Abstract
Background
Recent evidence suggests that low-level environmental exposure to manganese adversely affects child growth and neurodevelopment. Previous studies have addressed the effects of prenatal exposure, but little is known about developmental effects of early postnatal exposure.
Methods
We studied 448 children born in Mexico City from 1997 through 2000, using a longitudinal study to investigate neurotoxic effects of early life manganese exposure. Archived blood samples, collected from children at 12 and 24 months of age, were analyzed for manganese levels using inductively-coupled plasma mass spectrometry. Mental and psychomotor development were scored using Bayley Scales of Infant Development at 6-month intervals between 12 and 36 months of age.
Results
At 12 months of age, the mean (SD) blood manganese level was 24.3 (4.5) μg/l and the median was 23.7 μg/l; at 24 months, these values were 21.1 (6.2) μg/l and 20.3 μg/l, respectively. Twelve- and 24-month manganese concentrations were correlated (Spearman correlation = 0.55) and levels declined over time (β = −5.7 [95% CI = −6.2 to −5.1]). We observed an inverted U-shaped association between 12-month blood manganese and concurrent mental development scores (compared with the middle 3 manganese quintiles, for the lowest manganese quintile, β = −3.3 [−6.0 to −0.7] and for the highest manganese quintile, β = −2.8 [−5.5 to −0.2]). This 12-month manganese effect was apparent but diminished with mental development scores at later ages. The 24-month manganese levels were not associated with neurodevelopment.
Conclusions
These results suggest a possible biphasic dose-response relationship between early-life manganese exposure at lower exposure levels and infant neurodevelopment. The data are consistent with manganese as both an essential nutrient and a toxicant.
Recent epidemiologic evidence suggests that low-level environmental exposure to manganese may adversely affect child neurodevelopment. Inverse associations have been reported between manganese exposure, measured in environmental and biological samples, and child cognition, memory, behavior, and motor function. For example, 11-to-13-year-old Chinese children ingesting sewage-contaminated water had higher hair and blood manganese, as well as worse memory and motor function test scores and school grades, compared with children in an uncontaminated area.1,2 Among 10 year-old Bangladeshi children, water manganese and intellectual function were associated in a dose-dependent manner.3 Adverse effects of manganese on IQ, verbal learning, and memory have also been reported among children residing near a hazardous waste site in Oklahoma.4 Most recently, a study reported more hyperactive behavior among children with higher hair manganese.5 Because manganese is an essential nutrient and is critical to several neurologic processes, it is possible that both low and high manganese levels could be associated with toxicity.
Exposure timing is a critical contextual issue for neurotoxicology during development, as exposure during specific life stages (or developmental windows) commonly determines the dose-response curve for toxicity. For many toxicants, early-life exposures are more toxic per dose than later exposures.6,7 In the aforementioned studies, effects from early-life exposure could not be addressed because evaluations were limited to children over age 5. Furthermore, because these studies used a cross-sectional design, there remain concerns about temporality of manganese exposure and neurodevelopmental deficits. Two additional studies evaluated neurodevelopmental outcomes among children younger than age 3 years, using prenatal exposure markers.8,9
To our knowledge, effects on neurodevelopment from early childhood exposures (between birth and age 3) have not been studied. This is a period of rapid brain development and includes the initiation of the response to sensory input following fetal life.10 To address these issues of critical windows and possible non-linear effects, we utilized a prospective study design to examine whether early-life manganese exposure is associated with neurodevelopmental effects among children between ages 1 and 3 years.
METHODS
Study Population
Participants were enrolled from 1997 through 2000 in a larger study on plasma-lead biomarkers during pregnancy and neurodevelopment in Mexico City.11,12 Mother-infant pairs were enrolled either during pregnancy or 1 month postpartum from prenatal clinics and maternity hospitals affiliated with the Mexican Institute of Social Security; children were followed through age 3. Eligible participants lived within the Mexico City metropolitan area and were planning to remain there for 5 years. Participants were excluded if mothers had a history of infertility, diabetes, or psychosis; consumed alcoholic beverages daily during pregnancy; were addicted to illegal drugs; habitually used prescription drugs; or were diagnosed with high-risk pregnancy, pre-eclampsia, gestational diabetes, or renal or circulatory disease.
Eligible participants were informed about the study, and written consent was obtained before participation. The human subjects committees of the National Institute of Public Health of Mexico, Harvard School of Public Health, and participating hospitals approved all study materials and procedures.
Of 620 children born to women who satisfied these inclusion criteria, 493 provided a blood sample at 12 or 24 months of age or both (12 months: n = 301; 24 months: n = 482) and underwent neurodevelopmental testing. Participants were excluded due to lab error (n = 1); imprecise lab measurement (i.e., excluded if percent relative standard deviation >25%; n = 1); very low birth weight (<1.5 kg; n = 1); severely premature birth (<32 weeks gestation; n = 3); or missing data on gestational age (n = 2). There were a total of 486 eligible children (12 months: n = 296; 24 months: n = 475).
Sample Collection and Analysis
Venous whole-blood samples were collected in tubes free of trace metals from children at 12 and 24 months of age, and immediately frozen. Samples were analyzed for manganese concentrations at the Trace Metals Laboratory at Harvard School of Public Health in Boston, MA. Lead, a known neurodevelopmental toxicant, was also measured. Blood samples were prepared and analyzed for manganese and lead concentrations with a dynamic-reaction cell-inductively-coupled plasma mass spectrometer (DRC-ICP-MS, Elan 6100, Perkin Elmer, Norwalk, CT), using previously described methods and quality-control measures.13 Recovery rates for manganese and lead in quality-control and spiked samples were 78%-113%, and precision (percent relative standard deviation) was less than 5%. The average limits of detection were 0.09 μg/dl for manganese and 0.04 μg/dl for lead.
Measurement of Child Neurodevelopment and Potential Confounders
Child neurodevelopment was assessed at six-month intervals (i.e., 12, 18, 24, 30, 36 months of age) using the Bayley Scales of Infant Development-II, Spanish version14 and scores from the Mental Development Index and the Psychomotor Development Index were used as the primary outcomes. Trained study personnel, who were unaware of children’s manganese and lead levels, administered the test using a standardized protocol previously described by our research group.15
Information on demographic, socioeconomic, and other factors that could confound the relationship between manganese and neurodevelopment were collected at delivery, 1 month postpartum, and during subsequent study visits. These factors included: sex, hemoglobin (g/dl), ferritin (μg/l), umbilical cord blood lead (μg/dl), birth weight (kg), birth length (cm), head circumference at birth (cm), gestational age (weeks), maternal age at delivery (years), maternal and paternal education (years), maternal marital status (married, living with partner, separated/divorced/widowed), maternal blood lead at 1 month postpartum (μg/dl), duration of breastfeeding in infant’s first year (months), child nutrition (estimated iron and manganese dietary intake), and maternal IQ. Maternal IQ was assessed using Information, Comprehension, Similarities, and Block Design subscales of the Wechsler Adult Intelligence Scale, Spanish version.16
Statistical Analysis
Univariate summary statistics and distributional plots were examined for all variables. Neurodevelopment scores were approximately normally distributed and were modeled as continuous outcomes. Extreme values of manganese were identified using the generalized Extreme Studentized Deviate Many-Outlier procedure17 and were excluded from all analyses (12 months: n = 3; 24 months: n = 5). Covariates that are known predictors of neurodevelopment or strong potential confounders (including blood lead, sex, and maternal IQ and education) were included a priori in multivariable regression models, based on biologic plausibility. Possible associations of other potential confounders with manganese and neurodevelopment score were explored separately with bivariate regression. To the model with a priori covariates, we added, one at a time, covariates that were associated in bivariate models (P<0.10) with exposure and outcome at any time point. Additional covariates were selected separately for models of Mental Development Index and Psychomotor Development Index and included in the final model (hemoglobin, gestational age) if the manganese coefficient changed more than 10%. We examined potential nonlinearity of the association between covariates and neurodevelopment with penalized splines in generalized additive models, and found that all covariates assessed as potential confounders were linearly associated with neurodevelopment. For model building, we used all eligible children excluding outliers (12 months: n = 291; 24 months: n = 467). For final analyses, we additionally excluded participants missing data on covariates in the final model.
We also explored a potential nonlinear association between manganese and neurodevelopment by examining penalized splines for manganese using generalized additive models. Generalized cross validation was used to automatically select the degree of smoothing for splines. We used a likelihood ratio test comparing models with a smoothed manganese term to models with a linear manganese term to assess linearity of the manganese-neurodevelopment association. For linear associations, manganese was used as a continuous term in adjusted linear regression models. For nonlinear associations, indicator variables for quintiles of the manganese distribution were used in adjusted regression models. In this case, we assessed potential confounders for inclusion in the final model by examining the magnitude of change of these categorical manganese terms.
We fit separate models for each manganese measurement and for each time point of the Bayley assessment (i.e., 12-month manganese predicting 12-, 18-, 24-, 30-, and 36-month Bayley scores; and 24-month manganese predicting 24-, 30-, and 36-month Bayley scores). We also fit two linear mixed-effects models (i.e., one for each manganese time point) with repeated-outcome measurements. Additionally, for models of 24-month manganese predicting neurodevelopment, we considered 12-month manganese as a potential confounder. To eliminate concerns of multicollinearity between 12- and 24-month manganese, we used residual regression. Specifically, the residuals from a simple linear regression model of 24-month manganese regressed on 12-month manganese were calculated and then used in multivariable regression models in place of 24-month manganese to estimate effects on neurodevelopment.
Although fewer than 8% of eligible children (12 months: 21 of 291; 24 months: 37 of 467) were excluded due to missing covariate data, we conducted sensitivity analyses to evaluate the appropriateness of using complete data by comparing unadjusted estimates for manganese among all eligible children to those for eligible children with data available on all covariates. Statistical analyses were conducted using SAS version 9.1 (SAS Institute, Inc., Cary, NC, USA) and R version 2.7.1 (The R Foundation for Statistical Computing, www.r-project.org).
RESULTS
A total of 448 children (12 months: n = 270; 24 months: n = 430) had blood data, concurrent neurodevelopment scores, and complete covariate information. Characteristics of these children are summarized in Table 1, along with characteristics of nonparticipants (n = 172). Participants and nonparticipants were comparable regarding most characteristics, though participants had a higher percentage of boys and lower percentage of married mothers than nonparticipants.
TABLE 1.
Characteristicsa of Study Population
| Children with blood measurements at: |
|||
|---|---|---|---|
| Nonparticipants (n = 172)b |
12 months (n = 270) |
24 months (n = 430) |
|
| Children | |||
| Male sex; % | 44.8 | 51.5 | 50.9 |
| Birth weight (kg) | 3.1 (0.5) | 3.1 (0.4) | 3.1 (0.4) |
| Estimated gestational age (wks) | 38.5 (2.1) | 38.9 (1.3) | 38.9 (1.3) |
| Birth length (cm) | 49.5 (3.1) | 49.9 (2.2) | 49.8 (2.3) |
| Head circumference (cm) | 34.0 (1.6) | 34.1 (1.5) | 34.1 (1.6) |
| Blood manganese (μg/l) | |||
| 12 months | NA | 24.3 (4.5) | 24.4 (4.6)c |
| 24 months | NA | 18.7 (4.7)d | 21.1 (6.2) |
| Umbilical cord blood lead (μg/dl) | 4.9 (2.8) | 4.2 (2.7) | 4.7 (3.1) |
| Blood lead (μg/dl) | |||
| 12 months | NA | 5.1 (2.6) | 5.1 (2.6)c |
| 24 months | NA | 4.8 (2.5)d | 4.9 (2.5) |
| Hemoglobin (g/dl) | |||
| 12 months | 11.7 (1.3) | 11.9 (1.3) | 11.8 (1.3) |
| 24 months | 12.0 (1.4) | 12.6 (1.2) | 12.6 (1.1) |
| Mothers | |||
|
| |||
| Marital status (% married) | 76 | 71 | 72 |
| IQ | 88.8 (11.9) | 88.0 (12.5) | 88.1 (13.0) |
| Total years of school | 11.1 (3.6) | 10.7 (2.7) | 10.7 (2.8) |
| Age at delivery (yrs) | 27.7 (5.4) | 25.7 (5.3) | 25.9 (5.3) |
| Whole blood lead (μg/dl)e | 7.2 (4.1) | 6.9 (4.2) | 7.5 (4.7) |
Mean (SD) unless otherwise specified
Includes all children who did not meet eligibility criteria, or who had incomplete covariate data, outlying blood manganese, or unreliable blood data
262 of these 430 children also provided blood at 12-mo
261 of these 270 children also provided blood at 24-mo
At one month postpartum
NA indicates not applicable
At 12 months of age, the mean (SD) blood manganese level was 24.3 (4.5) μg/l and the median was 23.7 μg/l; at 24 months, these values were 21.1 (6.2) μg/l and 20.3 μg/l, respectively. These levels were similar for boys and girls. Twelve- and 24-month manganese concentrations were correlated (Spearman correlation = 0.55), and levels declined over time (β = −5.7 [95% confidence interval [CI] = −6.2 to −5.1]). Blood lead was positively associated with 24-month manganese (Table 2). Indicators of iron status (hemoglobin, ferritin), birth weight, and gestational age were inversely associated with manganese at both time-points.
TABLE 2.
Predictors of 12-month and 24-month Blood Manganese Levels (μg/l), from Unadjusted Regression Models
| Blood Manganese | ||||
|---|---|---|---|---|
| 12 months |
24 months |
|||
| No.a | Beta (95% CI) | No.a | Beta (95% CI) | |
| Blood lead (μg/dl) | ||||
| Umbilical cord | 179 | −0.03 (−0.28 to 0.22) | 273 | 0.44 (0.21 to 0.67) |
| 12 months | 270 | 0.11 (−0.09 to 0.32) | 262 | 0.23 (0.01 to 0.45) |
| 24 months | NA | NA | 430 | 0.34 (0.11 to 0.58) |
| Motherb | 251 | −0.04 (−0.17 to 0.10) | 387 | 0.19 (0.06 to 0.32) |
| Hemoglobin (g/dl) | ||||
| 12 months | 270 | −0.49 (−0.90 to −0.08) | 379 | −0.62 (−1.09 to −0.15) |
| 24 months | NA | NA | 430 | −0.74 (−1.26 to −0.23) |
| Ferritin (μg/l) | ||||
| 12 months | 270 | −0.03 (−0.06 to −0.0004) | 390 | −0.03 (−0.06 to 0.005) |
| 24 months | NA | NA | 430 | −0.05 (−0.08 to −0.01) |
| Birth weight (kg) | 270 | −1.48 (−2.68 to −0.28) | 430 | −1.98 (−3.31 to −0.65) |
| Gestational age (weeks) | 270 | −0.45 (−0.87 to −0.03) | 430 | −0.40 (−0.85 to 0.05) |
No. observations does not equal 270 for 12-mo data or 430 for 24-mo data due to incomplete covariate data.
Mother’s whole blood lead at 1 month postpartum.
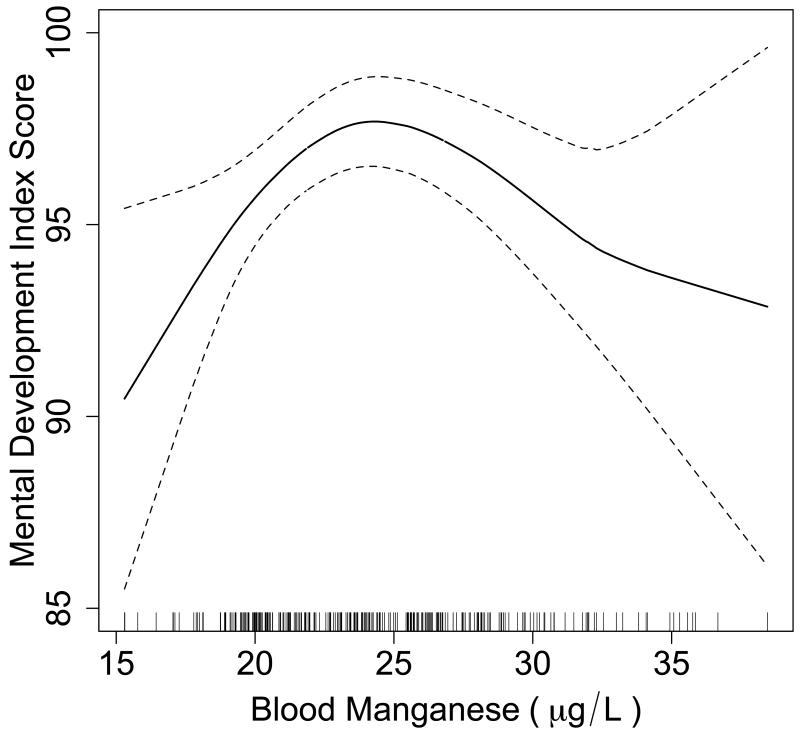
Using penalized splines of manganese, we observed a nonlinear association between 12-month manganese and 12-month Mental Development Index (P=0.04). Comparison with a model using a linear manganese term similarly suggested a better fit with a smoothed manganese term (likelihood ratio test, P=0.003). Based on the smoothed plot (Figure 1), the highest estimated Mental Development Index score of 97.7 points was observed at 24.4 μg/l manganese. The mental development score was estimated to be 93.9 points (95% CI = 91.7 to 96.0) at the 5th percentile of manganese (18.1 μg/l) and 94.2 points (95% CI = 91.6 to 96.8) at the 95th percentile (32.5 μg/l).
Figure.
Penalized spline for 12-month blood manganese (μg/l) predicting 12-month Mental Development Index, controlling for sex, 12-month blood lead, 12-month hemoglobin, gestational age, maternal IQ, and maternal education, among 270 children. The solid line represents the estimate; dotted lines represent 95% confidence limits. Vertical lines on the x-axis represent the distribution of blood manganese observations.
Because a nonlinear association was observed between 12-month manganese and 12-month Mental Development Index, we fit an adjusted multivariable regression model with indicator variables for each quintile of manganese. Children with manganese in quintile 3 (22.5-25.1 μg/l) demonstrated higher 12-month mental development scores than children in quintile 1 (15.3-20.1 μg/l; β = 4.8 [95% CI = 1.6 to 8.1]). To further describe the effects of manganese at the upper and lower extremes, we fit regression models comparing quintiles 1 and 5 to the middle three quintiles (i.e., quintiles 2, 3, and 4 collapsed together)(Table 3). We observed lower 12-month mental development scores among children with 12-month manganese in lowest and highest quintiles, compared with children in the middle three quintiles of manganese.
TABLE 3.
Crude and Adjusted Effect Estimates from Regression Models of 12-month Blood Manganese Quintiles Predicting Mental Development Index1
| 12-month (n = 270) |
Mental Development Index 18-month (n = 267) |
24-month (n = 268) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude |
Adjusted |
Crude |
Adjusted |
Crude |
Adjusted |
|||||||
| Covariates | Beta | (95% CI) | Beta | (95% CI) | Beta | (95% CI) | Beta | (95% CI) | Beta | (95% CI) | Beta | (95% CI) |
| Blood Manganese, Lowest Quintilea,b | −3.08 | (−5.84 to −0.32) | −3.35 | (−5.99 to −0.70) | −3.30 | (−5.99 to −0.62) | −3.40 | (−6.07 to −0.74) | −1.26 | (−4.98 to 2.46) | −1.51 | (−5.18 to 2.17) |
| Blood Manganese, Highest Quintilea,c | −3.30 | (−6.06 to −0.55) | −2.84 | (−5.51 to −0.17) | 0.24 | (−2.45 to 2.92) | −0.10 | (−2.80 to 2.59) | −0.40 | (−4.09 to 3.29) | −0.95 | (−4.64 to 2.74) |
| 12-mo Blood Lead (μg/dl) | −0.18 | (−0.58 to 0.22) | −0.27 | (−0.67 to 0.13) | −0.44 | (−1.00 to 0.11) | ||||||
| Child’s sexd | 3.15 | (1.07 to 5.24) | 1.85 | (−0.26 to 3.95) | 3.13 | (0.25 to 6.02) | ||||||
| Mother’s IQ | 0.07 | (−0.02 to 0.17) | −0.03 | (−0.12 to 0.07) | 0.02 | (−0.11 to 0.14) | ||||||
| Mother’s total years of school | 0.02 | (−0.41 to 0.46) | 0.52 | (0.08 to 0.97) | 0.66 | (0.06 to 1.25) | ||||||
| 12-mo Hemoglobin (g/dl) | 1.15 | (0.34 to 1.96) | −0.11 | (−0.93 to 0.71) | −0.24 | (−1.36 to 0.88) | ||||||
| Gestational age (wks) | 1.03 | (0.22 to 1.84) | 0.72 | (−0.10 to 1.54) | 0.25 | (−0.88 to 1.39) | ||||||
Comparison group is children in middle three quintiles of manganese distribution (i.e., quintiles 2, 3, and 4)
Lowest quintile of 12-month blood manganese is <20.2 μg/l
Highest quintile of 12-month blood manganese is >28.0 μg/l
Child sex coded as: 0 = male, 1 = female
At later time points (i.e., 18 and 24 months), the non-linear association was less apparent in smoothed plots, although lower scores were still observed at low manganese than at mid-levels of the manganese distribution (Table 3). Because relationships between 12-month manganese and the Mental Development Index at later time points were not significantly nonlinear, we fit regression models using manganese both as a categorical variable (quintiles 1 and 5 compared with the middle three quintiles) and as a continuous variable. When manganese was represented as a continuous term in linear regression models, manganese coefficients were positive but modest (0.2 point increase in Mental Development Index score per μg/l manganese increase). 12-month manganese did not appear to be associated with mental development scores at 30 or 36 months in adjusted linear regression models.
Mixed models of repeated outcome measures showed lower Mental Development Index scores among children with 12-month manganese in the lowest quintile (β = −2.4 [95% CI = −4.3 to −0.5]), and somewhat lower scores in the highest quintile (β = −0.9 [95% CI = −2.8 to 1.1]), compared with children in the middle three quintiles. We observed no association of 24-month manganese with 24- through 36-month mental development scores at any time point, or in repeated-measures models. There was no association of 12- or 24-month manganese with Psychomotor Development Index score at any time point.
We explored whether the rate of change in manganese between 12 and 24 months is associated with neurodevelopment score or change in neurodevelopment score (eAppendix, http://links.lww.com). There were no consistent associations.
In sensitivity analyses to evaluate the appropriateness of using complete data, we saw slightly stronger associations between 12-month manganese and 12-month Mental Development Index among children with all covariate data (n = 270) than among children who were missing some covariate data (n = 291). Among children with all data, in the lowest quintile β = −3.1 (95% CI = −5.8 to −0.3) and in the highest quintile β = −3.3 (−6.0 to −0.6). Among children who were missing some covariate data, in the lowest quintile β = −2.4 (−5.1 to 0.3) and in the highest quintile β = −2.5 (95% CI: −5.1 to 0.2). Our overall conclusions remained unchanged.
DISCUSSION
We observed an inverted U-shaped association between children’s blood manganese level at 12 months of age and concurrent mental development scores, suggesting that both low and high manganese levels may have adverse effects on neurodevelopment in young children. We saw no evidence of an association between blood manganese at either 12 or 24 months of age and psychomotor development. Observed declines of 3.4 and 2.8 Mental Development Index points for lowest and highest quintiles of blood manganese, respectively, relative to the middle three quintiles, correspond to declines of 0.37 and 0.31 SD units of 12-month Mental Development Index. The effect for 12-month manganese was apparent but diminished for mental development measures at older ages. Because blood manganese at 24 months of age was not associated with lower mental development scores, 12 months of age may be a critical developmental window for the effects of manganese exposure on child neurocognitive development.
The observed inverted U-shaped association between manganese and mental development may be explained by effects of manganese on oxidative stress at high and low levels. Manganese is a co-factor for enzymes that protect against oxidative stress. Deficiencies would presumably increase sensitivity to oxidative cellular injury, because manganese possesses anti-oxidant properties. Manganese is a key component of various metalloenzymes, such as mitochondrial superoxide dismutase, that are important for normal central nervous system function and protection against oxidative injury.18 Non-enzyme manganese complexes have also been shown to scavenge and block production of oxygen free radicals.19,20 This may explain the decrease in Mental Development Index scores in the lowest quintile of blood manganese.
At physiologic levels, manganese protects against oxidative injury, but at high levels, manganese itself is an oxidant. Neurotoxicity from manganese overexposure appears to involve oxidative damage to dopaminergic neurons in particular, as well as mitochondrial dysfunction, which limits energy production and increases oxidative stress and superoxide radical formation.21-23 As a transition metal, manganese catalyzes oxidative reactions in neurologic tissues via the Fenton reaction.24 The age-specific findings of our study may be due to developmental changes in gene expression that correlate with age. Indirect evidence of age-specific developmental effects would include the apparent decrease in blood manganese that corresponds with increasing age. Since manganese regulatory mechanisms such as biliary excretion are not fully developed in neonates,25,26 12-month-old children may be more sensitive to pro-oxidant effects of high manganese levels, but by 24 months of age, sufficient maturation of these systems may have occurred to limit absorption or enable more effective manganese metabolism and excretion.
Given the properties of manganese, adverse effects at high and low blood levels might be expected. An inverted U-shaped dose response curve between manganese and neurodevelopment has not been previously reported in children. However, Santos-Burgoa et al.27 observed U-shaped associations between blood manganese and reductions in both cognitive and motor function in adults. Another study reported nonlinear adverse effects of prenatal manganese and birth weight among a population of mother-infant pairs.13 While the age and outcome differ in these two other reports, the consistency of the findings lends weight to the plausibility of these results.
Blood manganese levels observed in this study are higher than reference normal values (4-14 μg/l) reported by the Agency for Toxic Substances Registry.21 However, these reference values are not age-specific, and manganese levels and requirements are known to vary by lifestage.28 Blood manganese levels in our study are in the range of values previously reported in the literature for children at various ages, some of which also measured cognitive outcomes. For example, Takser et al8 reported mean cord blood manganese of 38.5 μg/l in Parisian newborns, and found effects on attention and non-verbal memory at 3 years. Among older children in Bangladesh, Wasserman et al3 found no relation between blood manganese (mean 12.8 μg/l) and intellectual function, but nonlinear manganese models were not considered. A highly-exposed group of Chinese children consuming manganese-contaminated water had higher blood manganese (mean 33.9 μg/l) and lower school performance scores than controls.2 Although literature on children’s blood manganese is sparse, results are consistent with our findings of adverse cognitive effects with high blood concentrations.
There are several limitations to this study. Lack of data on prenatal manganese precludes our ability to assess whether exposure before birth or in early childhood might be even more predictive of neurodevelopment than 12-month exposure. With respect to generalizability and selection bias due to potential differences between participants and nonparticipants, the characteristics of these children were similar. Subjects were originally recruited to participate in a study of prenatal lead biomarkers and were unaware of hypotheses related to manganese. It is therefore unlikely that subjects participated or provided blood differentially based on both exposure and outcome. Furthermore, the attrition rate was low (9%) over the two-year follow-up period, and participants followed for the study duration (n = 406) were not notably different on exposure or outcome from the full 448 children who participated at either time point. Sensitivity analyses evaluating the appropriateness of using complete data showed moderate differences between children with complete covariate data and those missing some covariate data. However, the direction of associations was stable, suggesting that any potential selection bias would not alter our conclusions substantially.
While we lack data on a direct measure of socioeconomic status (SES) and stimulation of the child in the home environment, the study population arises from a relatively homogeneous low-to middle-income urban Mexican population, thus limiting unmeasured confounding by these factors. Furthermore, parental education is reported to be strongly correlated with SES among families in Mexico,29 and we therefore expect that any potential confounding by SES is removed by adjusting for maternal education. The observed association at 12 months appears fairly robust; controlling for sex, blood lead, hemoglobin, gestational age, and maternal IQ and education did not substantially change manganese coefficients. Nonetheless, possible residual or additional unmeasured confounding by maternal stress or other neurotoxicants cannot be ruled out. There is the possibility of laboratory measurement error independent of neurodevelopment scores. While this error typically attenuates effects, we cannot predict the direction of potential bias because exposure and outcome data were continuous or polychotomous.
In conclusion, we used a prospective design to examine linear and nonlinear neurodevelopmental effects of early-life manganese exposure at two distinct time points. Our study design allowed us to compare these two potential windows of susceptibility to manganese exposure. Together with findings of Takser et al.,8 who reported adverse effects from fetal manganese exposure, these results suggest that exposure during early life may have the strongest neurotoxic effects. If these findings of a nonlinear association between manganese and mental development are confirmed, it could indicate the need to focus on early infancy, as this appears to be a critical developmental stage during which children are sensitive to both low and high manganese levels.
Supplementary Material
Acknowledgements
We thank David Christiani for feedback and comments on analyses; Nick Lupoli for assisting with laboratory analyses; and our study participants.
Funding: Supported in part by the National Institute of Environmental Health Sciences grants R01-ES007821, R01-ES014930, R01-ES013744, and P30-ES0002, and by a STAR (Science to Achieve Results) Research Assistance Agreement No. FP-91690001 awarded by the U.S. Environmental Protection Agency (EPA). EPA has not officially endorsed this publication and the views expressed herein may not reflect the views of the EPA.
Footnotes
SDC Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.He P, Liu DH, Zhang GQ. [Effects of high-level-manganese sewage irrigation on children’s neurobehavior] Zhonghua Yu Fang Yi Xue Za Zhi. 1994;28(4):216–8. [PubMed] [Google Scholar]
- 2.Zhang G, Liu D, He P. [Effects of manganese on learning abilities in school children] Zhonghua Yu Fang Yi Xue Za Zhi. 1995;29(3):156–8. [PubMed] [Google Scholar]
- 3.Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, Kline J, van Geen A, Slavkovich V, LoIacono NJ, Cheng Z, Zheng Y, Graziano JH. Water manganese exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect. 2006;114(1):124–9. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright RO, Amarasiriwardena C, Woolf AD, Jim R, Bellinger DC. Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. Neurotoxicology. 2006;27(2):210–6. doi: 10.1016/j.neuro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Bouchard M, Laforest F, Vandelac L, Bellinger D, Mergler D. Hair manganese and hyperactive behaviors: pilot study of school-age children exposed through tap water. Environ Health Perspect. 2007;115(1):122–7. doi: 10.1289/ehp.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368(9553):2167–78. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- 7.Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sorensen N, Dahl R, Jorgensen PJ. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997;19(6):417–28. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- 8.Takser L, Mergler D, Hellier G, Sahuquillo J, Huel G. Manganese, monoamine metabolite levels at birth, and child psychomotor development. Neurotoxicology. 2003;24(4-5):667–74. doi: 10.1016/S0161-813X(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 9.Ericson JE, Crinella FM, Clarke-Stewart KA, Allhusen VD, Chan T, Robertson RT. Prenatal manganese levels linked to childhood behavioral disinhibition. Neurotoxicol Teratol. 2007;29(2):181–7. doi: 10.1016/j.ntt.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Nowakowski RS, Hayes NL. CNS development: an overview. Dev Psychopathol. 1999;11(3):395–417. doi: 10.1017/s0954579499002126. [DOI] [PubMed] [Google Scholar]
- 11.Surkan PJ, Schnaas L, Wright RJ, Tellez-Rojo MM, Lamadrid-Figueroa H, Hu H, Hernandez-Avila M, Bellinger DC, Schwartz J, Perroni E, Wright RO. Maternal self-esteem, exposure to lead, and child neurodevelopment. Neurotoxicology. 2008;29(2):278–85. doi: 10.1016/j.neuro.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tellez-Rojo MM, Bellinger DC, Arroyo-Quiroz C, Lamadrid-Figueroa H, Mercado-Garcia A, Schnaas-Arrieta L, Wright RO, Hernandez-Avila M, Hu H. Longitudinal associations between blood lead concentrations lower than 10 microg/dL and neurobehavioral development in environmentally exposed children in Mexico City. Pediatrics. 2006;118(2):e323–30. doi: 10.1542/peds.2005-3123. [DOI] [PubMed] [Google Scholar]
- 13.Zota AR, Ettinger AS, Bouchard M, Amarasiriwardena CJ, Schwartz J, Hu H, Wright RO. Maternal blood manganese levels and infant birth weight. Epidemiology. 2009;20(3):367–73. doi: 10.1097/EDE.0b013e31819b93c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayley N. Bayley Scales of Infant Development. 2nd ed. 2nd (Bayley-II) ed Psychological Corp.; San Antonio, TX.: 1993. [Google Scholar]
- 15.Gomaa A, Hu H, Bellinger D, Schwartz J, Tsaih SW, Gonzalez-Cossio T, Schnaas L, Peterson K, Aro A, Hernandez-Avila M. Maternal bone lead as an independent risk factor for fetal neurotoxicity: a prospective study. Pediatrics. 2002;110(1 Pt 1):110–8. doi: 10.1542/peds.110.1.110. [DOI] [PubMed] [Google Scholar]
- 16.Wechsler H. Wechsler Adult Intelligence Scale (WAIS) Spanish version Psychological Corporation; San Antonio, TX: 1968. [Google Scholar]
- 17.Rosner B. Percentage Points for a Generalized ESD Many-Outlier Procedure. Technometrics. 1983;25(2):165–172. [Google Scholar]
- 18.Aschner M, Aschner JL. Manganese neurotoxicity: cellular effects and blood-brain barrier transport. Neurosci Biobehav Rev. 1991;15(3):333–40. doi: 10.1016/s0149-7634(05)80026-0. [DOI] [PubMed] [Google Scholar]
- 19.Cheton PL, Archibald FS. Manganese complexes and the generation and scavenging of hydroxyl free radicals. Free Radic Biol Med. 1988;5(5-6):325–33. doi: 10.1016/0891-5849(88)90104-9. [DOI] [PubMed] [Google Scholar]
- 20.Hussain S, Ali SF. Manganese scavenges superoxide and hydroxyl radicals: an in vitro study in rats. Neurosci Lett. 1999;261(1-2)):21–4. doi: 10.1016/s0304-3940(98)01005-2. [DOI] [PubMed] [Google Scholar]
- 21.ATSDR . Toxicological Profile for Manganese. Vol. 2007. Agency for Toxic Substances & Disease Registry; 2000. [Google Scholar]
- 22.Normandin L, Hazell AS. Manganese neurotoxicity: an update of pathophysiologic mechanisms. Metab Brain Dis. 2002;17(4):375–87. doi: 10.1023/a:1021970120965. [DOI] [PubMed] [Google Scholar]
- 23.Verity MA. Manganese neurotoxicity: a mechanistic hypothesis. Neurotoxicology. 1999;20(2-3):489–97. [PubMed] [Google Scholar]
- 24.Watts RJ, Sarasa J, Loge FJ, Teel AL. Oxidative and Reductive Pathways in Manganese-Catalyzed Fenton’s Reactions. J Environmental Engineering. 2005;131(1):158–164. [Google Scholar]
- 25.Keen CL, Bell JG, Lonnerdal B. The effect of age on manganese uptake and retention from milk and infant formulas in rats. J Nutr. 1986;116(3):395–402. doi: 10.1093/jn/116.3.395. [DOI] [PubMed] [Google Scholar]
- 26.Miller ST, Cotzias GC, Evert HA. Control of tissue manganese: initial absence and sudden emergence of excretion in the neonatal mouse. Am J Physiol. 1975;229(4):1080–4. doi: 10.1152/ajplegacy.1975.229.4.1080. [DOI] [PubMed] [Google Scholar]
- 27.Santos-Burgoa C, Rios C, Mercado LA, Arechiga-Serrano R, Cano-Valle F, Eden-Wynter RA, Texcalac-Sangrador JL, Villa-Barragan JP, Rodriguez-Agudelo Y, Montes S. Exposure to manganese: health effects on the general population, a pilot study in central Mexico. Environ Res. 2001;85(2):90–104. doi: 10.1006/enrs.2000.4108. [DOI] [PubMed] [Google Scholar]
- 28.Institute of Medicine . Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academy Press; Washington D.C.: 2000. Food and Nutrition Board: National Panel on Micronutrients, Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Use of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. [PubMed] [Google Scholar]
- 29.Bronfman M, Guiscafre H, Castro V, Gutierrez G. Measuring unequality: a methodological approach, analysis of social and economic characteristics of the sample studied. Arch Invest Med (Mex.) 1988;19:351–360. [Google Scholar]
- 30.Spencer A. Whole blood manganese levels in pregnancy and the neonate. Nutrition. 1999;15(10):731–4. doi: 10.1016/s0899-9007(99)00144-6. [DOI] [PubMed] [Google Scholar]
- 31.Tholin K, Palm R, Hallmans G, Sandstrom B. Manganese status during pregnancy. Ann N Y Acad Sci. 1993;678:359–60. doi: 10.1111/j.1749-6632.1993.tb26146.x. [DOI] [PubMed] [Google Scholar]
- 32.Chan AW, Minski MJ, Lim L, Lai JC. Changes in brain regional manganese and magnesium levels during postnatal development: modulations by chronic manganese administration. Metab Brain Dis. 1992;7(1):21–33. doi: 10.1007/BF01000438. [DOI] [PubMed] [Google Scholar]
- 33.Collipp PJ, Chen SY, Maitinsky S. Manganese in infant formulas and learning disability. Ann Nutr Metab. 1983;27(6):488–94. doi: 10.1159/000176724. [DOI] [PubMed] [Google Scholar]
- 34.Takeda A, Ishiwatari S, Okada S. Manganese uptake into rat brain during development and aging. J Neurosci Res. 1999;56(1):93–8. doi: 10.1002/(SICI)1097-4547(19990401)56:1<93::AID-JNR12>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 35.McCracken JP, Schwartz J, Bruce N, Mittleman M, Ryan LM, Smith KR. Combining individual- and group-level exposure information: child carbon monoxide in the guatemala woodstove randomized control trial. Epidemiology. 2009;20(1):127–36. doi: 10.1097/EDE.0b013e31818ef327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.