Abstract
Ultrasound-based molecular imaging employs targeted microbubbles to image vascular pathology. This approach also has the potential to monitor molecularly targeted microbubble-based drug-delivery. We present an image-guided drug-delivery technique that uses multiple pulses to translate, image and cavitate microbubbles in real-time. This technique can be applied to both imaging of pathology in large arteries (sizes and flow comparable to those in humans), and guiding localized drug delivery in blood vessels. The microbubble translation (or pushing) efficacy of this technique was compared in a variety of flow media: saline, viscous saline (4 cp) and bovine blood. It was observed that the performance of this approach was marginally better (by 6, 4 and 2 dB) in viscous saline than in bovine blood with varying levels of hematocrit (40%, 30% and 10%). The drug delivery efficacy of this technique was evaluated by in vitro and ex vivo experiments. High-intensity pulses mediated fluorophore (DiI) deposition on endothelial cells (in vitro) without causing cell destruction. Ex vivo fluorophore-delivery experiments conducted on swine carotids of 2 and 5 mm cross-section diameter demonstrated a high degree of correspondence in spatial localization of the fluorophore-delivery between the ultrasound and composite fluorescence-microscopy images of the arterial cross-sections.
Keywords: Radiation force, drug delivery, real-time, molecular imaging, microbubbles
Introduction
Atherosclerosis is a progressive occlusive disease of the arterial vessels, and is the leading cause of death occurring from cardiovascular diseases. In the US, cardiovascular diseases are responsible for more than 1.7 million deaths annually (1). Current methods of imaging atherosclerosis in widespread clinical practice are restricted to detecting the anatomical features of macroscopic plaques– i.e. disease at a relatively late stage. However, it is well established that the early molecular markers of atherosclerosis may be expressed decades before this disease manifests itself at the scale at which current imaging technologies can detect (2). Imaging the molecular expressions of this arterial disease may help in early detection, thus facilitating more effective early therapy or lifestyle changes. In later stages, this approach may also help in characterizing different plaques for the purpose of treatment guidance or assessing the response to therapy.
Ultrasound based imaging with molecularly targeted microbubbles has been established in the literature as an effective approach for imaging the molecular expressions of pathology on the vascular endothelium in vivo and ex vivo (3–6). Additionally, in the past decade the therapeutic applications of microbubbles have emerged, motivated by the need for a focal drug delivery/gene delivery agent (7–13). Controlled oscillation of microbubbles under the influence of acoustic insonation causes transient changes in the permeability of cell membranes, enabling the transport of the extracellular molecules into the cytoplasm of viable endothelial/smooth muscle cells (EC/SMC) (14). An ultrasound-based real-time approach that combines molecular imaging and drug delivery may provide clinicians with a valuable method for diagnosing and treating (under image guidance) vascular diseases within a single procedure. Nevertheless, there are several issues that need to be addressed before such an approach can be formulated and applied to drug delivery. Some of these issues are discussed and addressed in this work.
It has been suggested by Jayaweera et al. (15) that the rheological behavior of microbubbles is similar to that of red blood cells (RBC) and hence microbubbles tend to travel along the central axis of the blood vessel. In our observation, while RBCs tend to travel along the central axis of the blood vessel, microbubbles remain buoyant even in the case when they are flowing through vessels (16). This selectively reduces the probability of microbubbles establishing contact with ECs on the lower wall of the blood vessels, thus impeding drug delivery. One solution to this problem is applying acoustic radiation force for guiding microbubbles (9, 10, 17). Zhao et al. (18) and Rychak et al.(19, 20) have successfully demonstrated the value of applying radiation force in small capillaries in vitro and in vivo. Gessner et al.(21) and Needles et al.(22) demonstrated a real-time application of radiation force-enhanced targeted imaging in small capillaries, while Patil et al. applied real-time sequences for simultaneously guiding and imaging microbubbles in vitro in large vessels (~2 mm) with flow velocities up to 15.9 cm/s. Those experiments, however, used saline as a flow medium, which in our observations lead to significantly different results from those obtained using blood (with varying hematocrit) as a flow medium. Thus, choice of flow media affects the efficacy of any approach used for drug delivery in vivo.
Application of ultrasound-assisted and microbubble-based drug delivery has been studied by different groups in vivo and ex vivo (14, 23, 24). However, those experiments were conducted without any real-time feedback or spatio-temporal monitoring of fluorophore or drug delivery. In this work, we introduce a modified real-time approach for selectively imaging and guiding the microbubbles in order to monitor/enhance drug delivery in large blood vessels. Furthermore, we quantify the performance of this method between varying blood (flow medium) hematocrit. Specifically, we conducted experiments to: a) Compare the performance of the proposed modified approach in saline with varying viscosity (1 cp and 4 cp) and in blood with varying hematocrit (10%, 30% and 40 %); b) Identify parameters in vitro to enable drug delivery without cell destruction; c) Evaluate the ability of our approach to perform selective imaging of adherent microbubbles and enable directed fluorophore/drug deposition at a desired location in ex vivo arteries (varying diameters) with flowing saline and blood. We also demonstrate that a real-time image guided drug delivery can be performed at diagnostic mechanical index (MI =< 1.9) and negligible spatial peak temporal average intensities (ISPTA) on clinical ultrasound scanners with minimal modifications implying the potential applicability of this approach in a clinical setting.
Methods
Real-time dual frequency imaging-drug delivery sequence
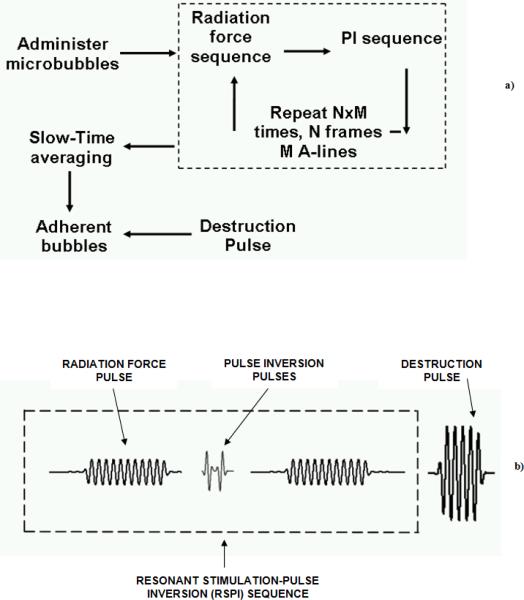
The flow diagrams of the proposed real-time imaging and drug delivery sequence are illustrated in Figures 1a and 1b. A twenty cycle low frequency pulse (centered at 4 MHz) with a high pulse repetition frequency (PRF) is interlaced between a series of single cycle high frequency (8 MHz) and low PRF pulse inversion (PI) pulses. The frequency of the radiation force pulse should typically match the resonance frequency of the microbubble in order to optimize the translational displacement and backscatter of the microbubble (17). The resonance frequency of the microbubble population (2.2 μm mean diameter) used in this work is approximately 4 MHz. (16, 25). The intermittent PI pulses provide high-resolution tracking of the temporal accumulation of microbubbles. By summing the echo responses of noninverted/inverted transmit signal sequences, high microbubble signal specificity is achieved (25). Note that the Mechanical Index (MI, MI = Peak Negative Pressure in MPa / √(Center Frequency in MHz)) of the radiation force pulse is always maintained below 0.2 in order to minimize microbubble destruction by ultrasound. Since equal peak negative pressures are used for imaging and pushing, the imaging MIs are significantly lower due to the higher imaging frequency.
Figure 1.
a) Flow diagram of the real-time algorithm used for selective image-guided drug delivery, b) Schematic of the pulse sequence used in the real-time imaging-drug delivery algorithm.
The received radio frequency (RF) PI data are slow-time filtered using a 10 tap infinite impulse response (IIR) filter. In slow-time filtering operation, a low pass filter is applied in the time direction over a fixed number of frames. Slow-time filtering retains the signal from static microbubbles and largely cancels the signal scattered by flowing microbubbles (5, 16, 26, 27). This allows selective imaging of static or adherent microbubbles in real-time (8 –10 Hz frame rate). At the end of this sequence, a high MI (MI = 1.0), high PRF destruction pulse is directed to the static or adherent microbubbles thus facilitating local microbubble destruction and a concomitant fluorophore delivery.
Programmable ultrasound scanner
An Ultrasonix RP (Richmond, BC, Canada) programmable research ultrasound scanner with a high bandwidth array transducer (4L12) was used in this study. Gaussian pulses with a programmed bandwidth and PRF can be generated using the scanner. This scanner provides focused and unfocused data with fixed transmit focusing and dynamic receive focusing. The transducer has a center frequency of 8 MHz and −6 and −10 dB fractional bandwidths of 80% and 110 %, respectively. This bandwidth allows varying transmit and receive frequencies between 4 and 12 MHz.
In vitro and ex vivo sample preparation
An agar/gelatin channel flow phantom was used in flow experiments to assess the performance of our technique in saline with different viscosities and in blood with varying levels of hematocrit. An overview of preparing gelatin-agar based wall-less phantoms is detailed in a paper by Patil et al. (16).
Experiments were conducted to determine the ability of our technique to deliver a potential drug to endothelial cells without destroying them. DiI (1,1',di-octadecyl-3,3,3'3'-tetramethylindocarbocyanine perchlorate, Molecular Probes, Eugene OR) was used as a model compound to mimic drug-delivery. SVEC4-10 endothelial cells were grown in the OptiCell cell culture system (Thermo Fisher Scientific, Rochester, NY) with 10% fetal bovine serum (FBS) in Dulbecco's Modified Eagle Medium (DMEM, Invitrogen, Carlsbad, CA). Plating density was 2 × 104 cells/cm2. Cells were cultured for 24 hours and allowed to reach approximately 80% confluence.
Swine carotids were used for ex vivo experiments testing fluorophore delivery under flow. Common carotid artery specimens were excised from recently sacrificed, farm-raised swine specimens. The arteries were excised proximal to the carotid bifurcation and ranged in size from 2 and 5 mm in diameter. Arteries were immediately stored in a physiological saline solution (PSS) containing Ca2+, Mg2+ phosphate buffered saline, MOPS, 2 μM EDTA, glucose and antibiotics at 4°C. The majority of connective tissue was removed from the outside of the artery. The arteries were sutured to the end of plastic tubing and immersed in a saline bath.
Microbubble preparation
Biotinylated lipid microbubbles with DiI fluorophore incorporated into the shell, and a non-fluorescent biotinylated microbubble of similar diameter (Targestar-B; Targeson Inc, San Diego, CA) were used in this study. Preparation and use of these microbubbles has been documented in depth by Klibanov et al. (3). Briefly, phosphatidylcholine biotin-PEG-lipid (Avanti Lipids, Alabaster, AL) and PEG stearate (Sigma-Aldrich, St. Louis, MO) were dispersed in saline. Fluorescent microbubbles were prepared with the inclusion of a small amount of DiI. The micellar lipid dispersion was then sonicated in the presence of gaseous decafluorobutane (F2 Corporation, Lancashire, UK). After preparation, the microbubble dispersion was washed with saline and aliquotted into glass vials with a decafluorobutane headspace.
Experimental design and apparatus
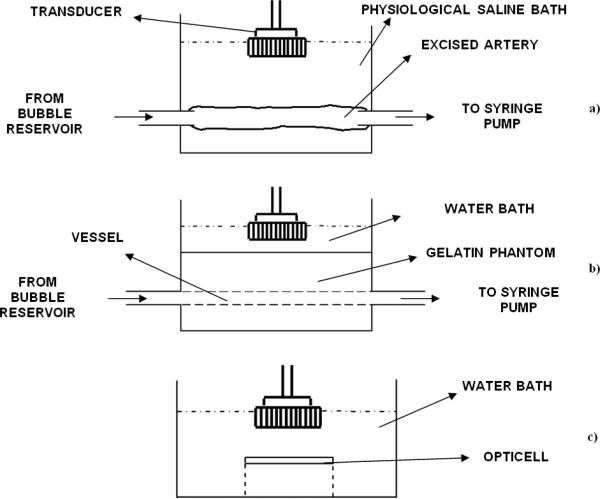
Table 1 lists the acoustic parameters and Table 2 lists the flow and the microbubble parameters used in the reported experiments. For the ex vivo carotid artery experiment, the swine carotid artery was immersed into a physiological saline bath and the ends were sutured to plastic tubing (Figure 2a). Microbubbles were drawn into the tubing and artery using a programmable syringe pump (PHD 2000, Harvard Apparatus, Holliston, MA, USA). The imaging and drug delivery sequences were applied using the Ultrasonix RP scanner. A wall-less phantom enclosing a 2.3 mm cross section diameter vessel was used for the flow experiments testing the effects of viscosity (Figure 2b). The interior of the gelatin phantom vessel channel was incubated with 0.05 mg/ml of streptavidin overnight and flushed with albumin before conducting experiments. For the static fluorophore delivery experiments the OptiCell was mounted on a plastic stand and the entire assembly was immersed in a 37° C water bath (Figure 2c).
Table 1.
Acoustic parameters used in various experiments.
| Radiation force pulse | Frequency = 4 MHz, 20 cycle, Pulse repetition frequency (PRF) = 42 kHz, MI =0.2, ~90 pulses per A-line |
| Imaging pulse | Frequency = 8 MHz, single cycle, Pulse repetition frequency (PRF) = 5 kHz, MI < 0.2, 2 pulses per A-line |
| Destruction pulse | Frequency = 4 MHz, 10 cycles, Pulse repetition frequency (PRF) = 42 kHz, MI= 1, ~90 pulses per A-line |
Table 2.
Wall-less flow phantom, Opticell and ex vivo swine carotid experiments (flow and microbubble parameters)
| Flow Phantom(streptavidin coated channel) | ex vivo swine carotid | OptiCell | |
|---|---|---|---|
| Microbubble conc. | 2 ×106/ml | 20 ×106/ml | 20 ×106/ml |
| Flow | 10 cm/s | 10 cm/s (approx.) | - |
| Hematocrit | 10%, 30% and 40% | 30 % (Whole Blood) | - |
| Saline | 1 cp and 4 cp | 1 cp | - |
| Vessel diameter | 2.3 mm | 2 and 5 mm | - |
| Microbubble type | Biotin | Dil | Dil |
Figure 2.
Schematic of the apparatus used in various experiments reported in this work, a) Experiment with ex vivo swine carotid artery, b) Wall-less flow phantom based experiments for comparing the efficacy of the algorithm in saline with varying viscosity and blood with varying hematrocrit, c) In vitro experiments with cultured endothelial cells.
Results
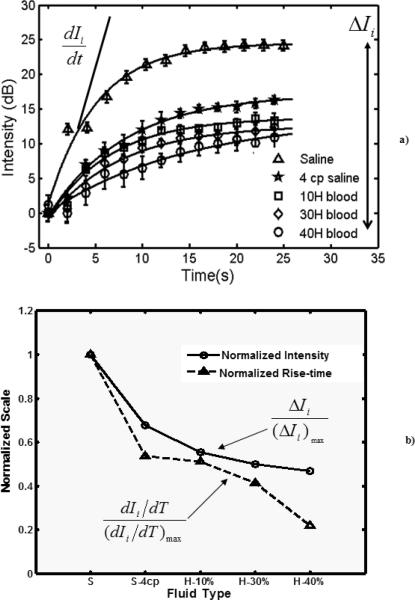
The temporal variation in the adherent microbubble signal from the distal (lower) vessel wall during the application of the selective molecular imaging sequence is presented in Figure 3a Signal from microbubbles in 5 different media (1 cp and 4 cp saline and blood with 10%, 30%, and 40% hematocrit) were plotted with the error bars indicating one standard deviation (n = 20). dIi/dt is the rate of change in the plotted signal (adherent microbubbles) and ΔIi indicates the absolute change in the plotted signal. An exponential model was superimposed on the experimental data (r2>0.99). The rate of change in signal and the absolute intensities for the various flow media were normalized using the highest values obtained from the saline curve and plotted in Figure 3b.
Figure 3.
a) Temporal variation in the signal obtained from static (or adherent) biotin microbubbles at the far wall of the streptavidin coated vessel in the flow phantom. The exponential model is superimposed on the experimental data. Radiation force was applied using a 20 cycle pulse with center frequency of 4 MHz and MI < 0.2. Imaging was performed at 8 MHz using single cycle pulse inversion pulses. The flow velocity of the fluid through the 2.3 mm cross-section vessel was 10 cm/s. The microbubble concentration was 2.0 × 106 /ml. The saline viscosity was maintained at 1 cp and 4 cp. The blood hematocrit was varied in steps from 10% and 30% to 40%. The mean and standard deviation were obtained over 20 independent renditions; b) Normalized absolute intensities and the maximum rate of change of the stationary (adherent) microbubble signal in Figure 3a. Note: S-Normal saline, S-4cp: Saline with viscosity 4 cp, H: Blood Hematocrit.
Composite bright field and fluorescence images of the SVEC4-10 endothelial cells insonated in the presence of DiI microbubbles (MI = 1) enabled verification of fluorophore delivery to endothelial cells (Figure 4). No significant difference was observed in the fluorescence signal between cells insonated at MI = 1.0 and MI = 1.9. No cell death was observed at MI= 1.0 and 1.9 (see Appendix I).
Figure 4.
Superimposed optical and fluorescence image of the SVEC4-10 endothelial cells. The endothelial cells were incubated with DiI microbubbles for 3 minutes and subjected to a destructive ultrasound sequence (4 MHz, 20 cycle, 42 kHz PRF and MI=1) for 3 minutes. The endothelial cells were washed and imaged under the microscope.
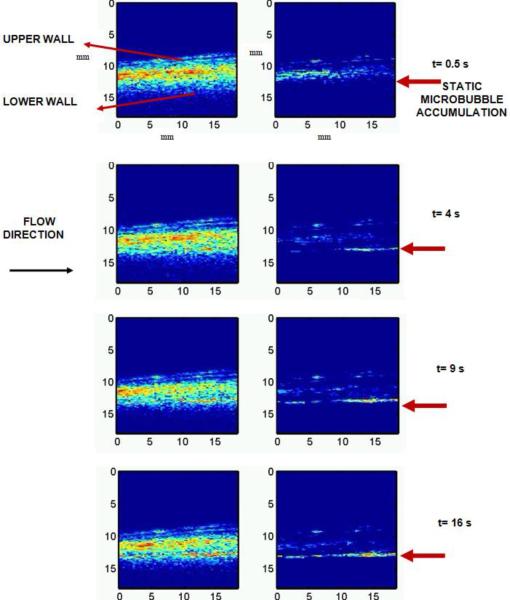
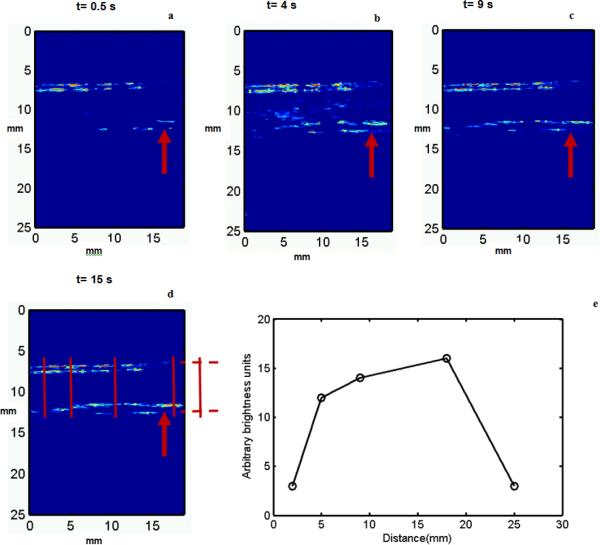
Ultrasound imaging data obtained during the image-guided drug delivery sequence enabled non-invasive verification of the retention of microbubbles to the endothelium (Figure 5). Data illustrate the presence of microbubbles in the vessel by the PI sequence (Figure 5, left), as well as the adherent bubbles only, determined after slow-time filtering (Figure 5, right). Saline was the flow media in this experiment and the flow direction was from the left to right of the images. The adherent microbubbles accumulated downstream of the flow at the lower or distal wall of the carotid artery. This spatial pattern of accumulation can be attributed to the effects of the fluid velocity and the radiation force exerted by the pulse on the microbubble. .At the end of 16 s, the destruction sequence was applied and the adhered DiI loaded microbubbles were destroyed.
Figure 5.
Images obtained from the selective molecular imaging sequence with (R) and without (L) slow-time filtering of the received pulse inversion data. The flow velocity of saline flowing through the ex vivo artery was approximately 10 cm/s. The concentration of the microbubbles was 20 × 106 /ml. Imaging was performed at 8 MHz (single cycle pulses) and radiation force was applied using 20 cycle 4 MHz pulses at PRF of 42 kHz (MI=0.2). For destroying microbubbles a MI of 1 was used with all other parameters similar to that of the radiation force pulse. The dynamic range of the images is 25 dB and the direction of flow of microbubbles is from left to the right of the image. The field of view is 18 × 18 mm.
Composite bright field and fluorescence images of cross-sections of the insonated swine carotid from upstream, downstream-stream and beyond the field of view (FOV) of insonation were used to verify the spatial location of fluorophore deposition (Figure 6 a–c). The red color on the composite images originates from the DiI flurophore in the shell of the destroyed microbubbles that were transferred to the inner wall of the swine carotid. The upstream, downstream and beyond the FOV of insonation locations are 1 mm, 13 mm and 25 mm from the left of Figure 6d, respectively. The microscopy images of the histologic sections were also compared to the corresponding locations on the slow-time filtered images (Figure 6d). The fluorescence signal from the lower wall was used to quantify the relative aggregation of adherent microbubbles to the endothelium of the carotid artery (Figure 7). In arbitrary brightness units, the fluorescence signal from upstream, downstream and beyond the FOV of insonation locations was 5, 30 and 6, respectively.
Figure 6.
Superimposed fluorescence and bright field images of the various cross-sections of the swine carotid artery in Figure 5; a) upstream, b) downstream and c) beyond the field of view of the applied sequence., d) Ultrasound image at the end of the applied sequence and the corresponding locations from where the sections (a, b and c) were excised.
Figure 7.
A plot of fluorescence DiI signal from the lower arterial wall in images 6 a to c quantified in arbitrary brightness units.
The composite bright field and fluorescence images of the cross section of the insonated swine carotid (~ 2 mm diameter) also demonstrated DiI delivery to the endothelium (Figure 8a–e). Histologic cross sections were obtained from 0 mm, 2 mm (both upstream), 9 mm (midstream) and 18 mm (downstream) from the left of the image (Figure 8 a–d respectively). Cross sections were also obtained from beyond the FOV of insonation (Figure 8e as indicated by the arrow in 8f). The fluorescence signals from upstream (0 mm and 2 mm) and mid-stream locations were 5, 40 and 45 arbitrary brightness units, respectively and the fluorescence signals recorded from downstream and beyond the FOV of insonation were 38 and 4 arbitrary brightness units (Figure 9).
Figure 8.
a–e) Superimposed fluorescence and bright field images of the various cross-sections of the swine carotid artery and the f) corresponding locations from where these sections were excised; the artery was 2 mm in cross-sectional diameter. The flow velocity of saline flowing through the ex vivo artery was approximately 10 cm/s. The concentration of the microbubbles was 20 × 106 /ml. Imaging was performed at 8 MHz (single cycle pulses) and radiation force was applied using 20 cycle 4 MHz pulses at PRF of 42 kHz (MI=0.2). When destroying microbubbles, MI was increased to 1.0 and all other parameters remained the same as that used during the radiation force pulse.
Figure 9.
Fluorescence DiI signal from the lower arterial wall in images 8 a to e quantified in arbitrary brightness unit.
Slow-time filtered images obtained from the application of the image guided-drug delivery sequence to the ex vivo swine carotid samples show the accumulation of the adherent microbubbles (Figure 10a–d). The flow media in this experiment was whole blood (30% hematocrit) and the flow was from the left to right of the images. At the end of 16 s, the destructive sequence was applied and the DiI loaded adhered microbubbles were destroyed. A plot quantifying the fluorescence signal obtained from the lower wall of the arterial sections corresponding to various locations is illustrated in Figure 10 e. The upstream (2mm, 5 mm) and the midstream (10 mm) fluorescence signals were 2.5, 12 and 14 arbitrary brightness units. The fluorescence signals from downstream and beyond the FOV insonation location were 16 and 3 arbitrary brightness units.
Figure 10.
a–d) Images obtained from the molecular imaging & drug delivery sequence with slow-time filtering of the received pulse inversion data, e) a plot of fluorescence DiI signal from the lower arterial wall in histology cross-sections obtained from various longitudinal positions denoted in Figure 10d. The flow velocity of blood (30 % H) flowing through the ex vivo artery was approximately 10 cm/s. The concentration of the microbubbles was 20 × 106 /ml. Imaging was performed at 8 MHz (single cycle pulses) and radiation force was applied using 20 cycle 4 MHz pulses at PRF of 42 kHz (MI=0.2). For destroying microbubbles a MI of 1 was used with all other parameters similar to that of the radiation force pulse. The dynamic range of the images is 25 dB and the direction of flow of microbubbles is from left to the right of the image. The field of view is 20 × 25 mm.
Discussion
In this paper, we described, implemented and validated ex vivo, a modified technique for selective real-time molecular imaging. We also demonstrated its application for enhancing localized fluorophore delivery in flow conditions that may be encountered in a clinical setting (blood, flow conditions and artery size). It is worth noting that both imaging and drug delivery sequences were implemented at MIs and ISPTAs that are within the diagnostic ultrasound limit (i.e. MI < 1.9 and ISPTA < < 740 mW/cm2). These sequences were implemented on a conventional ultrasound scanner and ultrasound probe with no change in transmit or beam forming interface and minor changes in the post-processing signal path and user interface. Previous research work on drug and gene delivery used significantly higher ultrasound intensities (9, 14, 28) and lacked real time spatio-temporal feedback (24). The imaging and radiation force parameters used in this work were based on previously conducted preliminary in vitro studies and are optimal for the thin shell lipid microbubbles and the instrumentation used in this work (16). The ultrasound parameters used for destroying microbubbles were significantly lower than those necessary for cell destruction (14). The microbubble dose concentration used in ex vivo and in vitro fluorophore delivery experiments (20 × 106 /ml) was at the higher end of the range (2 × 106–22 × 106/ml) tested in our previous work (16). This enabled higher signal-to-noise ratio (SNR) and aided visualization.
Our current research scanner (Ultrasonix RP) has limited capabilities in terms of ultimate microbubble sensitivity and specificity arising out of limitations with its hardware (i.e. ability to produce perfect inverted replica pulses for pulse inversion imaging). Newer versions of the Ultrasonix are believed to overcome these problems and will probably enable improved sensitivity and specificity even with lower microbubble concentrations and lower ultrasound intensities. Alternatively, higher performance ultrasound scanners (such as a Siemens Sequoia or similar) may, in principle, be adapted to use the techniques described here. Unfortunately, scanners other than the research user oriented Ultrasonix generally require engineering development by the scanner manufacturer and, in our experience, this expenditure of engineering resource is difficult to arrange.
Data presented in Figure 3a and 3b summarize the significant differences observed in the rate of accumulation and final concentration of accumulated microbubbles at the target site when high viscosity saline (4 cp) and blood with varying hematocrit were used. This observation underlines the significance of interaction between microbubbles and RBCs and needs further investigation. Slow-time filtering efficiently eliminates signals from moving microbubbles and retains signals from static or wall bound microbubbles thus aiding selective molecular imaging (Figure 5, right). The static microbubble signal in vessels where blood was the flow medium (Figure 10 a–d) is muted when compared with microbubble signal where saline was used (Figure 5 right). This is due to the interaction of microbubbles and RBCs, which impede the translation of microbubbles to the lower vessel wall. Nevertheless, measurable signal (ultrasound and fluorescence) can still be detected from the distal or lower arterial vessel wall (Figure 10e).
It should be noted that in Figures 6 and 8, the fluorescence signal from the inner wall of the swine carotid is localized in a sector. This is attributed to the directivity of the radiation force pulse. Whereas a 1D linear array cannot steer its beam in the elevation dimension, a 2D array can steer its beam in the elevation dimension. By taking advantage of the elevational beam steering, and translation of insonating aperture, it may be possible to direct microbubbles around a broader sector of an arterial cross-section, thus improving spatial localization of drug delivery. While the fluorescence signal from the inner wall of the smaller carotid (2 mm diameter) in Figure 8 is approximately uniform in intensity and spatial extent (upstream to downstream), the larger carotid artery exhibited reduced accumulation and lower signal (Figure 6). This is most likely due to the larger distances that the microbubbles must travel before coming in contact with the lower vessel wall.
The results presented in this work suggest that this approach provides real-time semi-quantitative tools for investigating early detection of molecular expression of pathology in large animal models such as swine. The arterial sizes and blood flow regimes in these models are closer to humans than in mice or rat models. The drug delivery sequence of our technique may also increase the efficacy of various drug delivery agents via higher spatial localization due to directivity of the radiation force pulse. This combined imaging-drug delivery approach may be further extended to intravascular ultrasound (IVUS) catheter based drug delivery systems that may provide higher localization and targeted drug delivery at lower dose concentration (24).
Acknowledgment
The authors are grateful to Brooks Taylor, Jr., for software implementation on the programmable scanner. The authors acknowledge Ali H. Dhanaliwala, Sunil Unnikrishnan, Jose Tlaxca and Bryce T. Lowrey for their help with experiments. Dr. Ann H. Macleod-Lambert (Gore's processing, Edinburg, VA) provided the swine tissue and bovine blood used in this study. This work was partially supported by NIH NIBIB grant EB 002185 and NHBLI grant HL090700 to JAH.
Appendix I
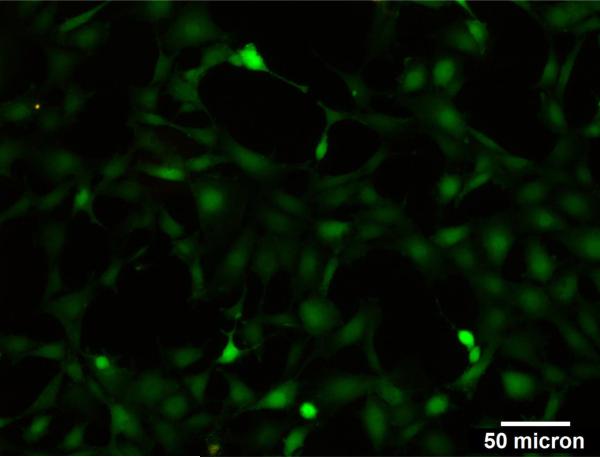
Cell viability after ultrasound was measured using a viability kit (LIVE/DEAD Viability/Cytotoxicity Assay Kit, Invitrogen). Briefly, SVEC4-10 endothelial cells were plated on Opticells and grown until 80% confluent as detailed in the methods section. The media was then replaced with a DPBS solution containing Calcein AM and Ethidium homodimer-1. The Opticell was then immersed in a 37 °C water bath and insonated (in the presence of microbubbles with a concentration of 20 ×106/ml) using the destruction pulse with parameters detailed in Table 1. The MI of insonation was 1.9. After insonation, the Opticells were imaged using fluorescence microscopy and analyzed. Images were taken from outside and within the area of insonation (Figures 11 a and b, respectively). The green color denotes live cells and the red color denotes dead cells. The superimposed red on green color indicates a viable cell whose cell membrane was transiently permeablized allowing Ethidium homodimer-1 to bind with the DNA. It is observed that most cells in the region of insonation are viable and/or permeablized and all cells outside the insonated region are viable.
Figure 11.
a) Fluorescence microscopy image of cells in the opticell a) outside and b) within the region of insonation. Green and red colors indicate viable and dead cells, respectively. Whereas, red superimposed on green color denotes permeablized cells.
References
- (1).Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- (2).Kaufmann BA, Sanders JM, Davis C, Xie A, Aldred P, Sarembock IJ, et al. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation. 2007;116:276–84. doi: 10.1161/CIRCULATIONAHA.106.684738. [DOI] [PubMed] [Google Scholar]
- (3).Klibanov AL, Rychak JJ, Yang WC, Alikhani S, Li B, Acton S, et al. Targeted ultrasound contrast agent for molecular imaging of inflammation in high-shear flow. Contrast Media Mol Imaging. 2006;1:259–66. doi: 10.1002/cmmi.113. [DOI] [PubMed] [Google Scholar]
- (4).Lindner JR. Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov. 2004;3:527–32. doi: 10.1038/nrd1417. [DOI] [PubMed] [Google Scholar]
- (5).Dayton PA, Rychak JJ. Molecular ultrasound imaging using microbubble contrast agents. Front Biosci. 2007;12:5124–42. doi: 10.2741/2553. [DOI] [PubMed] [Google Scholar]
- (6).Heppner P, Lindner JR. Contrast ultrasound assessment of angiogenesis by perfusion and molecular imaging. Expert Rev Mol Diagn. 2005;5:447–55. doi: 10.1586/14737159.5.3.447. [DOI] [PubMed] [Google Scholar]
- (7).Husseini GA, Pitt WG. Micelles and nanoparticles for ultrasonic drug and gene delivery. Adv Drug Deliv Rev. 2008;60:1137–52. doi: 10.1016/j.addr.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Unger EC, McCreery TP, Sweitzer RH, Caldwell VE, Wu Y. Acoustically active lipospheres containing paclitaxel: a new therapeutic ultrasound contrast agent. Invest Radiol. 1998;33:886–92. doi: 10.1097/00004424-199812000-00007. [DOI] [PubMed] [Google Scholar]
- (9).Shortencarier MJ, Dayton PA, Bloch SH, Schumann PA, Matsunaga TO, Ferrara KW. A method for radiation-force localized drug delivery using gas-filled lipospheres. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:822–31. doi: 10.1109/tuffc.2004.1320741. [DOI] [PubMed] [Google Scholar]
- (10).Dayton P, Klibanov A, Brandenburger G, Ferrara K. Acoustic radiation force in vivo: a mechanism to assist targeting of microbubbles. Ultrasound Med Biol. 1999;25:1195–201. doi: 10.1016/s0301-5629(99)00062-9. [DOI] [PubMed] [Google Scholar]
- (11).Frenkel V. Ultrasound mediated delivery of drugs and genes to solid tumors. Adv Drug Deliv Rev. 2008;60:1193–208. doi: 10.1016/j.addr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Ferrara K, Pollard R, Borden M. Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Annu Rev Biomed Eng. 2007;9:415–47. doi: 10.1146/annurev.bioeng.8.061505.095852. [DOI] [PubMed] [Google Scholar]
- (13).Mayer CR, Bekeredjian R. Ultrasonic gene and drug delivery to the cardiovascular system. Adv Drug Deliv Rev. 2008;60:1177–92. doi: 10.1016/j.addr.2008.03.004. [DOI] [PubMed] [Google Scholar]
- (14).Hallow DM, Mahajan AD, Prausnitz MR. Ultrasonically targeted delivery into endothelial and smooth muscle cells in ex vivo arteries. J Control Release. 2007;118:285–93. doi: 10.1016/j.jconrel.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Jayaweera AR, Edwards N, Glasheen WP, Villanueva FS, Abbott RD, Kaul S. In vivo myocardial kinetics of air-filled albumin microbubbles during myocardial contrast echocardiography. Comparison with radiolabeled red blood cells. Circ Res. 1994;74:1157–65. doi: 10.1161/01.res.74.6.1157. [DOI] [PubMed] [Google Scholar]
- (16).Patil AV, Rychak JJ, Allen JS, Klibanov AL, Hossack JA. Dual frequency method for simultaneous translation and real-time imaging of ultrasound contrast agents within large blood vessels. Ultrasound Med Biol. 2009;35:2021–30. doi: 10.1016/j.ultrasmedbio.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Dayton PA, Allen JS, Ferrara KW. The magnitude of radiation force on ultrasound contrast agents. J Acoust Soc Am. 2002;112:2183–92. doi: 10.1121/1.1509428. [DOI] [PubMed] [Google Scholar]
- (18).Zhao S, Borden M, Bloch SH, Kruse D, Ferrara KW, Dayton PA. Radiation-force assisted targeting facilitates ultrasonic molecular imaging. Mol Imaging. 2004;3:135–48. doi: 10.1162/1535350042380317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Rychak JJ, Klibanov AL, Hossack JA. Acoustic radiation force enhances targeted delivery of ultrasound contrast microbubbles: in vitro verification. IEEE Trans Ultrason Ferroelectr Freq Control. 2005;52:421–33. doi: 10.1109/tuffc.2005.1417264. [DOI] [PubMed] [Google Scholar]
- (20).Rychak JJ, Klibanov AL, Ley KF, Hossack JA. Enhanced targeting of ultrasound contrast agents using acoustic radiation force. Ultrasound Med Biol. 2007;33:1132–9. doi: 10.1016/j.ultrasmedbio.2007.01.005. [DOI] [PubMed] [Google Scholar]
- (21).Gessner R, Lukcas M, Lee M, Tsuruta F, Foster FS, Dayton PA. Radiationforce-enhanced targeted imaging and near real-time molecular imaging using a dual frequency high-resolution transducer. IEEE Ultrasonic symposium.2009. [Google Scholar]
- (22).Needles A, Couture O, Foster FS. A method for differentiating targeted microbubbles in real time using subharmonic micro-ultrasound and interframe filtering. Ultrasound Med Biol. 2009;35:1564–73. doi: 10.1016/j.ultrasmedbio.2009.04.006. [DOI] [PubMed] [Google Scholar]
- (23).Hwang JH, Brayman AA, Reidy MA, Matula TJ, Kimmey MB, Crum LA. Vascular effects induced by combined 1-MHz ultrasound and microbubble contrast agent treatments in vivo. Ultrasound Med Biol. 2005;31:553–64. doi: 10.1016/j.ultrasmedbio.2004.12.014. [DOI] [PubMed] [Google Scholar]
- (24).Phillips LC, Klibanov AL, Bowles DK, Ragosta M, Hossack JA, Wamhoff BR. Focused in vivo Delivery of Plasmid DNA to the Porcine Vascular Wall via Intravascular Ultrasound Destruction of Microbubbles. J Vasc Res. 2009;47:270–4. doi: 10.1159/000258905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Morgan KE, Allen JS, Dayton PA, Chomas JE, Klibaov AL, Ferrara KW. Experimental and theoretical evaluation of microbubble behavior: effect of transmitted phase and bubble size. IEEE Trans Ultrason Ferroelectr Freq Control. 2000;47:1494–509. doi: 10.1109/58.883539. [DOI] [PubMed] [Google Scholar]
- (26).de Ana FJ, O'Donnell M. Blood flow estimation error with intravascular ultrasound due to in-plane component of flow. Ultrason Imaging. 2003;25:193–212. doi: 10.1177/016173460302500306. [DOI] [PubMed] [Google Scholar]
- (27).Zhao S, Kruse DE, Ferrara KW, Dayton PA. Selective imaging of adherent targeted ultrasound contrast agents. Phys Med Biol. 2007;52:2055–72. doi: 10.1088/0031-9155/52/8/002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Taniyama Y, Tachibana K, Hiraoka K, Aoki M, Yamamoto S, Matsumoto K, et al. Development of safe and efficient novel nonviral gene transfer using ultrasound: enhancement of transfection efficiency of naked plasmid DNA in skeletal muscle. Gene Ther. 2002;9:372–80. doi: 10.1038/sj.gt.3301678. [DOI] [PubMed] [Google Scholar]