Abstract
Objective:
To collect information on the availability of five essential children's medicines in the public health facilities of India.
Materials and Methods:
A snap shot survey of the availability of five essential medicines for children was conducted. Five medicines which are included in the National Rural Health Mission (NRHM) list for subcentres were selected, i.e., vitamin A liquid solution, syrup cotrimoxazole, oral rehydration salt (ORS), syrup paracetamol, and zinc sulphate (oral liquid or tablets). Information about this survey was posted in two e-groups for pharmacologists and pharmacists and volunteers were requested to collect data on the availability of these five medicines and fill up a data sheet which was emailed back to the organizers. Data was collected from February 14 to 21, 2010.
Results:
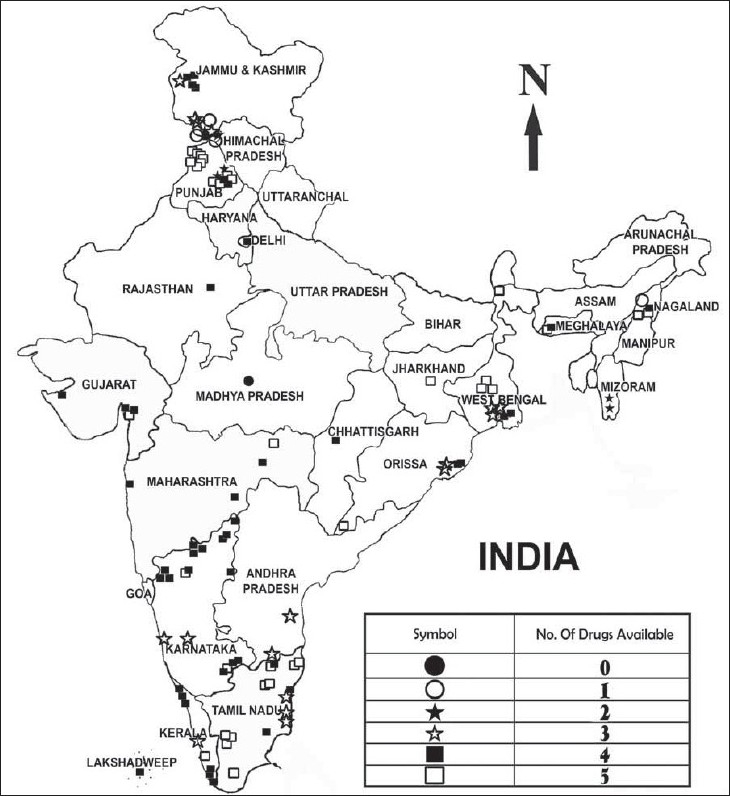
Data were collected from 129 public health facilities spanning 17 states, two union territories and NCT Delhi. The overall median availability was 80% (range: 0%-100%). Punjab, Tamilnadu, and Jharkhand showed 100% median availability (range: 40%-100%). Ninety percent of the facilities have ORS, paracetamol, and cotrimoxazole whereas zinc was available in only 36% of the public health facilities. Syrup cotrimoxazole and ORS have 100% availability in all states except in four and paracetamol has nearly 100% availability in all but six states.
Conclusion:
The availability of essential medicines for children in public health facilities is not satisfactory and needs to be improved.
Keywords: Access to medicine, better medicines for children, national rural health mission
INTRODUCTION
World Health Organisation (WHO) celebrated the 30th anniversary of the Model List of Essential Medicines in 2007 and reviewed the progress made over these years. It was noted that there was a need for more medicines specifically developed and tested for use in children.[1] It is estimated that eight million children under five years of age die every year worldwide.[2] These deaths are mainly due to causes like diarrhoea, pneumonia, neonatal sepsis, malaria, tuberculosis, etc., for which effective medicines exist. Hence, WHO launched the “Better Medicines for Children” initiative and “make medicines child size” campaign to raise awareness among policy-makers, pharmaceutical manufacturers, researchers, health-care professionals, and the public. Under this initiative, the Model List of Essential Medicines for Children (EMLc)[3] and WHO Model Formulary for Children[4] were developed.
The “Better Medicines for Children” initiative spearheaded by the WHO is focussed on addressing the access issues related to children's medicines. Data on the availability of essential medicines for children in the public sector health facilities in India are not available. Since the majority of children in India seek medical treatment from the public health facilities, any initiative on improving access to children's medicines, should be focussed on the public health facilities and it is necessary to have some baseline information. Hence, this study was done with the objective of collecting information on the availability of five selected essential medicines for children in public health facilities in India. The success of the global pill price snapshot survey of the price of ciprofloxacin and insulin conducted by Health Action International[5] gave an idea to use a similar methodology for this survey.
MATERIALS AND METHODS
Selection of the five medicines
Five essential medicines used for children, i.e., vitamin A solution, syrup cotrimoxazole, oral rehydration salt, syrup paracetamol, and zinc sulphate oral liquid or tablets were selected. The basis for their selection was that these five medicines have been included in the National Rural Health Mission's (NRHM) list of medicines to be dispensed at subcentres and so it was assumed that these medicines would also be available at higher levels of health care, i.e., primary health centres and community health centres. These medicines also necessarily figure either in the essential medicines list (EML) or on the procurement lists (or both) of all the states as NRHM covers all states. As this is a survey of essential medicines for children, child-friendly formulations were selected. The proven impact of each of these medicines for various indications in children and the fact that they are listed in the WHO Model EMLc were other factors that were considered.
Data collection
For collecting data spread over a vast geographical area, we decided to use volunteers who subscribe to two well known e-groups from India which are focussed on pharmacy and pharmacology. NETRUM (Network for Rational Use of Medicines) and GenXPharm (Generation X Pharmacologists) were the two e-groups that were used to publicize the survey and appeal to the members to go out to the field and collect data. The membership of both e-groups amount to nearly 1000 pharmacists and pharmacologists all over India.
A simple survey form prepared through Google documents was used to collect data. The survey form was tested with a small group of ten pharmacists at the Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER) Hospital, Puducherry. This survey form was posted in the two e-groups one week before the survey and, therefore, nearly one thousand pharmacologists and pharmacists working in India would have been able to access the email which gave details of the survey. Personal contacts of the organizers of the survey were also contacted and requests were made to postgraduate students and undergraduate students to collect data. Since the data collection had to be done on a working day and during working hours, it was decided to fix the time line for data collection as one week so that those collecting data could plan beforehand and visit the health centres on a date and time of their own convenience.
Data was collected from February 14th to 21st, 2010. An email request was sent out through NETRUM and GenXPharm on 12th February reminding the members to go to the nearest government health facility and ask whether the five medicines on the list were available or not. They were also asked to verify whether any list of medicines was displayed on the wall or notice board of the health facility and if possible to take a photograph of it and send it by email.
Availability of individual medicines is taken as the number of facilities having the particular medicine (on the day of data collection). The number of facilities (n) is given for each state/U.T. so that a false impression of 100% availability for states with poor representation (less than five data points/per state) is not created.
Statistical analysis
Data were represented as summary statistics and availability was described as median availability along with the range. Total availability was calculated as the median percentage of the number of medicines (out of the total five medicines) available in each center. Hence availability was 20, 40, 60, 80, or 100 % depending on whether 1, 2, 3, 4 or 5 drugs were available in each facility.
RESULTS
Data were collected from 129 public health facilities across 17 states, two union territories (Pondicherry, Lakshwadeep) and NCT Delhi. The overall availability was 80% with a range of 0%-100%. The state-wise availability is given in Table 1.
Table 1.
Median availability of fi ve essential children's medicines in public health facilities in 17 states, two union territories and NCT Delhi in India
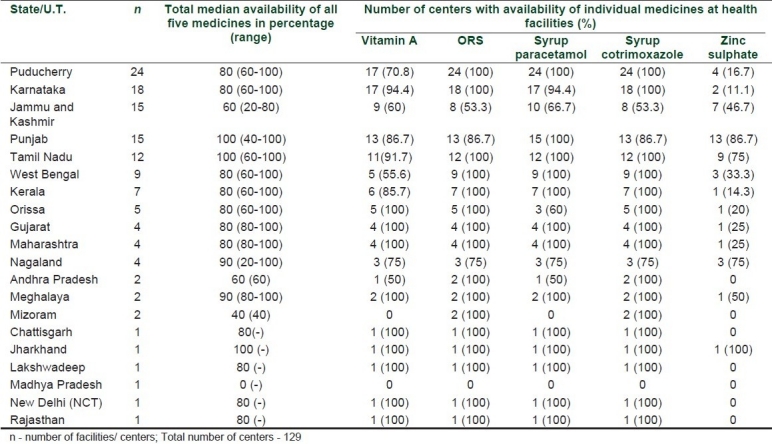
The map shows the number of drugs available in each health facility [Figure 1]. No data was collected from some of the larger states like Uttar Pradesh, Bihar, Arunachal Pradesh, Assam, Uttaranchal, and Himachal Pradesh.
Figure 1.
Number of drugs available in various public health care facilities
The total number of symbols on the map do not add to 129, as there are more than one data points from some places.
The doctor who visited the PHC at Goonga village in Madhya Pradesh (Bhopal district) said that on that day, not only there were no medicines available, even a doctor was not available. When the availability of individual medicines in the centres were analysed [Table 2], it was found that 90% of the facilities had ORS, paracetamol and cotrimoxazole. Zinc was poorly available with only one third of the facilities having it.
Table 2.
Availability of individual medicines (%) at public health facilities (n = 129)

The list of available medicines was displayed in few centres and very few volunteers sent photographs of it through email. In Tamilnadu two medical officers reported that they deliberately have stopped displaying the lists outside the PHCs since patients come and demand injections and intravenous fluids “to feel stronger”. If the doctor refuses they turn abusive. West Bengal, Punjab, Karnataka, Meghalaya, Jammu and Kashmir are some of the states where the list was displayed.
DISCUSSION
The good overall median rate of availability of 80 demonstrates that at least for these five essential medicines, availability is being ensured by the government, though the range of 0-100 shows that there are places where the availability is dismal. The median availability was 80 and above in all the sampled states except in Jammu and Kashmir, Andhra Pradesh, Mizoram and Madhya Pradesh. However, it must be stated that the information from Andhra Pradesh, Mizoram and Madhya Pradesh were based on very few data points, i.e., just 1 or 2. India has 28 states and seven union territories and this survey gives a rough estimate of a cross sectional view of the availability of five children's medicines in more than half the states. It must be kept in mind that data points in the central part of India and in the West were few and no data was collected from some of the larger states which are known to have very poor health care indicators.[6] Hence the high median availability has to be viewed with this in mind.
Punjab, Tamilnadu and Jharkhand show 100% median availability, but the range in Punjab is 40-100 and only one centre was sampled in Jharkhand. The high median availability of Punjab is offset by the fact that only 86% of the centres have ORS, whereas Mizoram with 40% median availability has ORS in both of the centres from where data was collected. The poor availability of oral zinc in all states raises concerns. Even though it has been included in the list, and is in the standard treatment guidelines for the management of diarrhoea in children, the lack of availability in public health centres raises questions as to whether it is freely available in the market. The availability of Vitamin A also is a cause for concern. The poor availability of zinc and vitamin A is similar to the availability pattern seen in 14 countries of Africa.[7]
Syrup cotrimoxazole and ORS have 100% availability in all states except in four, i.e., Jammu and Kashmir, Punjab, Nagaland, and Madhya Pradesh. Paracetamol also comes close to the other two with 100% availability in all but six states. Availability of zinc is less than 50% even in states with a median availability of 80% or more. Zinc was available in some of the states such as Jharkhand and Meghalaya (50%). However, since the sample is less no conclusion can be drawn from it.
The survey methodology used was a quick though not an established method of collecting some background information where none existed. However, it should be noted that the majority of people collecting data did so on personal email and phone call requests. The quality of the data can be considered high, since it was collected by motivated people who were pharmacologists, pharmacists or medical doctors. There were no incentives offered and hence we could be reasonably sure that there was no “false” data sent across. Whether the availability of these medicines was physically verified by all data collectors is not known and this is one of the limiting factors of this survey. The other limitation was that all states were not adequately represented, which could perhaps skew the results.
The response of members from the two e-groups was disappointing and even repeated requests did not change the quantum of data that came in. Many of the medical officers contacted did not have access to email and gave the information over phone and asked that it be recorded. Some sent emails giving a global scenario of the availability in their states. Such data was not included since there were no specific centers named in the emails and availability of each of the five medicines were not mentioned.
The 80% median availability seen in this survey, may be viewed as another example of improved availability if there is a limited list as all these five medicines were on the list to be made available in subcenters. However, it is sad that even with this very small list, all medicines were not available in all centres, a situation similar to that seen in Africa[7] and in neighbouring country Sri Lanka.[8] Lastly, it should be noted that this survey is by no means a substitute for a planned study on this topic, using standard survey methodology[9] and covering more number of children's medicines.
Acknowledgments
The authors would like to thank Dr. Vijay Thawani, moderator of NETRUM for permitting use of the e-group and for urging the members to participate and all those who went out to the facilities and collected the data.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.WHO Media centre. [Last accessed on 2011 Jan 3]. Available from: http://www.who.int/mediacentre/news/releases/2010/medicines_children_20100618/en/
- 2.World Health Organisation. [Last accessed on 2011 Jan 3]. Available from: http://www.who.int/en/
- 3.WHO Model list of essential medicines for children. Available from: http://www.who.int/selection_medicines/committees/expert/17/second_children_list_en.pdf .
- 4.WHO Model formulary for children. Available from: http://www.who.int/selection_medicines/list/WMFc_2010.pdf .
- 5.Health Action International. [Last accessed on 2011 Jan 3]. Available from: http://www.haiweb.org/
- 6.District Level Household and Facility Survey 2007-2008. Ministry of Health and Family Welfare, Government of India. Available from: http://www.rchiips.org/pdf/rch3/state/India.pdf .
- 7.Robertson J, Forte G, Trapsida JM, Hill S. What essential medicines for children are on the shelf. Bull World Health Organ. 2009;87:231–7. doi: 10.2471/BLT.08.053645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Availability and cost of selected paediatric essential medicines in Sri Lanka. [Last accessed on 2011 Jan 3]. Available from: http://whosrilanka.healthrepository.org/bitstream/123456789/337/1/Country%20survey%20-Paediatric%20medicine.pdf .
- 9.Overview of methods for medicines availability and pricing survey. [Last accessed on 2011 Jan 31]. Available from: http://www.who.int/childmedicines/progress/ChildMeds_pricing_surveys.pdf .


