Abstract
Background
Thoracic and thoracoabdominal aortic intervention carries a significant risk of spinal cord ischemia. The pathophysiologic mechanisms that cause hypoxic/ischemic injury to the spinal cord have not been totally explained. In normal spinal cord, neurons and glial cells do not express type IV collagen. Type IV collagen produced by reactive astrocytes is reported to participate in glial scar formation. Tissue inhibitors of metalloproteinases (TIMPs) are endogenous inhibitors that regulate the activity of the matrix metalloproteinases (MMPs). TIMP-2 binds strongly with MMP-2, facilitating activation by membrane-type MMP. Imbalance between TIMPs and MMPs can lead to excessive degradation of matrix components. Type IV collagen involved in the blood–brain barrier disruption and glial scar formation, TIMP-2 influences MMP-2 that controls degradation of collagen I and IV.
Objective
To examine the immunohistochemical analysis of TIMP-2 and collagen types I–IV in experimental spinal cord ischemia–reperfusion in rats.
Methods
Thirty-two male Wistar rats weighing 250–300 g were divided into four groups: group S: sham group (n = 8); group 0P: 30-minute occlusion without perfusion (n = 8); group 3P: 30-minute occlusion and 3-hour perfusion (n = 8); and group 24P: 30-minute occlusion and 24-hour perfusion (n = 8). Infrarenal aorta was cross-clamped at two sites by using two aneurysm clips for 30 minutes. Reperfusion was provided after removal of the clips. Lumbar spinal cord segments were removed for immunohistochemical analysis.
Results
TIMP-2 and collagen staining in 3-hour perfused (3P) group were nearly the same with sham group (S). TIMP-2 and collagen staining increased in the 24-hour perfused group.
Conclusion
Alterations in collagen levels may relate to the biphasic breakdown of the blood–brain barrier and collagen staining in new cell types with relation to glial scar formation. Our results demonstrate that 3-hour perfusion after occlusion in hypoxic/ischemic spinal cord injury seems to be the critical reversible period.
Keywords: Spinal cord ischemia, Collagen IV, TIMP-2, Paraplegia, Glial scar formation
Introduction
Spinal cord injury (SCI) has traumatic and non-traumatic origin and often leads to catastrophic dysfunction and disability. The pathophysiologic mechanisms that underlie hypoxic/ischemic injury to the spinal cord have not been totally explained yet.1–7 Thoracic and thoracoabdominal aortic intervention carries a significant risk of spinal cord ischemia, ranging from 5 to 20%.8–10 Pouw et al.11 compared the neurological outcome between paraplegia caused by acute spinal cord ischemia syndrome and traumatic SCI, and found that neurological outcome was independent of the diagnosis. In the literature, studies of spinal cord ischemia are based on biochemical markers of oxidative stress1,2,12,13 and there are various studies of matrix metalloproteinases (MMPs) in traumatic SCI.14–16 The extracellular matrix (ECM) of the blood–brain barrier (BBB) forms a basal lamina, which selectively filters blood elements. Collagen IV is the major structural component of all basement membranes. The response of the adult mammalian central nervous system (CNS) to injury results in a gliosis in the lesion and the formation of a glial scar. Type IV collagen produced by reactive astrocytes is reported to participate in glial scar formation.17–19 Type IV collagen involved in the BBB and glial scar and high levels of tissue inhibitor of metalloproteinase 2 (TIMP-2) inhibit MMP-2 that degrades collagen I and IV.
In this study, we investigated the levels of TIMP-2 and collagen IV and I that are involved in (non-traumatic) SCI pathogenesis after experimental spinal cord ischemia and reperfusion.
Methods
Thirty-two male Wistar rats weighing 250–300 g were housed in a temperature- and humidity-controlled room (22 ± 3 °C and 67 ± 7%, respectively) in which a 12:12 hours light–dark cycle was maintained. The animals were fed standard rat chow and tap water ad libitum. Ethical approval was granted by the University Ethics Committee.
The animals were anesthetized by intramuscular injection of 70 mg/kg ketamine (Ketalar, Parke-Davis, Eczacıbaşı, Istanbul, Turkey) and 5 mg/kg xylazine (Rompun, Bayer, Istanbul, Turkey), and allowed to breath spontaneously. Body temperatures were measured by rectal thermometry and maintained at 37 °C with a heating pad. Arterial pressure and heart rate were monitored continually. Animals were placed in supine position. After sterile preparation, a midline incision was made and the abdominal aorta was exposed through a transperitoneal approach; heparin 150 U/kg was administered for anticoagulation through an intravenous route 5 minutes before clamping. The aorta was cross-clamped at two sites by using two new aneurysm clips of 70 g/0.69 N closing force (Yasargil FE 721, Aesculap, Germany) for each group under surgical microscope. The occlusion sites were just caudal to the left renal artery and above the bifurcation. Cross-clamp time was 30 minutes. At the end of the occlusion period, the clips were removed and restoration of blood flow was visually verified. The incision was closed in layers. Spinal cord segments between L2 and S1 were harvested after total laminectomies from T12 to S1 and cutting the nerve roots. Spinal cord segments between L2 and L5 were removed for immunohistochemical analysis.
Thirty-two rats were divided into four groups:
Group S: Only laparotomy was performed in the sham group (n = 8).
Group 0P: Rats were sacrificed after the 30-minute occlusion without perfusion (n = 8).
Group 3P: 3-hour perfusion was provided after 30-minute occlusion (n = 8).
Group 24P: 24-hour perfusion was provided after 30-minute occlusion (n = 8).
Immunohistochemical analysis
After the survival period, all animals were perfused intracardially under deep anesthesia with 4% paraformaldehyde in phosphate buffer solution. After the completion of the perfusion process, all animals were decapitated, and the lumbar section of the spinal cord was removed from the vertebral column, postfixed in the same fixative.
The tissues were embedded in paraffin, coronally sectioned at 5 µm thickness. These sections were placed on positively charged glass slides, heated overnight at 37 °C, deparaffinized in xylene, and rehydrated through a graded series of ethanol. For antigen retrieval treatment, slides were boiled for 20 minutes in a microwave at 100 °C in sodium citrate buffer (10 mM, pH 6). Then the slides were immersed in 3% hydrogen peroxide for 20 minutes at room temperature to block endogenous peroxidase activity. To block non-specific binding sites, the slides were treated with block serum for 10 minutes at room temperature. Thereafter, the slides were incubated with antibodies against collagen I (1:100 dilution, rabbit polyclonal, ab34710), collagen IV (1:250 dilution, rabbit polyclonal, ab6586), and TIMP-2 (1:250 dilution, mouse monoclonal IgG2a, 2Q758, SC-73175) at 4 °C overnight. They were labeled using Universal HRP immunostaining kit (rabbit primer antibodies, KP-50AR). Sections were incubated in biotinylated secondary antibody and streptavidin-HRP for 20 minutes at room temperature. The bound antibodies were visualized using 3-amino-4-ethylcarbazole as chromogen. Finally, the sections were mounted for quantitative analysis. Negative controls consisted of tissue sections incubated without primary antibody.
Evaluation of collagen I and IV and TIMP-2 staining
Images of the immunohistochemically stained sections were captured with a Leica DFC290 HD color digital camera mounted on a Leica DM1000 microscope using a ×20 objective and stored as tagged image file format. Images were recorded in three parts as red, green, and blue (RGB format), each with 640 × 480-pixel resolution and each pixel having 256 possible gray levels. Images were then analyzed with Image J software. In each image, the parameters measured by the image analysis program were the percentage of antibody-stained area in relation to the whole area.
Statistical analyses
One-way analysis of variance and Tukey honestly significant difference post hoc test were applied to analyze the statistical significance of our results. Values were considered to be significant at P < 0.05.
Results
Statistical results: Comparison between groups 0P and 24P was not significant for collagen type IV and TIMP-2 (P > 0.05). Comparison between groups S and 3P was not significant for collagen types I and IV and TIMP-2 (P > 0.05). Comparison between the other groups was significant (P < 0.05, P < 0.0001). Details of the results with the standard deviations are listed in Table 1.
Table 1.
Statistical results with standard deviations of collagen types I and IV and TIMP-2 in groups S, 0P, 3P, and 24P
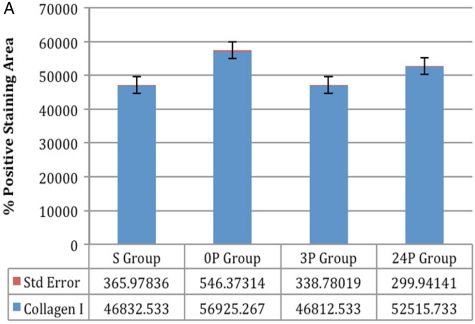 (A) Collagen I: comparison between groups S and 3P was not significant and comparison between all other groups was significant (P < 0.005, P < 0.0001) (A) Collagen I: comparison between groups S and 3P was not significant and comparison between all other groups was significant (P < 0.005, P < 0.0001) |
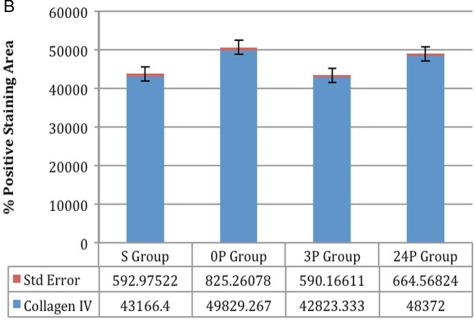 (B) Collagen IV: comparison between groups 0P and 24P, and comparison between groups S and 3P was not significant; comparison between all other groups was significant (P < 0.005, P < 0.0001) (B) Collagen IV: comparison between groups 0P and 24P, and comparison between groups S and 3P was not significant; comparison between all other groups was significant (P < 0.005, P < 0.0001) |
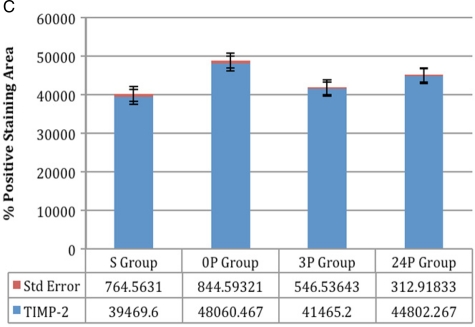 (C) TIMP-2: Comparisons between groups 0P and 24P and between groups S and 3P were not significant; comparison between all other groups was significant (P < 0.005, P < 0.0001) (C) TIMP-2: Comparisons between groups 0P and 24P and between groups S and 3P were not significant; comparison between all other groups was significant (P < 0.005, P < 0.0001) |
Immunohistochemical results: Immunohistochemical staining was strong in groups 0P and 24P for types IV and I collagen and TIMP-2. Weak vascular staining was observed in groups 3P and S for types IV and I collagen and TIMP-2 (Figs 1–3).
Figure 1.
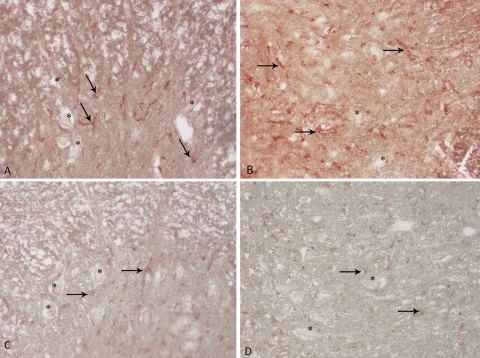
The anterior horn of spinal cord was positive for type IV collagen (A–D). Immunohistochemical staining for type IV collagen in 0P group (A). Strong vascular basement membrane staining of type IV collagen. Immunohistochemical staining for type IV collagen in 24P group (B). Weak vascular staining of type IV collagen in spinal cord in 3P (C) and S group (D). Asterisk (*) indicates neurons and arrows indicate type IV collagen positive stained blood vessels. Magnification ×20 in all panels.
Figure 3.
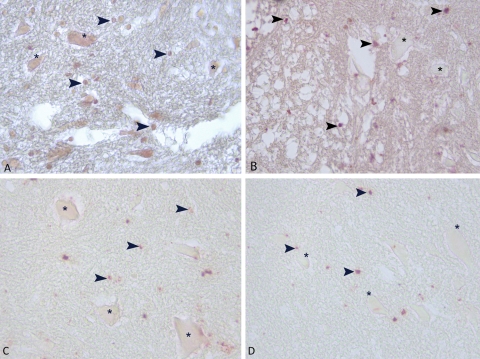
The anterior horn of spinal cord was positive for TIMP-2. Immunohistochemical staining for type TIMP-2 in 0P group (A). Strong positive staining of TIMP-2 (A). Immunohistochemical staining for TIMP-2 in 24P group (B). Weak vascular staining of TIMP-2 in spinal cord in 3P (C) and S groups (D). Asterisk (*) indicates neurons and arrowheads indicate TIMP-2 positive stained glia cells. Magnification ×40 in all panels.
Figure 2.
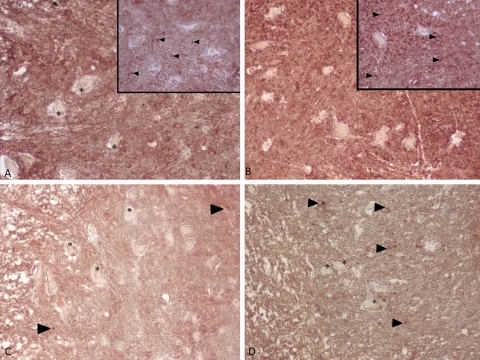
Immunohistochemical staining for type I collagen. Insets show a close-up of the spinal cord. Strong staining of type I collagen in 0P and 24P groups (A, B). Weak staining of type I collagen in spinal cord in 3P and S groups (C, D). Type I collagen staining is indicated by arrowheads. Asterisk indicates neurons. Magnification ×20 in all panels and ×40 in insets.
Discussion
The ECM of the BBB forms a basal lamina, which surrounds and anchors endothelial cells and astrocytes, which, in turn, provide a structural barrier that selectively filters blood elements. Major components of the cerebral microvascular basal lamina include collagen IV, laminin, and fibronectin. Collagen IV is the major structural component of all basement membranes. It provides the scaffolding onto which the rest of the basement membrane is built.20–26 In normal spinal cord, neurons and glial cells do not express type IV collagen. Type IV collagen produced by reactive astrocytes is reported to participate in glial scar formation.17–19
Relationship of TIMP-2 and collagen I and IV
Brain vascular tissue is an important source of TIMP-2, which is highly produced in brain microvessels. TIMPs are endogenous inhibitors that regulate the activity of the MMPs. MMP-2 degrades types IV and I collagen, and thus has the potential to modify constituents of basal laminae. TIMP-2 reduces the tracer uptake. Metalloproteinase inhibitors reduce ECM proteolysis and protect the BBB.15,27–33
There are four TIMPs, differentially expressed by cells in various tissues. TIMP-1, TIMP-2, and TIMP-4 are secreted, whereas TIMP-3 is bound to the ECM and TIMP-4 is mainly found in vascular tissue.32–35
An important feature of the MMPs is their latency. Secreted in a proform, they require activation by a variety of mechanisms before they can act. Matrix prometalloproteinase-2 (ProMMP-2) is activated by the membrane-type MMP (MT-MMP).36,37 TIMP-2 binds with MT-MMP and proMMP-2 to facilitate activation of MMP-2. MMP-2 degrades collagen IV and I. Low concentrations of TIMP-2 facilitate activation, while higher concentrations inhibit the processing of proMMP-2.36,38
In our study, TIMP-2 and collagen staining in 3-hour perfused group were nearly the same as in sham group, suggesting that 3 hours of perfusion is not sufficient to activate the process. However, as the perfusion continues, TIMP-2 and collagen staining increased in 24-hour perfused group due to an increase of TIMP-2. Elevated TIMP-2 levels inhibited MMPs (MMP-2 and MMP-9) and thus collagen staining (I and IV) increased.
We demonstrated that high percentage of antibody-stained area in relation to the whole area and staining density of TIMP-2 provided the increased levels of collagens, probably inhibiting the activation of MMPS as mentioned in the literature.36,38
Relationship of TIMP-2 and collagen I and IV to glial scar and breakdown of BBB
The response of the adult mammalian CNS to injury results in a gliosis in the lesion and the formation of a glial scar. The formation of the glial scar is a complex process, involving astrocytes, oligodendrocyte precursors, and meningeal cells. One of the most remarkable characteristics of astrocytes is their powerful response to various neurologic stimuli. Such activated astrocytes are called reactive astrocytes and are thought to be directly responsible for the glial scar formation. Thus, its final form is a structure composed of crowded, process-bearing reactive astrocytes and surrounded by ECM.39–42
TIMP-2 is a 21 kDa molecule that binds most strongly with MMP-2, facilitating activation by MT-MMP. MMP activity is required for the inflammatory cell infiltration that occurs following SCI and most likely contributes to early barrier disruption. The early inflammatory response involves an initial wave of infiltrating neutrophils, followed by migration of monocytes and macrophages into injured segment. Each of these inflammatory cells expresses MMPs, including MMP-2 (gelatinase A), MMP-8 (neutrophil collagenase), MMP-9 (gelatinase B), MMP-11 (stromelysin-3), and MMP-12 (metalloelastase). Imbalance between TIMPs and MMPs can lead to excessive degradation of matrix components.32 When the proteolytic activity is greater than the inhibition by the TIMPs, ECM breakdown occurs. Conversely, when the inhibitors are excessively expressed, and proteolysis is restricted, glial fibrosis occurs.36
Alteration of TIMP-2 during the hypoxia and reperfusion
The alternation of TIMP-2 expression in an acute stage of ischemia has not been identified in vivo. There are several mechanisms defining the initial expression of TIMP-2 in ischemia. TIMP-2 may influence BBB permeability independent of MMPs. TIMP expressions in the CNS can also be upregulated in response to MMPs under various pathologic conditions.43–45 However, the function of TIMPs in acute cerebral ischemia, especially with respect to their influence on MMP levels and BBB disruption, is less well understood. It is also defined that ECM breakdown products are additional regulators of MMPs and TIMPs.46,47 Pagenstecher et al.43 noted that some cytokines released during the ECM breakdown induce an increase of TIMP gene expression.
TIMP-2 levels may be increased due to both breakdown of BBB and release of cytokines or as a counter-regulator function of increased MMPs, consequently preventing the activation of proMMP-2 following the occlusion.
Cavdar et al.48 investigated the effects of hypoxia and hypoxia/reoxygenation on MMP-2 and MMP-9, and their inhibitor (TIMP-2) and activator (MT1-MMP), in human umbilical vein endothelial cells. They demonstrated a significant increase in both MMP-2 activity and TIMP-2 protein levels by reoxygenation for 24 hours following a short-term hypoxia. They defined that the increase in MMP-2 secretion caused by reoxygenation may be stimulated by the oxidative stress that occurs following reoxygenation and the increase in TIMP-2 protein level with the duration of reoxygenation may be interpreted as a compensation mechanism related to increased proMMP-2.
In studies of both Pagenstecher et al.43 and Cavdar et al.48 and according to our results, there is an induction of TIMP-2 levels during the 24-hour perfusion. Prolonged perfusion pressure may have adverse effects on the vascular structures, and inter-correlated multifactorial mechanisms (oxidative stress, breakdown of BBB, and release of cytokines) lead to an increase of TIMP-2 levels due to increased MMPs to prevent the activation of proMMP-2 following the occlusion. Thus, during the 24-hour perfusion, increased TIMP-2 levels cause blockage of MMP-2 activation, and this results in elevation of the collagen IV and I levels. Further studies are needed to define the mechanism of the increase of TIMP-2 during reoxygenation.
Biphasic pattern of BBB
Rosenberg demonstrated the biphasic BBB pattern of brain injury. The first phase includes the initial injury, which is thought to be related to the activation of the constitutively expressed MMP-2 by MT-MMP in the 3–6-hour period. Rosenberg defined that the early opening of the BBB secondary to the activation of MMP-2 can be blocked by treatment with an MMP inhibitor but, in the absence of treatment, the permeability reverts to normal in several hours. After 24–48 hours, there is a second, more severe disruption of the BBB.36
In another study, Rosenberg demonstrated that middle cerebral artery occlusion for 90 minutes with reperfusion caused a biphasic opening of the BBB with a transient opening at 3 hours and a second more severe injury at 48 hours. The early opening at 3 hours correlated with an increase in MMP-2.31
In our study, we investigated TIMP-2, which binds most strongly with MMP-2, controlling the activation by MT-MMP and collagen IV and I levels that participate in glial scar formation.
We observed that critical reversible period in hypoxic/ischemic spinal cord injury is 3 hours after the perfusion provided, which is similar to Rosenberg's results of hypoxic brain studies. TIMP-2 levels activate proMMP-2; thus, the collagens will not increase or stabilize in this 3-hour period. This period seems to represent the first phase of the process. Thus, possible ECM of spinal cord was recovered during the 3 hours of reperfusion. However, after the 24-hour perfusion, MMP-2 was probably increased by reactive astrocytes during the process as mentioned in the literature.17 This elevation induced TIMP-2 activation due to increased proMMP-2. Increased TIMP-2 provided the blockage of MMP-2 and increased collagens to the levels of the non-perfused group. This period may represent the beginning of the irreversible second phase causing the glial scar formation due to increased collagens. Further studies including measurement of MMPs by immunoassay and various groups researched for long time processes are needed to provide more clear results.
Encouraging results were provided with the use of MMP inhibitors in the studies including intracerebral hemorrhage, experimental allergic neuritis, delayed hypersensitivity, and bacterial meningitis.27,36,49–54
Ischemic injuries that are complicated by hypoxia induce cell pathways that lead to cell death, making ischemic injury more challenging to treat. The therapeutic goal in the ischemic lesion is to reverse the process at an early stage blocking the factors that lead to the production of MMPs.36
Conclusion
Biphasic opening of BBB plays an important role in ischemic spinal cord injury. Since TIMP-2 blocked the MMP-2-induced BBB opening in MMP-induced injury in the brain,36,55 synthetic MMP-2 inhibitors may help to recover BBB opening in spinal cord ischemic injury in the early stages.
References
- 1.Erten SF, Kocak A, Ozdemir I, Aydemir S, Colak A, Reeder BS. Protective effect of melatonin on experimental spinal cordischemia. Spinal Cord 2003;4(10):533–38 [DOI] [PubMed] [Google Scholar]
- 2.Emmez H, Yildirim Z, Kale A, Tönge M, Durdağ E, Börcek AO, et al. Anti-apoptotic and neuroprotective effects of alpha-lipoic acid on spinal cord ischemia-reperfusion injury in rabbits. Acta Neurochir (Wien) 2010;152(9):1591–600 [DOI] [PubMed] [Google Scholar]
- 3.Baptiste DC, Fehlings MG. Pharmacological approaches to repair the injured spinal cord. J Neurotrauma 2006;23(3–4):318–34 [DOI] [PubMed] [Google Scholar]
- 4.Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, et al. Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol 2001;24(5):254–64 [DOI] [PubMed] [Google Scholar]
- 5.Dumont RJ, Verma S, Okonkwo DO, Hurlbert RJ, Boulos PT, Ellegala DB, et al. Acute spinal cord injury, part II: contemporary pharmacotherapy. Clin Neuropharmacol 2001;24(5):265–79 [DOI] [PubMed] [Google Scholar]
- 6.Bowes MP, Swanson S, Zivin JA. The AMPA antagonist LY293558 improves functional neurological outcome following reversible spinal cord ischemia in rabbits. J Cereb Blood Flow Metab 1996;16(5):967–72 [DOI] [PubMed] [Google Scholar]
- 7.Genovese T, Cuzzocrea S. Role of free radicals and poly (ADP-ribose)polymerase-1 in the development of spinal cord injury: new potential therapeutic targets. Curr Med Chem 2008;15(5):477–87 [DOI] [PubMed] [Google Scholar]
- 8.Coselli JS, LeMaire SA, Miller CCR, Schmittling ZC, Köksoy C, Pagan J, et al. Mortality and paraplegia after thoracoabdominal aortic aneurysm repair: a risk factor analysis. Ann Thorac Surg 2000;69(2):409. [DOI] [PubMed] [Google Scholar]
- 9.Safi HJ, Hess KR, Randel M, Iliopoulos DC, Baldwin JC, Mootha RK, et al. Cerebrospinal fluid drainage and distal aortic perfusion: reducing neurologic complications in repair of thoracoabdominal aortic aneurysm types I and II. J Vasc Surg 1996;23(2):223. [DOI] [PubMed] [Google Scholar]
- 10.Drinkwater SL, Goebells A, Haydar A, Bourke P, Brown L, Hamady M, et al. On behalf of the Regional Vascular Unit, St Mary's Hospital, Imperial College NHS Trust. The incidence of spinal cord ischaemia following thoracic and thoracoabdominal aortic endovascular intervention. Eur J Vasc Endovasc Surg 2010;40(6):729–35 [DOI] [PubMed] [Google Scholar]
- 11.Pouw MH, Hosman AJ, van Kampen A, Hirschfeld S, Thietje R, van de Meent H. Is the outcome in acute spinal cord ischaemia different from that in traumatic spinal cord injury? A cross-sectional analysis of the neurological and functional outcome in a cohort of 93 paraplegics. Spinal Cord 2011;49(2):307–12 [DOI] [PubMed] [Google Scholar]
- 12.Chronidou F, Apostolakis E, Papapostolou I, Grintzalis K, Georgiou CD, Koletsis EN, et al. Beneficial effect of the oxygen free radical scavenger amifostine (WR-2721) on spinal cord ischemia/reperfusion injury in rabbits. J Cardiothorac Surg 2009;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao X, Phillis JW. The free radical scavenger, alpha-lipoic acid, protects against cerebral ischemia–reperfusion injury in gerbils. Free Radic Res 1995;23(4):365–70 [DOI] [PubMed] [Google Scholar]
- 14.Hsu JY, McKeon R, Goussev S, Werb Z, Lee JU, Trivedi A, et al. Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. J Neurosci 2006;26(39):9841–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esposito E, Genovese T, Caminiti R, Bramanti P, Meli R, Cuzzocrea S. Melatonin regulates matrix metalloproteinases after traumatic experimental spinal cord injury. J Pineal Res 2008;45(2):149–56 [DOI] [PubMed] [Google Scholar]
- 16.Buss A, Pech K, Kakulas BA, Martin D, Schoenen J, Noth J, et al. Matrix metalloproteinases and their inhibitors in human traumatic spinal cord injury. BMC Neurol 2007;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liesi P, Kauppila T. Induction of type IV collagen and other basement-membrane-associated proteins after spinal cord injury of the adult rat may participate in formation of the glial scar. Exp Neurol 2002;173(1):31–45 [DOI] [PubMed] [Google Scholar]
- 18.Stichel CC, Hermanns S, Luhmann HJ, Lausberg F, Niermann H, D'Urso D, et al. Inhibition of collagen IV deposition promotes regeneration of injured CNS axons. Eur J Neurosci 1999;11(2):632–46 [DOI] [PubMed] [Google Scholar]
- 19.Iseda T, Nishio T, Kawaguchi S, Kawasaki T, Wakisaka S. Spontaneous regeneration of the corticospinal tract after transection in young rats: collagen type IV deposition and astrocytic scar in the lesion site are not the cause but the effect of failure of regeneration. J Comp Neurol 2003;464(3):343–55 [DOI] [PubMed] [Google Scholar]
- 20.Timpl R, Wiedemann H, Van Delden V, Furthmayr H, Kuhn K. A network model for the organization of type IV collagen molecules in basement membranes. Eur Neurol 1981;41(1):35–43 [DOI] [PubMed] [Google Scholar]
- 21.Yurchenco PD, Schittny JC. Molecular architecture of basement membranes. FASEB J 1990;4(6):1577–90 [DOI] [PubMed] [Google Scholar]
- 22.Guo M, Lin V, Davis W, Huang T, Carranza A, Sprague S, et al. Preischemic induction of TNF-alpha by physical exercise reduces blood–brain barrier dysfunction in stroke. J Cereb Blood Flow Metab 2008;28(8):1422–30 [DOI] [PubMed] [Google Scholar]
- 23.Kose N, Asashima T, Muta M, Iizasa H, Sai Y, Terasaki T, et al. Altered expression of basement membrane-related molecules in rat brain pericyte, endothelial, and astrocyte cell lines after transforming growth factor-beta1 treatment. Drug Metab Pharmacokinet 2007;22(4):255–66 [DOI] [PubMed] [Google Scholar]
- 24.Grant DS, Kleinman HK. Regulation of capillary formation by laminin and other components of the extracellular matrix. EXS (Experientia Supplementum) 1997;79:317–33 [DOI] [PubMed] [Google Scholar]
- 25.Tilling T, Engelbertz C, Decker S, Korte D, Huwel S, Galla HJ. Expression and adhesive properties of basement membrane proteins in cerebral capillary endothelial cell cultures. Cell Tissue Res 2002;310(1):19–29 [DOI] [PubMed] [Google Scholar]
- 26.Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res 2005;97(11):1093–107 [DOI] [PubMed] [Google Scholar]
- 27.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol 2000;18(5):1135–49 [DOI] [PubMed] [Google Scholar]
- 28.La Fleur M, Underwood JL, Rappolee DA, Werb Z. Basement membrane and repair of injury to peripheral nerve: defining a potential role for macrophages, matrix metalloproteinases, and tissue inhibitor of metalloproteinases-1. J Exp Med 1996;184(6):2311–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shapiro SD. A concise yet informative stroll through matrix metalloproteinases and TIMPs. J Cell Sci 2000;113(Pt 19):3355–610984424 [Google Scholar]
- 30.Hashimoto T, Wen G, Lawton MT, Boudreau NJ, Bollen AW, Yang GY, et al. University of California, San Francisco BAVM Study Group. Abnormal expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in brain arteriovenous malformations. Stroke 2003;34(4):925–31 [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and TIMPs are associated with blood–brain barrier opening after reperfusion in rat brain. Stroke 1998;29(10):2189–95 [DOI] [PubMed] [Google Scholar]
- 32.Webster NL, Crowe SM. Matrix metalloproteinases, their production by monocytes and macrophages and their potential role in HIV-related diseases. J Leukoc Biol 2006;80(5):1052–66 [DOI] [PubMed] [Google Scholar]
- 33.Osman M, Tortorella M, Londei M, Quaratino S. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinase define the migratory characteristics of human monocyte-derived dendritic cells. Immunology 2002;105(1):73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer 2002;2(9):657–72 [DOI] [PubMed] [Google Scholar]
- 35.Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol 1997;74(2):111–22 [PubMed] [Google Scholar]
- 36.Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia 2002;39(3):279–91 Erratum in: Glia 2002;40(1):130 [DOI] [PubMed] [Google Scholar]
- 37.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 1994;370(6484):61–5 [DOI] [PubMed] [Google Scholar]
- 38.Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem 1995;270(10):5331–8 [DOI] [PubMed] [Google Scholar]
- 39.Hirano S, Yonezawa T, Hasegawa H, Hattori S, Greenhill NS, Davis PF, et al. Astrocytes express type VIII collagen during the repair process of brain cold injury. Biochem Biophys Res Commun 2004;317(2):437–43 [DOI] [PubMed] [Google Scholar]
- 40.Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull 1999;49(6):377–91 [DOI] [PubMed] [Google Scholar]
- 41.Shearer MC, Fawcett JW. The astrocyte/meningeal cell interface – a barrier to successful nerve regeneration. Cell Tissue Res 2001;305(2):267–73 [DOI] [PubMed] [Google Scholar]
- 42.Eddleston M, Mucke L. Molecular profile of reactive astrocytes – implications for their role in neurologic disease. Neuroscience 1993;54(1):15–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pagenstecher A, Stalder AK, Kincaid CL, Shapiro SD, Campbell IL. Differential expression of matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase genes in the mouse central nervous system in normal and inflammatory states. Am J Pathol 1998;152(3):729–41 [PMC free article] [PubMed] [Google Scholar]
- 44.Aoki T, Kataoka H, Moriwaki T, Nozaki K, Hashimoto N. Role of TIMP-1 and TIMP-2 in the progression of cerebral aneurysms. Stroke 2007;38(8):2337–45 [DOI] [PubMed] [Google Scholar]
- 45.Fujimoto M, Takagi Y, Aoki T, Hayase M, Marumo T, Gomi M, et al. Tissue inhibitor of metalloproteinases protect blood–brain barrier disruption in focal cerebral ischemia. J Cereb Blood Flow Metab 2008;28(10):1674–85 [DOI] [PubMed] [Google Scholar]
- 46.Huhtala P, Humphries MJ, McCarthy JB, Tremble PM, Werb Z, Damsky CH. Cooperative signaling by alpha 5 beta 1 and alpha 4 beta 1 integrins regulates metalloproteinase gene expression in fibroblasts adhering to fibronectin. J Cell Biol 1995;129(3):867–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tremble P, Damsky CH, Werb Z. Components of the nuclear signalling cascade that regulate collagenase gene expression in response to integrin-derived signals. J Cell Biol 1995;129(6):1707–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cavdar Z, Oktay G, Egrilmez MY, Genc S, Genc K, Altun Z, et al. In vitro reoxygenation following hypoxia increases MMP-2 and TIMP-2 secretion by human umbilical vein endothelial cells. Acta Biochim Pol 2010;57(1):69–73 [PubMed] [Google Scholar]
- 49.Leppert D, Hughes P, Huber S, Erne B, Grygar C, Said G, et al. Matrix metalloproteinase upregulation in chronic inflammatory demyelinating polyneuropathy and nonsystemic vasculitic neuropathy. Neurology 1999;53(1):62–70 [DOI] [PubMed] [Google Scholar]
- 50.Hamann GF, Okada Y, Fitridge R, del Zoppo GJ. Microvascular basal lamina antigens disappear during cerebral ischemia and reperfusion. Stroke 1995;26(11):2120–26 [DOI] [PubMed] [Google Scholar]
- 51.Rosenberg GA, Navratil M. Metalloproteinase inhibition blocks edema in intracerebral hemorrhage in the rat. Neurology 1997;48(4):921–6 [DOI] [PubMed] [Google Scholar]
- 52.Hughes PM, Wells GM, Clements JM, Gearing AJ, Redford EJ, Davies M, et al. Matrix metalloproteinase expression during experimental autoimmune neuritis. Brain 1998;121(Pt3):481–94 [DOI] [PubMed] [Google Scholar]
- 53.Lapchak PA, Chapman DF, Zivin JA. Metalloproteinase inhibition reduces thrombolytic (tissue plasminogen activator)-induced hemorrhage after thromboembolic stroke. Stroke 2000;31(12):3034–40 [DOI] [PubMed] [Google Scholar]
- 54.Leib SL, Clements JM, Lindberg RL, Heimgartner C, Loeffler JM, Pfister LA, et al. Inhibition of matrix metalloproteinases and tumour necrosis factor alpha converting enzyme as adjuvant therapy in pneumococcal meningitis. Brain 2001;124(Pt 4):1734–42 [DOI] [PubMed] [Google Scholar]
- 55.Rosenberg GA, Kornfeld M, Estrada E, Kelley RO, Liotta LA, Stetler-Stevenson WG. TIMP-2 reduces proteolytic opening of blood brain barrier by type IV collagenase. Brain Res 1992;576(2):203–7 [DOI] [PubMed] [Google Scholar]