Abstract
Context
Pressure ulcers are especially difficult to treat in patients with spinal cord injury (SCI) and recurrence rates are high. Prompted by encouraging results obtained using bone marrow stem cells to treat several diseases including chronic wounds, this study examines the use of autologous stem cells from bone marrow to promote the healing of pressure ulcers in patients with SCI.
Objective
To obtain preliminary data on the use of bone marrow mononuclear cells (BM-MNCs) to treat pressure ulcers in terms of clinical outcome, procedure safety, and treatment time.
Participants
Twenty-two patients with SCI (19 men, 3 women; mean age 56.41 years) with single type IV pressure ulcers of more than 4 months duration.
Interventions
By minimally invasive surgery, the ulcers were debrided and treated with BM-MNCs obtained by Ficoll density gradient separation of autologous bone marrow aspirates drawn from the iliac crest.
Results
In 19 patients (86.36%), the pressure ulcers treated with BM-MNCs had fully healed after a mean time of 21 days. The number of MNCs isolated was patient dependent, although similar clinical outcomes were observed in each case. Compared to conventional surgical treatment, mean intra-hospital stay was reduced from 85.16 to 43.06 days. Following treatment, 5 minutes of daily wound care was required per patient compared to 20 minutes for conventional surgery. During a mean follow-up of 19 months, none of the resolved ulcers recurred.
Conclusions
Our data indicate that cell therapy using autologous BM-MNCs could be an option to treat type IV pressure ulcers in patients with SCI, avoiding major surgical intervention.
Keywords: Pressure ulcers, Mononuclear cells, Stem cell therapy, Spinal cord injury
Introduction
Pressure ulcers arise from ischemic damage to soft tissues induced by unrelieved pressure over a bony prominence. Pressure ulcers are a major health problem for bedridden patients or persons with reduced mobility; individuals with spinal cord injury (SCI) are especially prone to developing pressure ulcers. This disabling condition includes involuntary muscle spasms that can cause shear injuries to tissue, loss of sympathetic tone affecting regional blood flow to tissue, and loss of the sensation that routinely leads to pressure relieving position changes in most people. In patients with SCI, ulcers are often a chronic problem1 and approximately 85% of these patients will develop pressure ulcers sometime during their lives. Among the patients with SCI who develop ulcers, in 11.5% these occur 1 year after injury2 and in 8% they are the cause of death.3
Pressure ulcers are difficult to treat with standard medical therapy (e.g. 5-amino-salicylates or corticosteroids) and often they recur. A four-stage classification system, proposed at the National Pressure Sore Advisory Panel Consensus Development Conference, grades pressure ulcers from least to most severe.4 Superficial stage I and stage II pressure ulcers should be treated conservatively with nutrition support, pressure relief, and local debridement to eliminate tissue that is a good substrate for infection.5,6 Ulcers classified as stage III show cutaneous and subcutaneous tissue involvement and those staged as IV show necrosis of muscles, bones, joints, and surrounding tissues.7,8 Small or superficial wounds can be debrided at the bedside, but debridement in the operating room is often the indicated treatment for stage III and IV ulcers,4 because deep ulcers are difficult to treat and usually have a poor prognosis.9 There are several surgical procedures to achieve pressure ulcer closure, mainly depending on the affected site. Thus, ulcer wounds can be closed using a fasciocutaneous flap, which is the most commonly used method in patients undergoing surgery for the second time, a myocutaneous flap created from surrounding tissue, which is the most effective method, or a more complex muscle flap.10
Given that treatment strategies for pressure ulcers can be both costly and complex,11 in this observational study, we examined the possibility of improving pressure ulcer healing using stem cell therapy. The use of bone marrow stem cells has yielded encouraging results in the treatment of several diseases such as acute myocardial infarction,12 stroke,13 or non-healing chronic wounds.14 Bone marrow mononuclear cells (BM-MNCs) include hematopoietic stem cells, mesenchymal stem cells (MSCs), endothelial progenitor cells, and precursor cells.15 Experimental and clinical studies have shown that BM-MNC transplantation has a neovascularization promoting effect16,17 mainly in ischemic tissues.18 It has been also reported that bone marrow MSCs secrete paracrine factors that could recruit macrophages and endothelial cells to enhance wound healing.19 The repair functions of MSC are thought to involve the secretion of factors such as Vascular Endothelial Growth Factor (VEGF)20 or Fibroblast Growth Factor (FGF)21 which could help prevent apoptosis, promote angiogenesis, assist in matrix reorganization, and increase the recruitment of circulating MSC.22 The findings of a recent study suggest that cooperation between MSCs and the remaining bone marrow cells improve their tissue-renewal or regenerating capacity.23 In this paper, we describe our initial experience with the use of mononuclear cells (MNCs) harvested from autologous bone marrow to treat pressure ulcers in 22 patients with SCI. Our main objective was to obtain preliminary data regarding this new therapy with special attention paid to standardizing the cell therapy procedure and assessing its safety and clinical outcome.
Methods
Patients
The patients enrolled in this study were 19 men and 3 women who on neurological examination had complete SCI based on the ASIA scale A. Mean patient age was 56.41 years (range 29–79 years). All patients had single type IV chronic pressure ulcers at different sites: 13 ischial, 4 sacral, 3 sacral-ischial, 1 ischial-trochanteric, and 1 plantar. Thirteen out of the 22 patients had undergone prior surgery for their ulcers. The inclusion criteria were a lack of response to 4 months of topical treatment and an ulcer size of about 5–6 cm meaning that surgery was usually indicated. Ulcers were required to be free of necrotic tissue and have good granulation tissue without local infection signs before treatment. To achieve this, antibiotic treatment was administered before stem cell therapy based on the microorganisms isolated in the case of positive cultures. Finally, the medical condition of the patient had to be compatible with conventional surgery and postoperative rehabilitation. The characteristics of the patients and their lesions are summarized in Table 1.
Table 1.
Characteristics of the patients treated and their corresponding pressure ulcers
| Patient | Sex | Age (years) | Date of cell therapy | Ulcer site | Ulcer size (cm) | Follow-up (months) |
|---|---|---|---|---|---|---|
| 1 | ♂ | 46 | 22/11/2007 | Ischial | 1 × 7 | 38 |
| 2 | ♂ | 62 | 22/02/2008 | Sacral | 5 × 4 | 35 |
| 3 | ♂ | 56 | 27/03/2008 | Ischial | 3 × 2 | 34 |
| 4 | ♂ | 34 | 11/04/2008 | Ischial | 5 × 3 | 33 |
| 5* | ♂ | 52 | 04/07/2008 | Ischial | 7 × 3 | 30 |
| 6* | ♂ | 51 | 02/10/2008 | Ischial | 3 × 2.5 | 27 |
| 7* | ♂ | 57 | 19/01/2009 | Ischial-trochanteric | 7 × 4 | 24 |
| 8 | ♂ | 54 | 13/03/2009 | Plantar | 5 × 3 | 22 |
| 9* | ♂ | 65 | 13/03/2009 | Ischial | 3 × 1.5 | 22 |
| 10 | ♂ | 60 | 17/06/2009 | Sacral | 1 × 2 | 19 |
| 11 | ♂ | 51 | 24/06/2009 | Ischial | 7 × 5 | 19 |
| 12 | ♂ | 64 | 18/08/2009 | Ischial | 6 × 3.9 | 17 |
| 13 | ♂ | 79 | 10/11/2009 | Ischial | 1 × 1 | 14 |
| 14 | ♂ | 53 | 10/11/2009 | Ischial | 1 × 1.5 | 14 |
| 15 | ♂ | 43 | 04/12/2009 | Ischial | 0.5 × 3 | 13 |
| 16 | ♀ | 51 | 08/01/2010 | Sacral-ischial | 3 × 2 | 12 |
| 17* | ♂ | 74 | 26/02/2010 | Sacral-ischial | 2.4 × 2 | 11 |
| 18 | ♂ | 76 | 07/04/2010 | Ischial | 1.5 × 4 | 9 |
| 19 | ♀ | 73 | 07/04/2010 | Sacral | 3 × 2.2 | 9 |
| 20 | ♂ | 29 | 03/05/2010 | Isquial (2) | 4.5 × 4.5 | 8 |
| 21 | ♂ | 37 | 11/05/2010 | Sacral-ischial (2) | 2 × 1.5 | 8 |
| 22 | ♀ | 74 | 28/06/2010 | Sacral | 1 × 3 | 7 |
*These patients required a second cell therapy intervention.
Preoperative assessment included a complete clinical examination, a blood test to determine nutritional status and oral protein support given if the biochemistry results indicated nitrogen deficiency, and an image study consisting of nuclear magnetic resonance (NMR) or computed tomography (CT) depending on hospital availability to determine lesion size, bone state, and calcifications. Prior to surgery, the patients were prepared to support the prone position by adopting this position at home or at the hospital each day for progressively longer time periods. All ulcers were photographed preoperatively.
All procedures were performed at the Hospital Universitario Central de Asturias (Spain). The therapeutic protocol was approved by the Hospital Ethics Committee in accordance with Spanish law: all patients signed a detailed informed consent form prior to intervention, and also gave their consent for publication of the study results. This study was conducted in accordance with the ethical standards of the Helsinki Declaration (1975).
Isolation of BM-MNCs
About 3 hours before cell therapy, autologous bone marrow (50–60 ml) was obtained by posterosuperior iliac crest aspiration under topical anesthesia (Mepivacaine 2%, B. Braun, Melsungen, Germany) using a heparin-rinsed trocar and syringe (Mayne heparin 25000 IU, CHI, USA). MNCs were isolated from the bone marrow aspirate on a Ficoll density gradient (Biocoll separating solution, 1.077 g/ml, Biochrom AG, Berlin, Germany) by centrifuging for 25 minutes at 400 g, 20°C. After washing twice in RPMI medium (Mediatech, VA, USA), the cells were finally resuspended in a heparinized (20 IU/ml) saline solution (Viaflo sodium chloride 0.9%, Baxter International Inc., Deerfield, IL, USA) until a final volume of 14 ml. Next, 10 ml of this cell suspension were introduced in a sterile syringe and transferred to the operating room; the remaining 4 ml were used for cell counts, sterility tests, and a small volume was deposited in the cell library. The entire procedure was performed according to Good Manufacturing Practices, establishing a required viability of 95% and minimum number of 60 million cells.
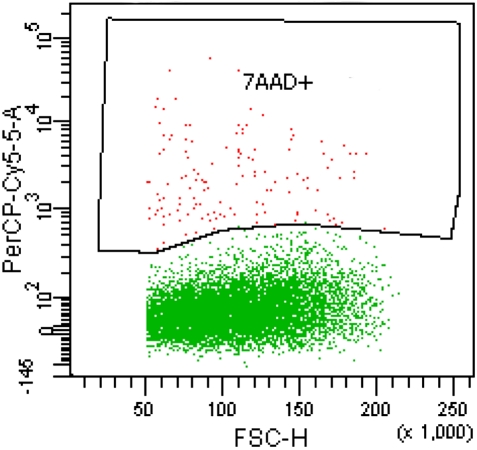
An aliquot (500 µl) of the final MNC preparation was processed in a ADVIA120 Haematology System (Bayer Healthcare, Leverkusen, Germany) for cell enumeration. Cell viability was determined by flow cytometry (FACSAria analyzer and FACSDiva v6.1.3 software, Becton Dickinson, Franklin Lakes, NJ, USA) using the 7-actynomicin D staining procedure (Becton Dickinson) (Fig. 1). Sterility tests served to confirm the absence of bacterial contamination of the final product by Gram staining and inoculating thioglycollate medium (Biomedics, Biomérieux, Marcy l'Etoile, France) to track bacterial growth.
Figure 1.
Flow cytometry image obtained after gating with 7-actynomicin D in order to eliminate non-viable cells.
Operating room
Cell infusion was performed in the operating room using an aseptic technique. The plastic surgeon washed the bursa repeatedly with saline solution (Viaflo sodium chloride 0.9%, Baxter International Inc.) to eliminate all traces of topical treatment and debrided the wound. Without removing the bursa, the ulcer margins were revitalized and the wound closed by a purse-string 2/0 silk suture (Vicryl, Ethicon, Somerville, NJ, USA) to create a pouch into which the cell suspension was injected using an Abocath 14–16-calibre catheter or 140 mm 14-caliber catheter for deeper wounds (Becton Dickinson). During the intervention, specimens were obtained for culture to test for the presence of microorganisms.
Follow-up
Mean follow-up for the 22 patients was 19.32 months, range 7–38 months. After the intervention, patients were required to adopt a prone position for 3 weeks to avoid wound contact. After these 3 weeks, movement was gradually increased. Follow-up sessions were conducted 1, 3, 6 months and 1 year after cell therapy, including image studies (NMR or CT scans) at 6 months and 1 year to assess wound progress. All ulcers were also photographed postoperatively.
Results
In this group of 22 patients treated with cell therapy, full healing was observed in 19 of the 22 ulcers for a resolution rate of 86.36% (Fig. 2). Ulcer resolution by the number of cell infusions administered was 70.37% since some patients required a second intervention following suture dehiscence. In these five cases (patients 5, 6, 7, 9, and 17), the second cell infusion was, respectively, performed 22 days, 1 month, 1 month, 7 months, and 4 months after the first intervention. In the 17 patients who did not need a second intervention, wound closure was achieved 3 weeks (average 21 days) after cell therapy. As soon as the wound had healed, the patients were started on a wheelchair program and approximately 1 month later they were discharged from the Plastic Surgery Unit, making the mean length of hospital stay postsurgery 43.06 days. In three patients (patients 4, 7, and 8), a delay in surface wound repair was observed at the ulcer margins. In a further three patients (patients 5, 10, and 19), the pressure ulcer did not respond to the treatment probably because of their underlying diseases: diabetes mellitus, hypertension, and cardiopathy, respectively. NMR and CT imaging confirmed the complete disappearance of the ulcer in the 19 patients (see Figs 3 and 4). Only one patient (patient 11) had the postoperative complication of bleeding from the wound 24 hours after the intervention. This patient underwent hemostatic treatment and the wound was cauterized and closed by suture. The number of MNCs infused in each intervention was 264.57 × 106 ± 70.71 (mean ± standard error). Cell viability was ≥95% for all the bone marrow extracts (Fig. 1). There were no cases of infection postsurgery and none of the 19 patients whose ulcers had resolved underwent ulcer recurrence during follow-up. The mean time to suture removal following surgery was 18 days (range 15–21 days). Postoperative wound care was topical treatment with 5% aqueous chlorhexidine daily. This procedure took a mean time of 5 minutes per patient.
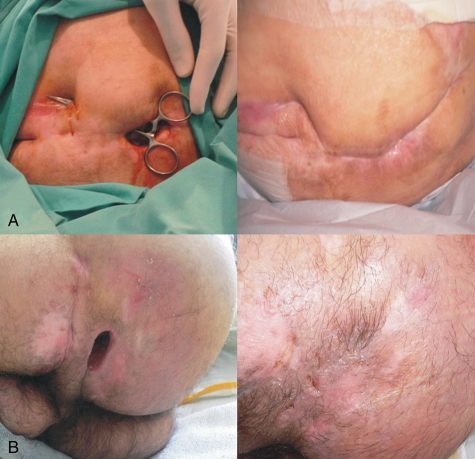
Figure 2.
Pressure ulcers before and after autologous MNC therapy. (A) Patient 2, images taken before (left) and after treatment (right). (B) Patient 4, images taken before (left) and after (right) treatment.
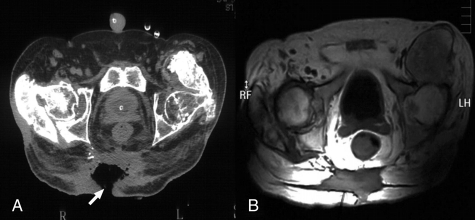
Figure 3.
RNM images obtained before and after autologous MNC therapy. Patient 2, images taken before (A) and 6 months after (B) treatment. Arrows point to the pressure ulcer.
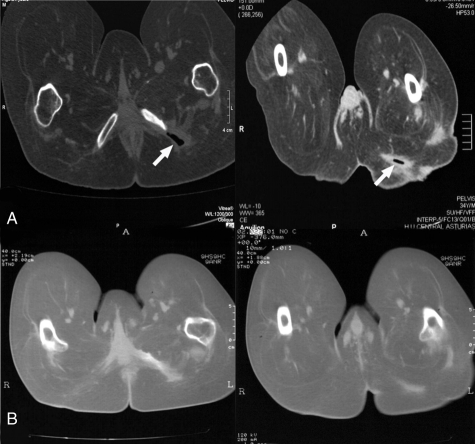
Figure 4.
RNM images obtained before and after autologous MNC therapy. Patient 4, images taken before (A) and 6 months after (B) treatment. Arrows point to the pressure ulcer.
Discussion
Pressure ulcers are a common yet still challenging problem in patients with SCI because they are difficult to treat with standard medical therapy and they often recur. Worldwide recurrence rates for pressure ulcers in SCI patients have been estimated at 80%.24 Pressure ulcers also increase length of hospital stay and the cost of treatment but, most importantly, they have devastating effects on quality of life.25 In current clinical practice, deep pressure ulcers are candidates for surgery although in some patients surgery is contraindicated because of underlying disease.26 In effect, patients with SCI are not optimal candidates because ulcers often recur after surgery due to their spinal injury or other patient factors, and there may also be a lack of tissue due to prior surgical interventions and/or infection. Complications during the immediate postoperative course have been also remarkably high, particularly in patients with SCI.27 Prompted by the successful use of bone marrow stem cells to treat several conditions,12–14 we decided to assess this alternative therapy for pressure ulcers in patients with SCI.
The use of autologous BM-MNCs was designed to avoid problems of immunological rejection. The BM-MNCs isolation procedure took about 3 hours so that the whole intervention could be completed in a morning. Cell viability determined in the 27 bone marrow aspirates was consistently high at ≥95%, supporting the efficacy of our cell isolation method. In almost all the patients treated (86.36%) good wound healing was achieved in a maximum time of 3 weeks and confirmed by imaging techniques revealing the complete closure of the pressure ulcer. Five patients (5, 6, 7, 9, and 17) required a second intervention as a consequence of suture dehiscence. As a limitation of our study, we should mention the variation among the 27 extracts in the number of isolated MNCs that was patient dependent. However, the results observed in terms of ulcer healing were independent of the number of cells infused. In three patients, cell therapy failed to resolve the ulcer probably due to their underling diseases: diabetes, hypertension, and cardiopathy, respectively. Another limitation is the lack of a control group although our prior experience with this type of patient is that following debridement and closure of the ulcer without using stem cells, 100% of trochanteric and 30% of ischial ulcers recur within 1 year.28 Although direct suture to achieve closure is the simplest way of managing a pressure ulcer, this frequently leads to wound dehiscence29 and this method is today seldom used except for small, superficial ulcers.30 Several authors, such as Sorensen26 or Conway,31 have reported negative results with the direct closure method and thus advocate surgical treatment for sacral, trochanteric, and ischial ulcers.
BM-MNCs can be easily obtained in large numbers by aspiration without extensive manipulation or cultivation before transplant and cells can be transplanted directly without in vitro expansion.32 Using the entire mononuclear fraction, no potentially beneficial cell type is omitted and the functional response will depend on the equilibrium between cell types.33 In contrast, before their uses for cell therapy, autologous MSCs need to be cultured and this determines contamination risks and treatment delay, which increase treatment costs. Moreover, the culture process may interfere with optimal transplantation protocols that requires acute or subacute delivery for best possible results.34
MNCs from a patient's own bone marrow promote angiogenesis35 and this seems to be a key factor for optimal healing of skin wounds.19 Recent studies have identified the role of bone marrow cells in host defence and inflammatory processes in the skin, including wound healing.36,37 Improved skin flap survival with BM-MNC transplantation has recently been reported in a rat model.38 Moreover, bone marrow MSCs, which make up a small proportion of BM-MNCs, secrete paracrine factors that could recruit macrophages and endothelial cells to enhance wound healing.19 The repair functions of MSC are thought to involve the secretion of factors such as VEGF20 or FGF,21 which could help prevent apoptosis, promote angiogenesis, assist in matrix reorganization, and increase the recruitment of circulating MSCs.22 In addition, the findings of a recent study suggest that cooperation between MSCs and the remaining bone marrow cells improve their tissue-renewal or regenerating capacity.23
The intervention proposed has two major advantages in terms of nursing workload and costs. Thus, mean hospital stay after stem cell therapy was significantly reduced from the mean value for conventional surgery of 85.16 days recorded at our center to 43.06 days, and the mean daily time of wound care required postsurgery was 5 minutes compared to 20 minutes for conventional surgery. Moreover, the simplicity of this method means it is indicated for use in patients in whom anesthesia and surgery are contraindicated. Based on the present preliminary observational data regarding the use of stem cells to treat pressure ulcers, we are now in a position to design a Phase I/II clinical trial to confirm these promising findings.
Conclusion
The findings of our study indicate that the use of autologous BM-MNCs plus surgical debridement of the wound could be a feasible option for the treatment of type IV pressure ulcers. The stem cell therapy proposed could avoid major surgical intervention, especially if these sores have not responded to conservative and/or conventional surgical treatment.
Acknowledgments
We would like to thank to M A Blanco Gelaz for his technical support in the flow cytometry assay. This work was supported by the Obra Social Cajastur and the project grant FICYT PEST08–12.
References
- 1.Kierney PC, Engrav LH, Isik FF, Esselman PC, Cardenas DD, Rand RP. Results of 268 pressure sores in 158 patients managed jointly by plastic surgery and rehabilitation medicine. Plast Reconstr Surg 1998;102(3):765–72 [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, DeVivo MJ, Jackson AB. Pressure ulcer prevalence in people with spinal cord injury: age-period-duration effects. Arch Phys Med Rehabil 2005;86(6):1208–13 [DOI] [PubMed] [Google Scholar]
- 3.Byrne DW, Salzberg CA. Major risk factors for pressure ulcers in spinal cord disabled: a literature review. Spinal Cord 1996;34(5):255–63 [DOI] [PubMed] [Google Scholar]
- 4.Bergstrom N, Allman RM, Bennett MA. ‘Treatment of pressure ulcers: clinical practice guideline’, ‘Treatment of pressure ulcers’, US Dept of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research, Rockville, MD, 1994. ‘Clinical practice guideline’. Technical Report N°15. AHCPR Publication 96-N014–96-N015 [Google Scholar]
- 5.Agren M, Strömberg HE. Topical treatment of pressure ulcers: a randomized comparative trial of Varidase and zinc oxide. Scand J Plast Reconstr Surg 1985;19(1):97–100 [DOI] [PubMed] [Google Scholar]
- 6.Galpin JE, Chow AW, Bayer AS, Guze LB. Sepsis associated with decubitus ulcers. Am J Med 1976;61(3):346–50 [DOI] [PubMed] [Google Scholar]
- 7.Thompson D. A critical review of the literature on pressure ulcer aetiology. J Wound Care 2005;14(2):87–90 [DOI] [PubMed] [Google Scholar]
- 8.Cannon BC, Cannon JP. Management of pressure ulcers. Am J Health-Syst Pharm 2004;61(18):1895–905 [PubMed] [Google Scholar]
- 9.Mortenson WB, Miller WC. A review of scales for assessing the risk of developing a pressure ulcer in individuals with SCI. Spinal Cord 2008;46(3):168–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maslauskas K, Samsanavicius D, Rimdeika R, Kaikaris V. Surgical treatment of pressure ulcers: an 11-year experience at the department of plastic and reconstructive surgery of Hospital of Kaunas University of Medicine. Medicina Kaunas 2009;45(4):269–75 [PubMed] [Google Scholar]
- 11.Reddy M, Gill SS, Kalkar SR, Wu W, Anderson PJ, Rochon PA. Treatment of pressure ulcers: a systematic review. JAMA 2008;300(22):2647–62 [DOI] [PubMed] [Google Scholar]
- 12.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev 2005;85(4):1373–416 [DOI] [PubMed] [Google Scholar]
- 13.Nakano-Doi A, Nakagomi T, Fujikawa M, Nakagomi N, Kubo S, Lu S, et al. Bone marrow mononuclear cells promote proliferation of endogenous neural stem cells through vascular niches after cerebral infarction. Stem Cells 2010;28(7):1292–302 [DOI] [PubMed] [Google Scholar]
- 14.Wu Y, Wang J, Scott PG, Tredget EE. Bone marrow-derived stem cells in wound healing: a review. Wound Repair Regen 2007;15Suppl. 1:S18–26 [DOI] [PubMed] [Google Scholar]
- 15.Taylor DA, Zenovich AG. Cardiovascular cell therapy and endogenous repair. Diabetes Obes Metab 2008;10Suppl. 4:5–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uitterdijk A, Groenendijk BC, van Der Giessen WJ. Stem cell therapy for chronic heart failure. Hellenic J Cardiol 2009;50(2):127–32 [PubMed] [Google Scholar]
- 17.Zhang H, Zhang N, Li M, Feng H, Jin W, Zhao H, et al. Therapeutic angiogenesis of bone marrow mononuclear cells (MNCs) and peripheral blood MNCs: transplantation for ischemic hindlimb. Ann Vasc Surg 2008;22(2):238–47 [DOI] [PubMed] [Google Scholar]
- 18.Saito Y, Sasaki K, Katsuda Y, Murohara T, Takeshita Y, Okazaki T, et al. Effect of autologous bone-marrow cell transplantation on ischemic ulcer in patients with Buerger's disease. Circ J 2007;71(8):1187–92 [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 2008;3(4):e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang YL, Zhao Q, Zhang YC, Cheng L, Liu M, Shi J, et al. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept 2004;117(1):3–10 [DOI] [PubMed] [Google Scholar]
- 21.Ono I, Yamashita T, Hida T, Jin HY, Ito Y, Hamada H, et al. Local administration of hepatocyte growth factor gene enhances the regeneration of dermis in acute incisional wounds. J Surg Res 2004;120(1):47–55 [DOI] [PubMed] [Google Scholar]
- 22.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006;98(5):1076–8416619257 [Google Scholar]
- 23.Urbán VS, Kiss J, Kovács J, Gócza E, Vas V, Monostori E, et al. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells 2008;26(1):244–53 [DOI] [PubMed] [Google Scholar]
- 24.Bansal C, Scott R, Stewart D, Cockerell CJ. Decubitus ulcers: a review of the literature. Int J Dermatol 2005;44(10):805–10 [DOI] [PubMed] [Google Scholar]
- 25.Srivastava A, Gupta A, Taly AB, Murali T. Surgical management of pressure ulcers during impatient neurologic rehabilitation: outcomes for patients with spinal cord disease. J Spinal Cord Med 2009;32(2):125–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sørensen JL, Jørgensen B, Gottrup F. Surgical treatment of pressure ulcers. Am J Surg 2004;188Suppl. 1A:42–51 [DOI] [PubMed] [Google Scholar]
- 27.Disa JJ, Carlton JM, Goldberg NH. Efficacy of operative cure in pressure sore patients. Plast Reconstr Surg 1992;89(2):272–8 [DOI] [PubMed] [Google Scholar]
- 28.González Sarasua J, Pérez Arias A, Fueyo Lorente A, Arranz JL, Alzaga FB, Campano JG. Selección de técnicas quirúrgicas y prevención de las complicaciones en el tratamiento de las úlceras por presión. Cirugía Plástica Ibero-Latinoamericana 1992;XVIII(4):411–8 [Google Scholar]
- 29.Casas LA, Lewis VL., Jr A reliable approach to the closure of large acquired midline defects of the back. Plast Reconstr Surg 1989;84(4):632–41 [PubMed] [Google Scholar]
- 30.Anthony JP, Huntsman WT, Mathes SJ. Changing trends in the management of pelvic pressure ulcers: a 12-year review. Decubitus 1992;5(3):44–7, 50–1. [PubMed] [Google Scholar]
- 31.Conway H, Griffith BH. Plastic surgery for closure of decubitus ulcers in patients with paraplegia; based on experience with 1,000 cases. Am J Surg 1956;91(6):946–75 [DOI] [PubMed] [Google Scholar]
- 32.Minnerup J, Seeger FH, Kuhnert K, Diederich K, Schilling M, Dimmeler S, et al. Intracarotid administration of human bone marrow mononuclear cells in rat photothrombotic ischemia. Exp Transl Stroke Med 2010;2(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathieu M, Bartunek J, El Oumeiri B, Touihri K, Hadad I, Thoma P, et al. Cell therapy with autologous bone marrow mononuclear stem cells is associated with superior cardiac recovery compared with use of nonmodified mesenchymal stem cells in a canine model of chronic myocardial infarction. J Thorac Cardiovasc Surg 2009;138(3):646–53 [DOI] [PubMed] [Google Scholar]
- 34.Samdani AF, Paul C, Betz RR, Fischer I, Neuhuber B. Transplantation of human marrow stromal cells and mono-nuclear bone marrow cells into the injured spinal cord: a comparative study. Spine (Phila Pa 1976) 2009;34(24):2605–12 [DOI] [PubMed] [Google Scholar]
- 35.Kamata Y, Takahashi Y, Iwamoto M, Matsui K, Murakami Y, Muroi K, et al. Local implantation of autologous mononuclear cells from bone marrow and peripheral blood for treatment of ischaemic digits in patients with connective tissue diseases. Rheumatol Oxford 2007;46(5):882–4 [DOI] [PubMed] [Google Scholar]
- 36.Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, et al. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells 2004;22(5):812–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng W, Han Q, Liao L, Li C, Ge W, Zhao Z, et al. Engrafted bone marrow-derived flk-1+. mesenchymal stem cells regenerate skin tissue. Tissue Eng 2005;11(1–2):110–9 [DOI] [PubMed] [Google Scholar]
- 38.Yang M, Sheng L, Li H, Weng R, Li QF. Improvement of the skin flap survival with the bone marrow-derived mononuclear cells transplantation in a rat model. Microsurgery 2010;30(4):275–81 [DOI] [PubMed] [Google Scholar]