Abstract
Objective
Electrical stimulation of the urethra can evoke bladder contractions in persons with spinal cord injury (SCI). The objective of this study was to determine whether electrical stimulation of the urethra could evoke bladder contractions that empty the bladder.
Methods
The first patient was a 45-year-old man with a T6 ASIA A SCI secondary to a gunshot wound 15 years prior. The second patient was a 51-year-old man with a T2 ASIA A SCI secondary to a fall from scaffolding 2 years prior. Both patients demonstrated neurogenic detrusor overactivity on urodynamics and managed their bladder with clean intermittent catheterization and oxybutynin medication. Following informed consent, each patient discontinued oxybutynin 2 days prior to urodynamic testing. Urodynamics were performed with a custom 12 French balloon catheter mounted with ring-shaped electrodes (3 mm) positioned in the prostatic urethra. After filling the bladder to approximately three-fourth of capacity at a rate of 25 ml/minute, the urethra was stimulated with a range of parameters to determine whether electrical stimulation could evoke a bladder contraction and empty the bladder.
Results
Electrical stimulation of the prostatic urethra evoked bladder contractions (peak detrusor pressures of 60–80 cm H2O) that emptied the bladder in both subjects. In the first subject, stimulation (9–12 mA, 20 Hz) emptied 64–75%, leaving post-void residual volumes (PVRs) of 41–20 ml. In the second subject, stimulation (20 mA, 20 Hz) emptied 68–77%, leaving PVRs of 56–45 ml.
Conclusion
Urethral stimulation evoked bladder emptying in persons with SCI.
Keywords: Spinal cord injury, Electrical stimulation, Neurogenic bladder, Oxybutynin
Introduction
The inability to empty the bladder can result in medical complications, such as urinary tract infections and damage, a decrease in quality of life, and social frustration.1,2 Voiding dysfunction often follows spinal cord injury (SCI) and can include neurogenic acontractile detrusor or detrusor over-activity with detrusor-sphincter dyssynergia resulting in high-pressure low-efficiency voiding. There is a large unmet need for a simple system to empty the bladder in persons with SCI. Recent studies have demonstrated that electrical stimulation of the urethra and pudendal afferents can evoke bladder contractions in humans,3,4 and bladder emptying in animals.5–12 However, the feasibility of emptying the bladder efficiently with electrical stimulation of the urethra has not been established in persons with SCI.
Activation of receptors in the urethra via turbulent fluid flow generates activity in urethral afferents,13,14 which mediates a spinal cord reflex (Barrington's ‘augmenting reflex’) that coordinates the bladder contraction and sphincter relaxation necessary to produce micturition.15,16 Likewise, electrical stimulation of urethral afferents leads to activation of the bladder and inhibition of urethral sphincter activity5,17 before and after spinalization6 and can produce bladder emptying in cats.7–11,18 Analysis of the bladder and sphincter responses across a range of stimulus parameters12 indicates that parameters can be optimized to empty the bladder efficiently and reduce post-void residual volume to a minimum in both the acute8,18 and chronic SCI animal model.11
The objective of this case study was to determine whether intraurethral electrical stimulation could evoke bladder emptying in two men with SCI. This is the first published account of efficient bladder emptying evoked by electrical stimulation of the urethra in persons with SCI. Ultimately the goal is to develop a medical device using electrical stimulation to restore bladder control. It is known that stimulation of genital nerves can improve bladder capacity in persons with and without SCI,19–23 and if electrical stimulation can activate genital nerves to provide continence and urethral afferents to provide bladder emptying, it may be possible to develop a system that restores complete bladder control.
Methods
The research study followed the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board. Each participant provided written informed consent prior to enrolling in the study.
Urodynamics were performed with a custom 12 French balloon catheter (uncoated silicone Foley catheter with 5 cc balloon, Bard Inc., Covington, GA, USA) mounted with ring-shaped electrodes (304 stainless steel, 3 mm wide). In both cases (1 and 2), ring electrodes were mounted at 1, 3, and 5 cm from the balloon corresponding to the bladder neck, the proximal portion of the prostatic urethra, and the distal portion of the prostatic urethra. In case 2, additional ring electrodes were mounted further along the catheter in 2 cm increments to allow stimulation of the distal penile urethra. The inflated balloon was placed against the bladder neck to stabilize the catheter electrodes in place along the urethra but limited potential emptying during contractions. All measurements of bladder emptying were performed while the 12 French catheter was in place and the 5 cc balloon was inflated with the exception of the voiding trial pictured in Fig. 5 in which the stimulation-evoked bladder contraction expelled the catheter during urination. Bladder pressure was recorded via the custom 12 French balloon catheter, and abdominal pressure was recorded via a 10 French balloon catheter (Bard Inc.) placed in the rectum. Abdominal pressure was subtracted from bladder pressure to obtain detrusor pressure. Data were recorded and stored on an urodynamic monitoring system (Laborie Aquarius TT, Triton Williston, VT, USA).
The bladder was emptied and then filled at a rate of 25 ml/minute until a distention-evoked bladder contraction occurred, and this volume was labeled threshold (Vth). The bladder was then drained and filled in increments, expressed as a percentage of Vth, to begin the stimulation trials. The urethra was stimulated over a range of parameters (location, amplitude, and frequency) to determine whether electrical stimulation could evoke a bladder contraction and empty the bladder. Voided volumes were collected in a graduated cylinder, and the residual volume remaining in the bladder was measured. Voiding percentages (%) were calculated by dividing the voided volume by the sum of the voided volume and residual volume. Electrical stimulation was generated by a regulated current stimulator (SMP-plus, Medi-Stim Inc., Wabasha, MN, USA). A 2 in. round self-adhering surface electrode (Rehabilicare, New Brighton, MN, USA) placed on the hip of the patient served as the return electrode.
For each stimulation trial, one of the ring electrodes in the urethra was chosen as the active contact. Stimulation was applied in trains of asymmetric charge-balance biphasic pulses with cathodic pulse width of 200 microseconds. The duration of the stimulus trains was typically at least 30–60 seconds.
Results
Electrical stimulation of the urethra evoked bladder contractions and emptying in two men with SCI. The bladder contractions evoked by urethral stimulation emptied up to 60–77% of the bladder, leaving post-void residual volumes of 20–55 ml. No adverse events were reported by the subjects or observed during this study.
Case 1
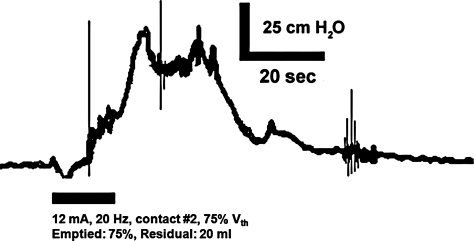
The first participant was a 46-year-old male with a T6 ASIA A SCI secondary to a gunshot wound 15 years prior. The participant demonstrated neurogenic detrusor overactivity on urodynamics and managed his bladder with clean intermittent catheterization approximately 6 times per day and anticholinergic medication (oxybutynin 5 mg 3 times a day). After the participant provided informed consent, he stopped taking oxybutynin 2 days prior to the experiment. The bladder was emptied via catheter and then filled at a rate of 25 ml/minute until distention-evoked contractions (peak detrusor pressure of approximately 55 cm H2O) at approximately 110 ml (volume threshold (Vth)). The bladder was filled to 80 ml (approximately 75% of Vth) and electrical stimulation (20 Hz, 12 mA) was delivered to contact #2 on the urethral catheter (in the mid-prostatic urethra). Stimulation evoked a bladder contraction that emptied 60 ml (75%) and left a post-void residual of 20 ml (Fig. 1).
Figure 1.
Electrical stimulation (12 mA, 20 Hz) of the proximal portion of the prostatic urethra (via contact #2) evoked a bladder contraction that emptied approximately 75% of the initial bladder volume around the inflated balloon and urethral catheter, leaving a post-void residual volume of 20 ml. Prior to stimulation, the bladder was filled to approximately 75% of volume threshold for distention-evoked contractions (Vth). These data are from case 1: a male patient with T-6 ASIA A SCI.
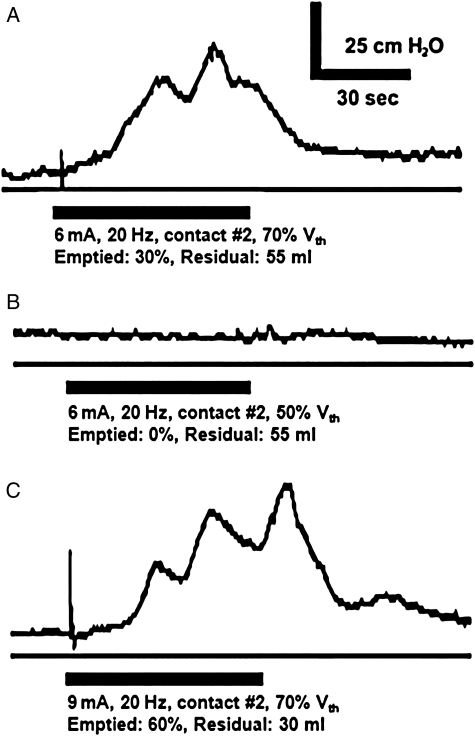
The bladder volume influenced whether urethral stimulation evoked a bladder contraction. Stimulation did not evoke a contraction when the bladder contained 55 ml (50% of Vth), but evoked a bladder contraction after the bladder was filled to 75 ml (70% of Vth) (Fig. 2).
Figure 2.
Detrusor pressure in response to electrical stimulation (bar) of the urethra. Electrical stimulation (via contact #2) of the proximal portion of the prostatic urethra evoked a bladder contraction when the bladder was filled to at least 70% of Vth (A and C), but did not evoke a contraction when the bladder was filled to 50% of Vth (B). The contractions in A and C emptied approximately 30 and 60% of the initial bladder volumes around the inflated balloon and urethral catheter, leaving post-void residual volumes of approximately 55 and 30 ml, respectively. These data are from case 1.
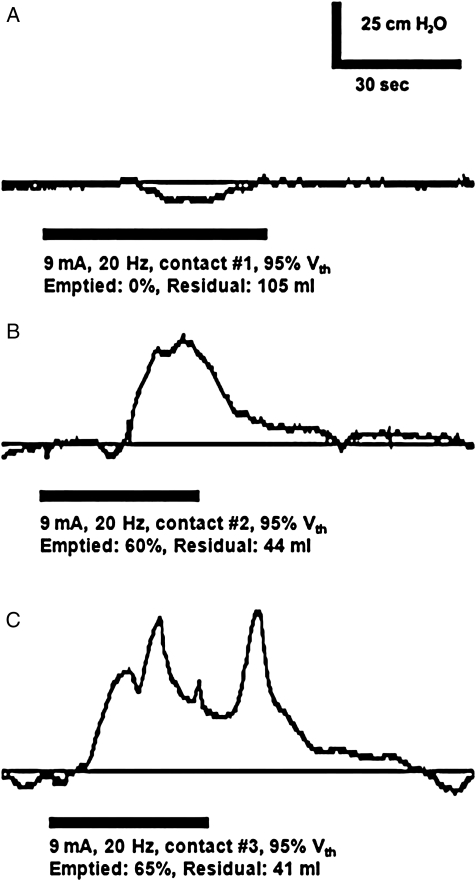
Whether urethral stimulation evoked a bladder contraction was also influenced by the location of stimulation. When the bladder contained more than two-third of Vth, stimulation (20 Hz, 9 mA) of the bladder neck (contact #1) evoked no contractions (0% of three trials), stimulation of the proximal portion of the prostatic urethra (contact #2) evoked three contractions (50% of six trials), and stimulation of the distal portion of the prostatic urethra (contact #3) evoked three contractions (100% of three trials) (Fig. 3). Stimulation evoked peak bladder detrusor pressures of 51 ± 10 cm H2O (mean ± s.d., n = 6 evoked contractions) across all parameters (20 Hz, 6–12 mA, contacts #2 and #3). Without electrical stimulation, contractions evoked by bladder distention or leg spasms generated peak detrusor pressures of 60 ± 12 cm H2O, which emptied 49 ± 36% of the volume in the bladder, leaving post-void residuals of approximately 44 ± 30 ml (n = 5 contractions).
Figure 3.
Detrusor pressure in response to electrical stimulation depended on the location of urethral stimulation. Stimulation of the bladder neck (via contact #1) did not evoke a bladder contraction (A), but bladder contractions were evoked by stimulation of the proximal portion of the prostatic urethra (via contact #2) (B) and by stimulation of the distal portion of the prostatic urethra (via contact #3) (C). These data are from case 1.
Case 2
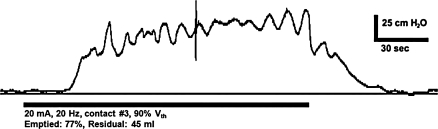
The second participant was a 51-year-old male with a T2 ASIA A SCI secondary to a fall 2 years prior. The participant demonstrated neurogenic detrusor overactivity on urodynamics and managed his bladder with anticholinergic medication (oxybutynin 5 mg 3times a day) in combination with clean intermittent catheterization approximately 4 times per day during the day and with a condom and leg-bag at night. After the participant provided informed consent, he stopped taking oxybutynin 2 days prior to the experiment. The bladder was emptied via catheter and then filled at a rate of 25 ml/minute until distention of the bladder evoked contractions (peak detrusor pressure of approximately 90 cm H2O) at approximately 215 ml (Vth). When the bladder was filled to approximately 90% of Vth (195 ml), electrical stimulation (20 Hz, 20 mA via contact #3 in the distal portion of the prostatic urethra) evoked a bladder contraction that emptied 77% (150 ml) of the bladder, leaving a post-void residual of approximately 45 ml (Fig. 4). Stimulation at bladder volumes less than 165 ml failed to evoke bladder contractions, suggesting that the bladder needed to be filled to greater than approximately 75% of Vth to evoke a bladder contraction.
Figure 4.
Electrical stimulation of the distal portion of the prostatic urethra (via contact #3) evoked a bladder contraction that emptied approximately 77% of the initial bladder volume. These data are from case 2: a male patient with a T2 ASIA A SCI.
An interesting finding was that electrical stimulation of the urethra produced inhibition or excitation of bladder contractions depending on the stimulation parameters and location. A distention-evoked contraction that followed bladder filling was inhibited by stimulation of the penile urethra (3 cm from the external meatus, 40 mA, 10 Hz) prior to leaking. After bladder pressure returned to baseline for approximately 1 minute, stimulation was changed to the distal portion of the prostatic urethra (contact #3, 20 mA, 20 Hz), and evoked a bladder contraction that emptied approximately 68% of the volume in the bladder (Fig. 5). Without electrical stimulation, contractions evoked by bladder distention generated peak detrusor pressures of 73 ± 11 cm H2O, which emptied 55 ± 36% of the volume in the bladder, leaving post-void residuals of approximately 102 ± 90 ml (n = 7 contractions).
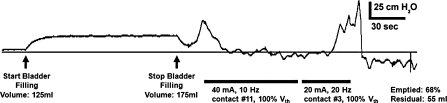
Figure 5.
Electrical stimulation of the urethra produced inhibition or excitation of bladder contractions depending on the stimulus parameters. Stimulation of the penile urethra at 10 Hz inhibited a distention-evoked contraction and prevented leaking. Stimulation of the distal prostatic urethra at 20 Hz evoked a bladder contraction that emptied approximately 68% of the volume in the bladder. Stimulation and the recording of the pressure trace were terminated prematurely because the urethral catheter was expelled from the urethra during urination. Bladder volume was approximately 125 ml at the beginning of the trace, and the bladder was filled (25 ml/minute) to approximately 175 ml although the catheter lumen used to monitor bladder pressure, causing an offset in baseline pressure during filling. These data are from case 2.
Discussion
There are three lower urinary tract problems associated with SCI: neurogenic detrusor overactivity, which can lead to incontinence, and underactive bladder and detrusor-sphincter dyssynergia, which can prevent efficient bladder emptying. Several previous studies suggest that it may be possible to treat incontinence caused by neurogenic detrusor overactivity and increase cystometric capacity with electrical stimulation of genital afferents of the pudendal nerve.19–25 The goal of the present study was to determine whether electrical stimulation of urethral afferents of the pudendal nerve could generate efficient bladder emptying.
The present study reports the first time electrical stimulation of the urethra evoked bladder contractions that voided up to 60–77% of the bladder volume in patients with complete SCI. These data suggest that an excitatory urethra-to-bladder spinal reflex may be used to empty the bladder in patients with complete SCI.
These results complement previous studies in patients with SCI, in which electrical stimulation of the urethra or the pudendal nerve, evoked bladder contractions.3,4 The present study also builds upon pre-clinical studies of electrical stimulation of the urethral afferents and the pudendal nerve that evoked bladder contractions and emptying (64–78%) in cats after acute and chronic spinal transection.6,8,10,11
When considering bladder emptying, detrusor-sphincter dyssynergia is a substantial challenge. Although electromyogram activity was not recorded in the present study, it has been recorded during previous studies of urethral stimulation, and these data demonstrate that urethral stimulation can evoke bladder contractions and suppress sphincter activity in subjects with SCI.26 These reports of coordinated bladder activation and sphincter suppression is supported by animal studies in which stimulation of the pudendal nerve or its branches evoked coordinated bladder contraction and sphincter suppression following spinal cord transection.6,7,9 Although these data are encouraging, it remains unclear how challenging it will be to achieve a long-term solution that provides efficient bladder emptying in patients with SCI. Electrical stimulation of the urethra may be activating the same urethra-to-bladder reflex evoked in response to fluid flow along the urethra. The reflex was initially observed by Barrington15,16 in cats and later documented in humans.27 Urethral fluid flow can evoke a bladder contraction when the bladder contains a minimum volume, approximately 65% of the volume threshold (Vth) for distention-evoked contractions.28 This minimum volume matches the volume (approximately 70–80% of Vth) required to evoke a bladder contraction in the present study and falls within the range (60–80%) in previous clinical and preclinical studies.8,9,12,18,29
The dependence of the bladder response on bladder volume suggests that pelvic afferents are involved in the reflex activation of the bladder. The lack of bladder response to electrical stimulation in the bladder neck (contact #1) compared to the bladder contractions evoked by stimulation of the prostatic urethra (contacts #2 and #3), and as reported recently,26 suggests that urethral stimulation activates urethral afferents in the pudendal nerve in addition to or rather than afferents in the pelvic nerve. These data are consistent with previous studies of urethral stimulation in SCI patients,3 suggesting that the excitatory bladder reflex was mediated by urethral afferents located at the prostatic urethra. Further, bilateral transection of the pudendal nerve in cats abolishes the bladder contractions evoked by electrical stimulation of the prostatic urethra, indicating that pudendal afferents are required to evoke bladder contractions with urethral stimulation.
Prior data strongly support that generation of bladder contractions is mediated by stimulation of urethral pudendal afferents, rather than by activation (and fatigue) of the external urethral sphincter (EUS). First, animal studies indicate that electrical stimulation of both the proximal (prostatic) urethra and the distal (penile) can evoke bladder contraction and profile of thresholds to evoke reflex activation have maxima in the vicinity of the EUS, suggesting that this is not the site of activation.30,31 Second, electrical stimulation of pudendal afferents, including by intraurethral stimulation, can still evoke bladder contractions following administration of neuromuscular blocking agents,31 indicating that activation or modulation of activity in the striated EUS is not required for intraurethral stimulation to evoke bladder contractions. Finally, animal studies indicate that proximal urethral stimulation with higher stimulation frequencies (20–50 Hz), which would generate greater fatigue of the EUS, are less effective in generating bladder contractions than stimulation with lower frequencies.31
Previous studies have suggested that the mechanism of evoking bladder contractions may be by excitation or by release of inhibition (rebound), a mechanism thought to operate during magnetic stimulation of sacral nerve roots and possibly during stimulation of the pudendal nerve in previous studies.32–34 It is unlikely that urethral stimulation evoked bladder contractions by release of inhibition in the present study because the increases in detrusor pressure were generated while stimulation was still on, the increase in pressure was maintained for the duration of stimulation (for up to approximately 2 minutes), and the decrease in pressure was time locked to the offset of stimulation (Fig. 4).
Autonomic dysreflexia is a risk in persons with SCI, and increases in bladder pressure or pudendal nerve activity may lead to increases in blood pressure, which is a potential limitation of activating the bladder via stimulation of pudendal afferents. While some studies indicate that electrical stimulation of the dorsal genital nerve with surface electrodes may lead to autonomic dysreflexia,35 other studies have described electrical stimulation of the urethra in subjects with SCI without reports of autonomic dysreflexia,3,26,36 and no signs or symptoms of autonomic dysreflexia were observed during the present study.
Stimulation of genital nerves is known to improve bladder capacity in persons with and without SCI,19–23 and it is likely that stimulation of the penile urethra inhibited bladder activity and prevented leaking by activating genital afferents in case 2 of the present study (Fig. 5).
Conclusion
The electrode catheter provides a minimally invasive method to determine whether stimulation of pudendal urethral afferents can produce bladder emptying in persons with SCI. These data suggest that it may be possible for an implantable stimulator to restore bladder function by stimulating the pudendal nerve or its branches. By changing stimulus parameters, urethral stimulation could either inhibit the bladder (preventing leaking) or excite the bladder (providing micturition). The ability to activate selectively inhibitory or excitatory bladder reflexes via electrical stimulation holds promise for eventual restorations of both bladder functions: continence and micturition.
Acknowledgements
The authors would like to thank Kimberly Arena, PA-C, Nell Shaffer, RN, and Robbin Clark Scott, RN for their technical assistance. This investigation was conducted with extramural financial support from the National Institute of Neurological Disorders and Stroke (R43NS055393) and the pelvic health business of NDI Medical, LLC, which is now Medtronic Urinary Solutions.
All authors (1) contributed substantially to the conception and design, (2) participated in the drafting and revising of the intellectual content of the article, and (3) provided final approval of the version to be published.
Conflicts of interest statement
The authors' conflicts of interest include: (Code: 2) four of the authors (M.E.B., W.M.G., J.H.G., and J.W.B.) are named inventors on patents controlled by Medtronic; (Code: 3) four of the authors (M.E.B., W.M.G., J.H.G., and J.W.B.) are directors or employees of NDI Medical; (Code: 4) two of the authors (W.M.G. and J.W.B.) may receive royalties related to the patents; (Code: 5) four of the authors (M.E.B., W.M.G., J.H.G., and J.W.B.) have equity interest in NDI Medical; (Code: 6) one author (M.J.K.) received a research grant from NDI Medical.
Institution
The work was performed at Carolinas Rehabilitation, Urology Department, in Charlotte, NC after approval by the IRB.
References
- 1.Shingleton WB, Bodner DR. The development of urologic complications in relationship to bladder pressure in spinal cord injured patients. J Am Paraplegia Soc 1993;16(1):14–17 [DOI] [PubMed] [Google Scholar]
- 2.Watanabe T, Rivas DA, Chancellor MB. Urodynamics of spinal cord injury. Urol Clin North Am 1996;23(3):459–73 [DOI] [PubMed] [Google Scholar]
- 3.Gustafson KJ, Creasey GH, Grill WM. A urethral afferent mediated excitatory bladder reflex exists in humans. Neurosci Lett 2004;360(1–2):9–12 [DOI] [PubMed] [Google Scholar]
- 4.Yoo PB, Klein SM, Grafstein NH, Horvath EE, Amundsen CL, Webster GD, et al. Pudendal nerve stimulation evokes reflex bladder contractions in persons with chronic spinal cord injury. Neurourol Urodyn 2007;26(7):1020–3 [DOI] [PubMed] [Google Scholar]
- 5.Mazieres L, Jiang C, Lindstroem S. Bladder parasympathetic response to electrical stimulation of urethral afferents in the cat. Neurourol Urodyn 1997;16(5):471–2 [Google Scholar]
- 6.Shefchyk SJ, Buss RR. Urethral pudendal afferent-evoked bladder and sphincter reflexes in decerebrate and acute spinal cats. Neurosci Lett 1998;244(3):137–40 [DOI] [PubMed] [Google Scholar]
- 7.Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Frequency-dependent selection of reflexes by pudendal afferents in the cat. J Physiol 2006;577(Pt 1):115–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Bladder emptying by intermittent electrical stimulation of the pudendal nerve. J Neural Eng 2006;3(1):43–51 [DOI] [PubMed] [Google Scholar]
- 9.Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Spinal micturition reflex mediated by afferents in the deep perineal nerve. J Neurophysiol 2005;93(5):2688–97 [DOI] [PubMed] [Google Scholar]
- 10.Tai C, Smerin SE, de Groat WC, Roppolo JR. Pudendal-to-bladder reflex in chronic spinal-cord-injured cats. Exp Neurol 2006;197(1):225–34 [DOI] [PubMed] [Google Scholar]
- 11.Tai C, Wang J, Wang X, de Groat WC, Roppolo JR. Bladder inhibition or voiding induced by pudendal nerve stimulation in chronic spinal cord injured cats. Neurourol Urodyn 2007;26(4):570–7 [DOI] [PubMed] [Google Scholar]
- 12.Yoo PB, Woock JP, Grill WM. Bladder activation by selective stimulation of pudendal nerve afferents in the cat. Exp Neurol 2008;212(1):218–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talaat M. Afferent impulses in the nerves supplying the urinary bladder. J Physiol 1937;89:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Todd JK. Afferent impulses in the pudendal nerves of the cat. Q J Exp Physiol Cogn Med Sci 1964;49:258–67 [DOI] [PubMed] [Google Scholar]
- 15.Barrington FJF. The component reflexes of micturition in the cat, Parts I and II. Brain 1931;54:177–88 [Google Scholar]
- 16.Barrington FJF. The component reflexes of micturition in the cat III. Brain 1941;64:239–43 [Google Scholar]
- 17.Jiang CH, Lindstrom S. Prolonged enhancement of the micturition reflex in the cat by repetitive stimulation of bladder afferents. J Physiol 1999;517(Pt 2):599–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woock JP, Yoo PB, Grill WM. Activation and inhibition of the micturition reflex by penile afferents in the cat. Am J Physiol Regul Integr Comp Physiol 2008;294(6):R1880–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vodusek DB, Light JK, Libby JM. Detrusor inhibition induced by stimulation of pudendal nerve afferents. Neurourol Urodyn 1986;5(4):381–9 [Google Scholar]
- 20.Wheeler JS, Jr, Walter JS, Sibley P. Management of incontinent SCI patients with penile stimulation: preliminary results. J Am Paraplegia Soc 1994;17(2):55–9 [DOI] [PubMed] [Google Scholar]
- 21.Wheeler JS, Jr, Walter JS, Zaszczurynski PJ. Bladder inhibition by penile nerve stimulation in spinal cord injury patients. J Urol 1992;147(1):100–3 [DOI] [PubMed] [Google Scholar]
- 22.Lee YH, Creasey GH. Self-controlled dorsal penile nerve stimulation to inhibit bladder hyperreflexia in incomplete spinal cord injury: a case report. Arch Phys Med Rehabil 2002;83(2):273–7 [DOI] [PubMed] [Google Scholar]
- 23.Horvath EE, Yoo PB, Amundsen CL, Webster GD, Grill WM. Conditional and continuous electrical stimulation increase cystometric capacity in persons with spinal cord injury. Neurourol Urodyn 2010;29(3):401–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirkham AP, Shah NC, Knight SL, Shah PJ, Craggs MD. The acute effects of continuous and conditional neuromodulation on the bladder in spinal cord injury. Spinal Cord 2001;39(8):420–8 [DOI] [PubMed] [Google Scholar]
- 25.Dalmose AL, Rijkhoff NJ, Kirkeby HJ, Nohr M, Sinkjaer T, Djurhuus JC. Conditional stimulation of the dorsal penile/clitoral nerve may increase cystometric capacity in patients with spinal cord injury. Neurourol Urodyn 2003;22(2):130–7 [DOI] [PubMed] [Google Scholar]
- 26.Yoo PB, Horvath EE, Amundsen CL, Webster GD, Grill WM. Multiple pudendal sensory pathways reflexly modulate bladder and urethral activity in patients with spinal cord injury. J Urol 2011;185(2):737–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garry RC, Roberts TD, Todd JK. Reflexes involving the external urethral sphincter in the cat. J Physiol 1959;149:653–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robain G, Combrisson H, Mazieres L. Bladder response to urethral flow in the awake ewe. Neurourol Urodyn 2001;20(5):641–9 [DOI] [PubMed] [Google Scholar]
- 29.Yoo PB, Grill WM. Minimally-invasive electrical stimulation of the pudendal nerve: a pre-clinical study for neural control of the lower urinary tract. Neurourol Urodyn 2007;26(4):562–9 [DOI] [PubMed] [Google Scholar]
- 30.Woock JP, Yoo PB, Grill WM. Finite element modeling and in vivo analysis of electrode configurations for selective stimulation of pudendal afferent fibers. BMC Urol 2010;10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woock JP, Yoo PB, Grill WM. Intraurethral stimulation evokes bladder responses via 2 distinct reflex pathways. J Urol 2009;182(1):366–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bycroft JA, Craggs MD, Sheriff M, Knight S, Shah PJ. Does magnetic stimulation of sacral nerve roots cause contraction or suppression of the bladder? Neurourol Urodyn 2004;23(3):241–5 [DOI] [PubMed] [Google Scholar]
- 33.Craggs M, Shah N, Sheriff M, Khastgir J, Shah P. Contraction or suppression of the bladder by magnetic stimulation of the sacral roots? Resolving the paradox. Neurourol Urodyn 1999;18(9):279–80 [Google Scholar]
- 34.Possover M, Schurch B, Henle KP. New strategies of pelvic nerves stimulation for recovery of pelvic visceral functions and locomotion in paraplegics. Neurourol Urodyn 2010;29(8):1433–8 [DOI] [PubMed] [Google Scholar]
- 35.Reitz A, Schmid DM, Curt A, Knapp PA, Schurch B. Autonomic dysreflexia in response to pudendal nerve stimulation. Spinal Cord 2003;41(10):539–42 [DOI] [PubMed] [Google Scholar]
- 36.Kennelly MJ, Arena KC, Shaffer N, Bennett ME, Grill WM, Grill JH, et al. Electrical stimulation of the urethra evokes bladder contractions in a woman with spinal cord injury. J Spinal Cord Med 2010;33(3):261–5 [DOI] [PMC free article] [PubMed] [Google Scholar]