Sir,
Deoxynivalenol [DON] (3 α, 7 α, and 15 α trihydroxy 12, 13-epoxytrichothec-9-en-8-one) is a naturally occurring trichothecene toxin produced by several Fusarium fungi that parasitize numerous cereal grains. This is the most prevalent toxin in contaminated corps infected through food and feed production.[1] It is a member of a group of metabolites of Fusarium fungi known as trichothecenes[2] and it has been reported to cause feed refusal and vomiting in swine.[3] In vitro, DON has been shown by interfering with the intestinal epithelial cell lines viz. Caco-2 and T-84.[4]
However, the effect of DON in blood is not reported so far or it is not clear from the previous data whether the changes in blood parameters are direct result of the toxicity of the ingested toxin. Thus, purpose of the present investigation is to elucidate further the reason for blood parameters and hemostatic effect after oral administration of DON.
Standard DON was purchased from Sigma-Aldrich (Madrid, Spain). Other chemicals in analytical grade were obtained from Merck (Darmstadt, Germany). BLAB/c mice of either sex with average weight of 30–40 g were procured from Laboratory Animal Resources, Defence Research Laboratory, Tezpur, Assam, India. Animals were maintained under temperature-controlled rooms at animal house with 12 h alternating light and dark cycles and were given adequate nutrition and water ad libitum. All experimental protocols using animals were performed according to the “Principles of Laboratory Animal care” (NIH publication 85-23, revised 1985) and all animals received human care in compliance as per Guide for the care and use of Laboratory Animals. Experimental protocols were reviewed and approved by institutional ethical committee. Twelve BLAB/c mice were divided into sham-operated control group (1) given 0.9% saline with 1% gum acacia (2 ml/kg, p.o.) for 7 days (n = 6) (administered 1 ml 1% gum acacia suspension only), and DON-treated group (2) (10 μg/kg body weight/daily by oral route for 7 days).
Blood samples were withdrawn from tail vein and were collected in sterile syringes containing ethylene di-amine tetra acetic acid (EDTA) (1 mg/ml). For estimation of white blood corpuscles (WBC), 2 ml blood was added with 0.5 ml WBC fluids (Hymedia, Mumbai, India) followed by spined at 1000 rpm for 10 min at 4°C and supernatant was discarded. After 10 min, pallets were stained with eosin blue and calculated the different composition of WBC in microscope with Naubers chamber. Red blood corpuscles (RBC) were determined in similar way except RBC fluids were added instead of WBC fluid. The bleeding time was determined using a modified tail cutting method, as described.[5] Bleeding time was noted from the moment transection was done until bleeding stopped completely; it was expressed in seconds. The blood was collected in a capillary tube by retro-orbital puncture. A stop clock was started immediately, and the time taken to form thread-like structure of the blood while breaking the capillary tube was noted in seconds.
All values were expressed as mean ± SD. Differences in mean values were compared by one-way ANOVA and Student-Newman-Keul (SNK) test. P<0.05 was considered statistically significant.
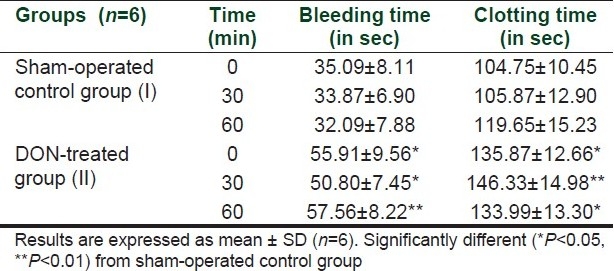
The bleeding time after 0, 30, and 60 min of sham-operated control mice were 55.09±8.11, 53.87±6.90, and 52.09±7.88, respectively. After administration of DON, the bleeding time were significantly increased to 55.91±9.56, 50.80±7.45, and 57.56±8.22, respectively. Further, the clotting times after 0, 30, and 60 min of sham-operated control mice were 104.75±10.45, 105.87±12.90, and 119.65±15.23, respectively, whereas after administration of DON the clotting times were significantly increased to135.87±12.66, 146.33±14.98, and 146.33±14.98, respectively. Thus, administration of DON increased bleeding time and clotting time.
The activity of hematocrit percentage was increased whereas percentages of neutrophils, lymphocytes and monocytes and RBC cu mm were decreased. Eosinophils % activity was increased significantly (P<0.05) in DON-administered group of mice as compared to control group [Table 1].
Table 1.
Effect of DON in bleeding time and clotting time
Earlier study had shown that DON induces apoptosis in several leukocyte cell lines such as RAW 264.7, U937, and Jurkat cells[6] and our study in agreement with the previous study shows decreased hematocrit value and lymphocytes value which supported that equally DON is toxic in cell lines. In the present study, DON significantly (P<0.05) decreased hematocrit value and significantly increased (P<0.05) blood clotting time and bleeding time. This result confirms that DON decreased the natural hemostatic activity, and this result provided clue as De Walle and colleagues demonstrated a dual toxicological effect of DON on differentiated Caco-2 cells inhibition of protein synthesis as well as an increase in in monolayer permeability was obtained.[7] Further, the present study showed the rapid loss of RBC after administration of DON resulted in decreased hematocrit percentage which indicates that DON inhibited protein synthesis leading to cell proliferation including WBC and RBC1 and abnormality of RBC production. Another, important finding that DON compromised the basophilic function including increased clotting time and bleeding time which supported that DON has immunosuppressive action. Previous study shows that four trichothecenes T-2 toxin, diacetoxyscirpenol (DAS), nivalenol (NIV), and DON inhibited mutagen-induced lymphocyte proliferation. Combinations of NIV with T-2, DAS, or DON resulted in additive toxicity in lymphocyte proliferation while combinations of DON with T-2 or DAS resulted in an inhibition that was slightly lower than that could have been expected from inhibition produced by the individual toxins.[8]
In conclusion, our study shows that DON is a potential hemato-toxin and is known as toxic contaminants in crops of many areas of the world and shows potential toxicity. Since DON is commonly toxicant in cereal foods, there are major issues regarding the risks of acute poisoning and chronic effects posed to persons ingesting this trichothecene. A further challenge is persistently adopting the procedure of best management of perceived risks without rendering critical food staples unavailable to an ever-expanding world population. Further, the regulatory guidelines should be imposed for controlling this toxin concerning human health.
Acknowledgments
This research was fully supported by Defence Research Laboratory, DRDO, for providing facilities and financial assistance for completion of the investigation.
REFERENCES
- 1.Rotter BA, Prelusky DB, Pestka JJ. Toxicology of deoxynivalenol (vomitoxin) J Toxicol Environ Health. 1996;48:1–34. doi: 10.1080/009841096161447. [DOI] [PubMed] [Google Scholar]
- 2.Pathre SV, Mirocha CJ. Two new trichothecenes produced by Fusarium roseum. J Agric Food Chem. 1978;26:649–53. doi: 10.1021/jf60217a030. [DOI] [PubMed] [Google Scholar]
- 3.Forsyth DM. Emetic and Refusal Activity of Deoxynivalenol to Swinet. Applied and Environ Microb. 1977;34:547–52. doi: 10.1128/aem.34.5.547-552.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasuga F, Hara-Kudo Y, Saito N, Kumagai S, Sugita-Konishi Y. In vitro effect of deoxynivalenol on the differentiation of human colonic cell lines Caco-2 and T84. Mycopathologia. 1998;142:161–7. doi: 10.1023/a:1006923808748. [DOI] [PubMed] [Google Scholar]
- 5.Dejana E, Villa S, De Gartano G. Bleeding time in rats: A comparison of different experimental conditions. Thromb Haemostasis. 1982;48:108–11. [PubMed] [Google Scholar]
- 6.Pestka JJ, Smolinski AT. Deoxynivalenol: Toxicology and potential effects on humans. J Toxicol Environ Health B Crit Rev. 2005;8:39–69. doi: 10.1080/10937400590889458. [DOI] [PubMed] [Google Scholar]
- 7.De Walle JV, Sergent T, Piront N, Toussaint O, Schneider YJ, Larondella Y. Deoxynivalenol affects in vitro intestinal epithelial cell barrier integrity through inhibition of protein synthesis. Toxicol Appl Pharmacol. 1998;245:291–8. doi: 10.1016/j.taap.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Thuvander A, Wikman C, Gadhasson I. In vitro exposure of human lymphocytes to trichothecenes: Individual variation on sensitivity and effects of combined exposure on lymphocyte function. Food Chem Toxicol. 1999;37:639–48. doi: 10.1016/s0278-6915(99)00038-1. [DOI] [PubMed] [Google Scholar]


