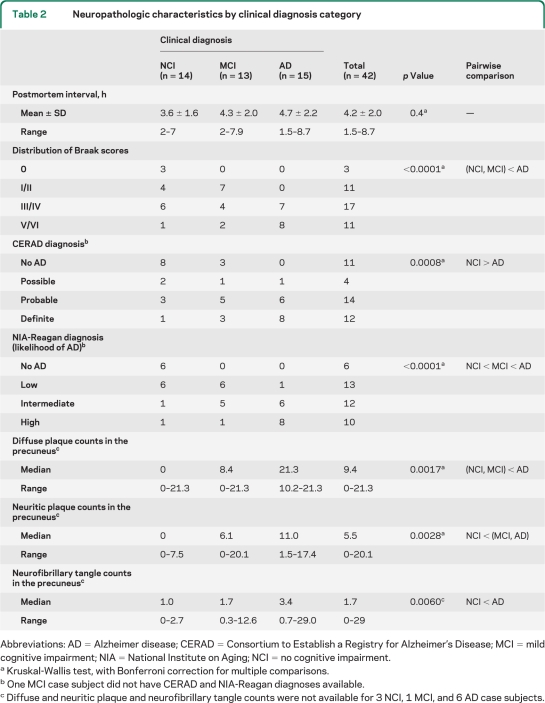
Formalin-fixed precuneus tissue was available from 32 of 42 cases examined. Samples were fixed in 10% buffered formalin for 48 hours, paraffin-embedded, cut into 8-μm-thick sections, and stained with the Bielschowsky silver method. A neuropathologist blinded to clinical diagnosis and demographic data performed the analyses. Diffuse plaques (DPs) and neuritic plaques (NPs) were counted using a 10 × objective (2.35 mm2 field of view), and neurofibrillary tangles (NFTs) were counted using a 20 × objective (0.586 mm2 field of view) in the 5 most severely involved areas. The results are shown as number of lesions per square millimeter. The upper limit for counting NPs and DPs was 50 plaques/2.35 mm2 field or 21.3/mm2 (table 2), whereas NFT counts had no upper limit.
Table 2.
Neuropathologic characteristics by clinical diagnosis category
Abbreviations: AD = Alzheimer disease; CERAD = Consortium to Establish a Registry for Alzheimer's Disease; MCI = mild cognitive impairment; NIA = National Institute on Aging; NCI = no cognitive impairment.
Kruskal-Wallis test, with Bonferroni correction for multiple comparisons.
One MCI case subject did not have CERAD and NIA-Reagan diagnoses available.
Diffuse and neuritic plaque and neurofibrillary tangle counts were not available for 3 NCI, 1 MCI, and 6 AD case subjects.
In vitro [3H]PiB binding assay.
The [3H]PiB binding assay was performed using a protocol published previously.23 Unlabeled PiB was dissolved in dimethyl sulfoxide (DMSO) at 400 μM to yield <1% DMSO in the final assay; this stock solution was diluted with PBS to achieve the desired concentration. [3H]PiB (1 nM, specific activity 72.4 Ci/mmol; American Radiolabeled Chemicals, St. Louis, MO) was incubated with 100 μg tissue in 1 mL PBS. Nonspecific binding was defined as the number of counts remaining in the presence of 1 μM unlabeled PiB. The binding mixtures were filtered through a Whatman GF/B glass filter and rapidly washed 5 times with 3 mL PBS. After thorough vortexing and resting overnight, the filters were counted in CytoScint ES, and results were corrected for nonspecific, nondisplaceable binding in the presence of 1 μM PiB. Values are expressed as picomoles of [3H]PiB bound per gram of wet tissue weight. The amount of [3H]PiB bound by this method correlates highly with the total number of PiB binding sites (i.e., the Bmax; r = 0.99)24 and mimics the low nanomolar concentrations of radioactive PiB achieved in human brain during a PET study.
Aβ ELISA.
The Aβ42 peptide concentration was quantified in diethylamine (DEA)-soluble Aβ fractions as described previously.23 The DEA-soluble fraction was prepared by centrifuging the precuneus homogenate aliquot described above at 135,000 × g at 4°C for 1 hour and neutralizing the supernatant with 0.5 M Tris-Cl. The Aβ concentration was assayed using a fluorescent-based ELISA (Biosource, Camarillo, CA) with a capture antibody specific for the NH2 terminus of human Aβ (amino acids 1–16) and detection antibodies specific for the neoepitope at the 42–amino acid end of Aβ. Values were determined from standard curves using synthetic Aβ42 peptide (Biosource) and are expressed as picomoles per gram wet brain tissue.
ChAT activity assay.
ChAT activity was measured using a modification of the Fonnum method.25 Aliquots were rehomogenized in buffer containing 10 mM disodium EDTA and 0.5% Triton X-100 in distilled water. All samples were run in triplicate. The reaction was initiated by the addition of 5 μL of sample or blanks (homogenizing buffer) to Eppendorf tubes containing 10 μL of assay mixture consisting of 250 μL of incubation buffer (100 mM sodium phosphate buffer, 600 mM sodium chloride, 20 mM choline chloride, and 10 mM disodium EDTA), 250 μL of [14C]acetyl coenzyme A (0.4 mM, 40–60 mCi/mmol; New England Nuclear, Boston, MA), and 5 μL of 20 mM eserine salicylate. Tubes were incubated for 30 minutes at 37°C and transferred into scintillation vials. The reaction was stopped by adding 4 mL of 10 mM sodium phosphate followed by the addition of 1.6 mL of aqueous extraction solution (acetonitrile and 5 mg/mL tetraphenylboron) and 8 mL of organic extraction fluid (Econofluor). Vials were mixed by inversion and counts per minute were obtained 24 hours later. Protein content was determined using BCA protein assay kits (Pierce, Rockford, IL). ChAT activity values were expressed as micromoles per hour per gram of protein.
Statistical analysis.
Primary outcome measures were levels of ChAT activity, [3H]PiB binding, and soluble Aβ42 concentration in the precuneus. Predictor variables comprised demographic, clinical, and neuropathologic factors. Comparisons of demographic and clinical/neuropathologic characteristics, biochemical measures, and neuropathology counts among the clinically defined groups were performed using the Kruskal-Wallis test or Fisher exact test with Bonferroni correction for multiple comparisons. Associations between biochemical measures, demographic and clinical characteristics, and neuropathology scores were assessed by Spearman rank correlation or Wilcoxon rank sum test. Although cases were matched on age, gender, years of education, and postmortem interval, the residual effects of these potential confounders were explored in multivariate analyses including partial correlation or multiple regression; the findings remained essentially unchanged and are presented as unadjusted (univariate) results. Nonparametric methods were used whenever possible because they are more robust to outliers and non-normality in the data. No subgroup analysis was conducted, and statistical analyses were based on all available data, with any missing value documented by a footnote in tables 1 and 2. The level of statistical significance was set at 0.05 (2-sided).
Table 1.
Demographic and clinical characteristics by clinical diagnosis category
Abbreviations: AD = Alzheimer disease; MCI = mild cognitive impairment; MMSE = Mini-Mental State Examination; NCI = no cognitive impairment.
Kruskal-Wallis test, with Bonferroni correction for multiple comparisons.
Fisher exact test, with Bonferroni correction for multiple comparisons.
RESULTS
Case demographics and clinical and neuropathologic characteristics.
NCI, MCI, and mAD groups differed by MMSE scores, Braak scores, and CERAD and NIA-Reagan diagnoses of AD (all p < 0.001), but not by age, gender, years of education, APOE allele status, or postmortem delay (tables 1 and 2). Post hoc analyses revealed that the mAD group had lower MMSE scores and a more advanced Braak stage of NFT pathology than the MCI and NCI groups, whereas the latter 2 groups were not statistically different. NIA-Reagan scores were higher in the mAD group than in the NCI and MCI groups and higher in the MCI than in the NCI group. The mAD group had more advanced CERAD diagnoses than the NCI group, but differences between the mAD and MCI groups were not statistically significant (table 1). The 3 clinical groups differed in numbers of DPs, NPs, and NFTs in the precuneus (all p < 0.01; table 2). Post hoc analysis showed that compared with the NCI group, the MCI group had significantly more NPs, whereas there were no significant increases in precuneus DPs and NFTs. Compared with the mAD group, the MCI group had fewer DPs, but the 2 groups were not statistically different when compared for NPs or NFTs. The mAD group had more DPs, NPs, and NFTs in the precuneus than the NCI group.
ChAT activity, [3H]PiB binding, and soluble Aβ42 in clinical groups.
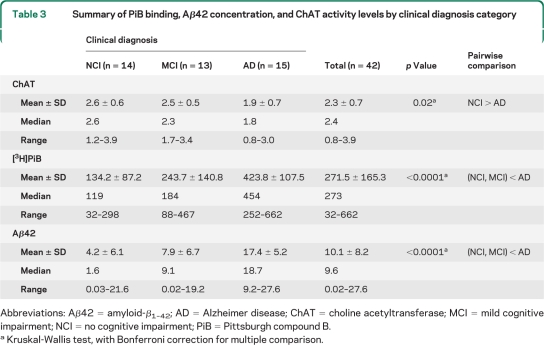
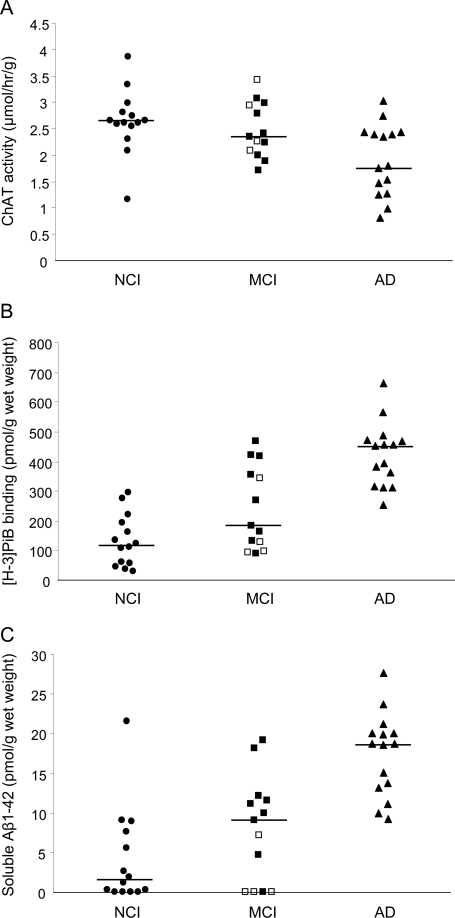
Precuneus ChAT activity differed among the 3 clinical groups (p = 0.02). ChAT activity in the mAD group was 31% lower than that in the NCI group (p = 0.007) and 22% lower than that in the MCI group; however, the latter difference was not significant statistically. ChAT activity was not different between the NCI and MCI groups (table 3, figure 1). The 3 clinical groups also differed by precuneus [3H]PiB binding and Aβ42 concentration (both p < 0.0001; table 3), with the median level of PiB binding in the mAD group 3.8-fold higher than that in the NCI group (p < 0.0001) and 2.5-fold higher than that in the MCI group (p < 0.003; figure 1). The median level of PiB binding in the MCI group was 1.5-fold higher than that in the NCI group, but this difference was not statistically significant. The precuneus Aβ42 concentration showed an 11.7-fold increase in the mAD group compared with that in the NCI group (p < 0.0001) and a 2-fold increase in mAD compared with that in the MCI group (p < 0.0008; figure 1). The median level of soluble Aβ42 concentration in the MCI group was 5.7-fold higher than that in the NCI group, but this difference was not statistically significant (table 3).
Table 3.
Summary of PiB binding, Aβ42 concentration, and ChAT activity levels by clinical diagnosis category
Abbreviations: Aβ42 = amyloid-β1–42; AD = Alzheimer disease; ChAT = choline acetyltransferase; MCI = mild cognitive impairment; NCI = no cognitive impairment; PiB = Pittsburgh compound B.
Kruskal-Wallis test, with Bonferroni correction for multiple comparison.
Figure 1. Quantitative analyses of precuneus choline acetyltransferase (ChAT) activity, Pittsburgh compound B (PiB) binding, and soluble amyloid-β1–42 (Aβ1–42) in the 3 clinical groups.
Scatterplots comparing precuneus ChAT activity levels (A), [3H]PiB binding (B), and soluble Aβ1–42 concentration (C) in no cognitive impairment (NCI), mild cognitive impairment (MCI), and mild Alzheimer disease (AD) groups. The horizontal line indicates the group median value. In the MCI group: ■, amnestic MCI case subjects (n = 9); □, nonamnestic MCI case subjects (n = 4).
Associations of biochemical, clinical, and neuropathologic measures.
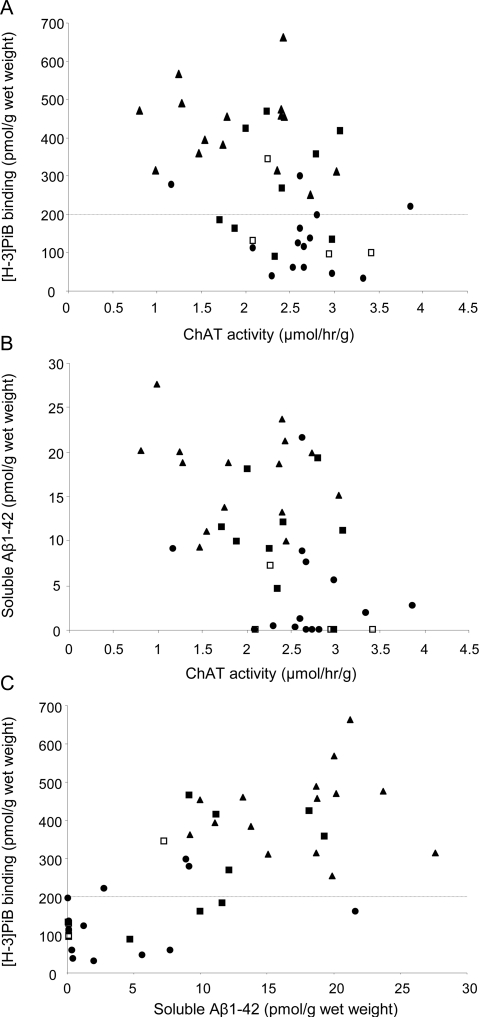
Across all cases, we observed a decrease in levels of ChAT activity in the precuneus in parallel with increases in [3H]PiB binding (r = −0.46, p = 0.003; figure 2A) and soluble Aβ42 concentration (r = −0.46, p = 0.0013; figure 2B). Increased [3H]PiB binding in the precuneus correlated directly with the soluble Aβ42 concentration (r = 0.72, p < 0.0001; figure 2C). Lower MMSE scores correlated with reduced ChAT activity (r = 0.41, p = 0.008), higher [3H]PiB binding (r = −0.76, p < 0.0001), and higher soluble Aβ42 concentration (r = −0.74, p < 0.0001) in the precuneus. More advanced AD neuropathologic diagnosis by CERAD criteria was associated with lower ChAT activity (r = −0.50, p = 0.0027) and higher [3H]PiB binding (r = 0.64, p < 0.0001) and soluble Aβ42 (r = 0.62, p < 0.0001) in the precuneus. A higher likelihood of AD diagnosis by the NIA-Reagan criteria and higher Braak staging neurofibrillary pathology scores were associated with lower ChAT activity (r = −0.49, p = 0.0027 and r = −0.29, p = 0.05, respectively), higher [3H]PiB binding (r = 0.74 and 0.68, respectively, both p < 0.0001), and higher concentration of soluble Aβ42 (r = 0.63, p < 0.0001 and r = 0.55, p = 0.0005). Precuneus ChAT activity correlated inversely with numbers of regional NPs (r = −0.56, p = 0.0009), whereas associations with DPs and NFTs were weak (r = −0.43, p = 0.015 and r = −0.36, p = 0.044). Precuneus [3H]PiB and Aβ42 levels correlated directly with regional DPs and NPs (r = 0.83 and r = 0.67, both p < 0.0001) and NFTs (r = 0.61, p < 0.0002). The presence of the APOE*4 allele was associated with lower ChAT activity and higher levels of [3H]PiB binding and soluble Aβ42 concentration (all p < 0.0001) in the precuneus.
Figure 2. Relationships among biochemical measures of precuneus choline acetyltransferase (ChAT) activity, Pittsburgh compound B (PiB) binding, and soluble amyloid-β1–42 (Aβ1–42).
Scatterplots show associations between ChAT activity levels and either [3H]PiB binding (A) or soluble Aβ1–42 concentration (B) and between soluble Aβ1–42 concentration and [3H]PiB binding (C) in the precuneus of no cognitive impairment (NCI), mild cognitive impairment (MCI), and mild Alzheimer disease (mAD) case subjects. ●, NCI; ■, amnestic MCI; □, nonamnestic MCI; ▴, mAD. The horizontal line at the [3H]PiB value of 200 pmol/g wet weight of tissue (A and B) marks a cutoff for a PiB PET-positive signal based on a previous analysis.23
DISCUSSION
The present study demonstrates stable ChAT activity despite accumulating amyloid load in the precuneus of MCI, expanding previous observations in other neocortical regions in prodromal AD.26,27 Interestingly, the superior frontal cortex (SFC), which also exhibits high levels of PiB PET retention and amyloid pathology early in AD,3,28 displays increased ChAT activity in MCI, suggesting that upregulated SFC cholinergic activity is a compensatory response during the transition to AD.27 The precuneus appears to lack a similar plasticity. Furthermore, our finding of reduced precuneus ChAT activity in mild AD cases is in contrast with reports of stable cholinergic levels in other neocortical regions in early AD.26,27 The mechanisms underlying cortical region–specific differences in amyloid pathology and cholinergic plasticity changes during early clinical stages of disease remain unclear.
Although numbers of precuneus NPs were higher in MCI than in NCI, biochemical measures of amyloid overlapped substantially, consistent with reports of amyloid-burdened individuals in both clinical groups.8,29–34 Herein, one NCI case subject exhibited normal global cognition (MMSE score = 30) despite advanced amyloid pathology. Another NCI case subject with an unusually high DEA-soluble Aβ42 concentration was the only APOE*4/4 homozygous subject in our study, supporting the fact that APOE*4 status affects soluble Aβ42 levels.35 However, its [3H]PiB binding, a marker of insoluble amyloid load and in vivo PiB PET retention,23 was average for the NCI group. A previous report of an APOE*3/4 AD case subject with low [3H]PiB binding and high Aβ concentrations suggested that this disparity was due to a unique profile of Aβ oligomers, truncated Aβ species, and high vascular Aβ pathology.36 In our study, excessive vascular pathology was an exclusion criterion for case selection. The spectrum of truncated Aβ species in our PiB-refractory NCI case subject remains to be investigated. Despite these occasional mismatches, precuneus [3H]PiB binding and soluble Aβ42 concentration correlated strongly with each other and with MMSE scores. MMSE, a measure of global cognitive function, relies on integration of activity from multiple cortical associational areas including the precuneus as either a part of the cortical default mode network or a complex brain circuitry involved in attention and working memory. Because the present findings were generated from 3 clinical cohorts, they suggest general applicability of the data across subject populations. However, the relatively small number of cases in the present study preclude a definite conclusion regarding the relationship between amyloid measures and cognition.
The heterogeneity of the current MCI cohort, with 4 of 13 cases classified as nonamnestic MCI (naMCI), may have contributed to the lack of differences in ChAT activity and the low magnitude of differences in PiB binding and soluble Aβ42 concentration between the NCI and MCI groups. Three of the 4 naMCI case subjects had the lowest detectable Aβ42 concentrations, low PiB binding levels, and high ChAT activity values (figure 1), suggesting that these subjects with naMCI may not be at risk to convert to AD or would develop AD only much later in life.8 When the MCI group was restricted to those with aMCI (n = 9), [3H]PiB binding did not differ from that in the AD group, and a trend toward increased soluble Aβ42 levels in aMCI was seen compared with that for the NCI group. In contrast, ChAT activity levels in the aMCI group did not differ significantly from those in the NCI and AD groups, suggesting that during the clinical progression from NCI to aMCI to AD, soluble Aβ42 increases before a decline in cholinergic enzyme activity and is, therefore, an important therapeutic target. However, similar to other cross-sectional neuropathologic studies, we cannot determine the time course or a causal relationship between these pathologic events in the precuneus. The associations between cholinergic and global cognitive deficits and buildup of Aβ pathology in the precuneus are reminiscent of the relationship between increased precuneus amyloid load on PiB PET scans and decreased regional metabolism measured by [18F]fluoro-2-deoxy-d-glucose PET (considered to be a surrogate marker of synaptic activity) in AD but not in MCI and amyloid-positive NCI case subjects.37 It would be of value to examine changes in synaptic markers in relation to [3H]PiB binding in the precuneus and other neocortical regions.
In a previous clinicopathologic study, the [11C]PiB PET imaging signal and postmortem [3H]PiB binding in the same subject correlated strongly and [3H]PiB binding levels greater than 200 pmol/mg corresponded to PiB PET-positive values above a conservative positive cutoff determined as an atrophy-corrected distribution volume ratio of 1.4.23,38 In the present study, precuneus [3H]PiB binding determined postmortem was greater than 200 pmol/mg in all AD case subjects (figure 1), suggesting that they would have been PiB PET-positive if imaged before death. Interestingly, 46% of MCI and 21% of NCI case subjects in the present study also had precuneus [3H]PiB binding greater than 200 pmol/mg (figure 1), suggesting that they would probably have been PiB PET-positive as well. These observations are comparable to reports from imaging studies in live subjects.8,34,37,39)
Our study demonstrates that precuneus ChAT activity decreases in parallel with increases in PiB binding and soluble Aβ42 concentration. Thus, the positive PiB PET signal in the precuneus of amyloid-burdened NCI and MCI case subjects probably reflects ongoing impairment of cholinergic neurotransmission, which becomes detectable only after the onset of clinical AD. This finding suggests a temporal window for the use of cholinomimetic therapies, alone or in combination with antiamyloid therapies, to slow the progression of AD. Such therapy may prove to be effective in individuals with preclinical cases in whom the cortical amyloid load has not reached a plateau.
ACKNOWLEDGMENT
The authors thank William R. Paljug and Manik L. Debnath for technical assistance, Dr. Sue E. Leurgans for discussion of statistical analyses, and members of the Rush Alzheimer's Disease Center and the Religious Orders Study clinical and pathology cores. The authors also thank the participants in the University of Kentucky and University of Pittsburgh ADRC and the Religious Orders Study; for a list of participating groups in the Religious Orders Study see the Web site http://www.rush.edu/rumc/page-R12394.html.
Footnotes
- Aβ
- amyloid-β
- Aβ42
- amyloid-β1–42
- AD
- Alzheimer disease
- mAD
- mild Alzheimer disease
- aMCI
- amnestic mild cognitive impairment
- CERAD
- Consortium to Establish a Registry for Alzheimer's Disease
- ChAT
- choline acetyltransferase
- DEA
- diethylamine
- DMSO
- dimethyl sulfoxide
- DP
- diffuse plaque
- MCI
- mild cognitive impairment
- MMSE
- Mini-Mental State Examination
- naMCI
- nonamnestic MCI
- NCI
- no cognitive impairment
- NFT
- neurofibrillary tangle
- NP
- neuritic plaque
- PBS
- phosphate-buffered saline
- PiB
- Pittsburgh compound B
- SFC
- superior frontal cortex
AUTHOR CONTRIBUTIONS
M.D.I., S.W.S., and E.J.M. obtained funding. M.D.I. and E.J.M. designed and conceptualized the study. M.D.I. supervised the study. M.D.I., W.E.K., E.E.A., and S.W.S. acquired data. M.D.I., W.E.K., E.E.A., J.W., and S.W.S. analyzed data. M.D.I., W.E.K., E.E.A., J.W., C.A.M., and S.W.S. interpreted the data. J.W. conducted statistical analysis. M.D.I., W.E.K., E.E.A., J.W., C.A.M., S.W.S., and S.T.D. drafted and revised the manuscript.
DISCLOSURE
Dr. Ikonomovic has served as a consultant for and received funding for travel from GE Healthcare; serves as an Associate Editor for Cardiovascular Psychiatry and Neurology; and receives research support from GE Healthcare, Forest Research Institute, Inc., and the NIH. Dr. Klunk serves on scientific advisory boards and as a consultant for GE Healthcare, Neuroptix Corporation, Elan/Janssen AI, Roche, Wyeth/Pfizer, and AstraZeneca; has received funding for travel from Elan/Janssen AI, Roche, and AstraZeneca; is author on patents re: PiB PET imaging and chrysamine-G derivatives for imaging and therapy; receives research support from GE Healthcare, Neuroptix Corporation, the NIH (NIA, NIBIB), Anonymous Foundation, and Cure Alzheimer's Fund; holds stock in Neuroptix Corporation; and receives license fee and royalty payment from GE Healthcare. Dr. Abrahamson reports no disclosures. J. Wuu has received research support from the NIH/NIA, the FDA, the CDC, the ALS Association, the Muscular Dystrophy Association, and the Consolidated Anti-Aging Foundation. Dr. Mathis serves on a scientific advisory board for Neuroptix Corporation; has received funding for travel and speaker honoraria from Elan/Janssen AI, GE Healthcare, Bayer Schering Pharma, Biogen Idec, IBA, and Takeda Pharmaceutical Company Limited; serves on the editorial board of Nuclear Medicine and Biology; serves/has served as a consultant for GE Healthcare, Elan/Janssen AI, Wyeth/Pfizer, and Novartis; is author on numerous US and international patents re: Amyloid imaging agents; receives/has received research support from GE Healthcare, Neuroptix Corporation, the NIH, the US Department of Energy, the Dana Foundation, and Anonymous Foundation; holds stock options in Neuroptix Corporation; and has received license fees and royalty payments from GE Healthcare and Neuroptix Corporation for patents re: Amyloid imaging agents. Dr. Scheff serves on a scientific advisory board for TIRR; serves on the editorial board of the Journal of Neurotrauma; and receives research support from the NIH. Dr. Mufson has served as a consultant for Ceregene and NeuroPhage and receives research support from the NIH. Dr. DeKosky has served on a scientific advisory board for Pfizer Inc and as a consultant for Eisai, PsychoGenics Inc., Merck, Elan/Wyeth, Novartis, Eli Lilly, and Janssen; is the site principal investigator at the University of Virginia Memory Disorders Clinics for experimental therapeutic trials of Alzheimer's disease medications for Elan, Novartis, Forest, and Janssen Pharmaceuticals; serves on the editorial boards of Annals of Neurology, Archives of Neurology, Neurodegenerative Diseases, Journal of Alzheimer's Disease, and Alzheimer Disease and Associated Disorders: An International Journal, and as Editor of Up to Date; receives research support from the NIH; and serves as a board member for the National Center for Complementary and Alternative Medicine, the American Board of Psychiatry and Neurology, and the Alzheimer's Association.
REFERENCES
- 1. Buxbaum JD, Oishi M, Chen HI, et al. Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer β/A4 amyloid protein precursor. Proc Natl Acad Sci USA 1992;89:10075–10078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liskowsky W, Schliebs R. Muscarinic acetylcholine receptor inhibition in transgenic Alzheimer-like Tg2576 mice by scopolamine favors the amyloidogenic route of processing of amyloid precursor protein. Int J Dev Neurosci 2006;24:149–156 [DOI] [PubMed] [Google Scholar]
- 3. Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh compound-B. Ann Neurol 2004;55:306–319 [DOI] [PubMed] [Google Scholar]
- 4. Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of Aβ42(43) and Aβ40 in senile plaques with end-specific Aβ monoclonals: evidence that an initially deposited species is Aβ42(43). Neuron 1994;13:45–53 [DOI] [PubMed] [Google Scholar]
- 5. Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 2005;25:7709–7717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sperling RA, Laviolette PS, O'Keefe K, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 2009;63:178–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karas G, Scheltens P, Rombouts S, et al. Precuneus atrophy in early-onset Alzheimer's disease: a morphometric structural MRI study. Neuroradiology 2007;49:967–976 [DOI] [PubMed] [Google Scholar]
- 8. Wolk DA, Price JC, Saxton JA, et al. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol 2009;65:557–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci 2005;9:445–453 [DOI] [PubMed] [Google Scholar]
- 10. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioral correlates. Brain 2006;129:564–583 [DOI] [PubMed] [Google Scholar]
- 11. Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Hum Brain Mapp 2005;26:231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sheline YI, Raichle ME, Snyder AZ, et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry 2010;67:584–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol 1983;214:170–197 [DOI] [PubMed] [Google Scholar]
- 14. Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology 2002;59:198–205 [DOI] [PubMed] [Google Scholar]
- 15. Davis DG, Schmitt FA, Wekstein DR, Markesbery WR. Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropath Exp Neurol 1999;58:376–388 [DOI] [PubMed] [Google Scholar]
- 16. Lopez OL, Becker JT, Klunk W, et al. Research evaluation and diagnosis of probable Alzheimer's disease over the last two decades: I. Neurology 2000;55:1854–1862 [DOI] [PubMed] [Google Scholar]
- 17. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944 [DOI] [PubMed] [Google Scholar]
- 18. Petersen RC, Smith GE, Waring SC. Mild cognitive impairment. Arch Neurol 1999;56:303–308 [DOI] [PubMed] [Google Scholar]
- 19. Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol 2001;58:397–405 [DOI] [PubMed] [Google Scholar]
- 20. National Institute on Aging and Reagan Institute Working Group on Diagnosis Criteria for the Neuropathological Assessment of Alzheimer's Disease Consensus recommendations for the postmortem diagnosis of AD. Neurobiol Aging 1997;18:S1–S2 [PubMed] [Google Scholar]
- 21. Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD): part II: standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479–486 [DOI] [PubMed] [Google Scholar]
- 22. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–259 [DOI] [PubMed] [Google Scholar]
- 23. Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer's disease. Brain 2008;131:1630–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klunk WE, Lopresti BJ, Ikonomovic MD, et al. Binding of the positron emission tomography tracer Pittsburgh compound-B reflects the amount of amyloid-β in Alzheimer's disease brain but not in transgenic mouse brain. J Neurosci 2005;25:10598–10606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DeKosky ST, Scheff SW, Markesbery WR. Laminar organization of cholinergic circuits in human frontal cortex in Alzheimer's disease and aging. Neurology 1985;35:1425–1431 [DOI] [PubMed] [Google Scholar]
- 26. Davis KL, Mohs RC, Marin D, et al. Cholinergic markers in elderly patients with early signs of Alzheimer's disease. JAMA 1999;281:1401–1406 [DOI] [PubMed] [Google Scholar]
- 27. DeKosky ST, Ikonomovic MD, Styren S, et al. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol 2002;51:145–155 [DOI] [PubMed] [Google Scholar]
- 28. Thal DR, Rub U, Orantes M, Braak H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 2002;58:1791–1800 [DOI] [PubMed] [Google Scholar]
- 29. Mufson EJ, Chen EY, Cochran EJ, Beckett LA, Bennett DA, Kordower JH. Entorhinal cortex β-amyloid load in individuals with mild cognitive impairment. Exp Neurol 1999;158:469–490 [DOI] [PubMed] [Google Scholar]
- 30. Price JL, McKeel DWJ, Buckles VD, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging 2009;30:1026–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 2006;67:446–452 [DOI] [PubMed] [Google Scholar]
- 32. Forsberg A, Engler H, Almkvist O, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging 2008;29:1456–1465 [DOI] [PubMed] [Google Scholar]
- 33. Kemppainen NM, Aalto S, Wilson IA, et al. PET amyloid ligand [11C]PIB uptake is increased in mild cognitive impairment. Neurology 2007;68:1603–1606 [DOI] [PubMed] [Google Scholar]
- 34. Pike KE, Savage G, Villemagne VL, et al. β-Amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain 2007;130:2837–2844 [DOI] [PubMed] [Google Scholar]
- 35. Lue LF, Kuo YM, Roher AE, et al. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol 1999;155:853–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosen RF, Ciliax BJ, Wingo TS, et al. Deficient high-affinity binding of Pittsburgh compound B in a case of Alzheimer's disease. Acta Neuropathol 2010;119:221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cohen AD, Price JC, Weissfeld LA, et al. Basal cerebral metabolism may modulate the cognitive effects of Aβ in mild cognitive impairment: an example of brain reserve. J Neurosci 2009;29:14770–14778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aizenstein HJ, Nebes RD, Saxton JA, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 2008;65:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hatashita S, Yamasaki H. Clinically different stages of Alzheimer's disease associated by amyloid deposition with [11C]-PIB PET imaging. J Alzheimers Dis 2010;21:995–1003 [DOI] [PubMed] [Google Scholar]