Abstract
Spinal cord injury (SCI) causes loss of neurological function and, depending on serverity, may cause paralysis. The only recommended pharmacotherapy for the treatment of SCI is high-dose methylprednisolone and its use is controversial. We have previously shown that estrogen treatment attenuated cell death, axonal and myelin damage, calpain and caspase activities, and inflammation in acute SCI. The aim of this study was to examine whether post-treatment of SCI with estrogen would improve locomotor function by protecting cells and axons and reducing inflammation during chronic phase following injury. Moderately severe injury (40 g.cm force) was induced in male Sprague-Dawley rats following laminectomy at T10. Three groups of animals were used: sham (laminectomy only), vehicle (dimethyl sulfoxide or DMSO) treated injury group, and estrogen treated injury group. Animals were treated with 4 mg/kg estrogen at 15 min and 24 h post-injury followed by 2 mg/kg estrogen daily for the next 5 days. Following treatment, animals were sacrificed at the end of 6 weeks following injury, and 1-cm segments of spinal cord (lesion, rostral to lesion, and caudal to lesion) were removed for biochemical analyses. Estrogen treatment reduced COX-2 activity, blocked NF-κB translocation, prevented glial reactivity, attenuated neuron death, inhibited activation and activity of calpain and caspase-3, decreased axonal damage, reduced myelin loss in the lesion and penumbra, and improved locomotor function when compared with vehicle treated animals. These findings suggest that estrogen may be useful as a promising therapeutic agent for prevention of damage and improvement of locomotor function in chronic SCI.
Keywords: axonal damage, cell death, chronic spinal cord injury, estrogen, locomotor function
INTRODUCTION
Spinal cord injury (SCI) is a devastating medical problem in young individuals who are otherwise healthy. The currently available pharmacotherapy is methylprednisolone (Bracken et al. 1998; Sekhon and Fehlings 2001), which has limited efficacy and is controversial (Hurlbert 2000; Sribnick et al. 2009b). There are many destructive pathways involved in secondary damage. Such processes include increases in reactive oxygen species (ROS), reperfusion, glutamate concentration, and mitochondrial damage (Barut et al. 1993; Carlson et al. 1998; Mills et al. 2000). Mitochondrial damage in SCI may not only change the Na+/K+-ATPase activity, potentiating increase in intracellular [Ca2+], and the activation of glutamate receptors also facilitate Ca2+ influx (Agrawal and Fehlings 1996; Agrawal et al. 2000; Li et al. 2000; Wingrave et al. 2004). Increases in intracellular free [Ca2+] following SCI leads to activation of the Ca2+-actvated protease calpain and phospholipases (Dhillon et al. 1999; Ray and Banik 2002). Ubiquitous calpain exists in two forms, μ-calpain and m-calpain, which require μM and mM concentrations of Ca2+ for activation, respectively (Ray and Banik 2002). Increased calpain activity has been found in SCI and traumatic brain injury (TBI) (Posmantur et al. 1997; Ray and Banik 2002), and activated calpain degrades myelin basic protein and the cytoskeletal protein α-spectrin (Ray and Banik 2002). Greater calpain activity also degrades calpastatin, the endogenous specific inhibitor of calpain, leading to unrestricted proleolysis (Nath et al. 1996; Pang et al. 2003). Calpain activity leads to apoptosis via the mitochondrial pathway through activation of pro-apoptotic Bax and calcineurin, leading to release of cytochrome c from mitochondria and thus promoting activation of caspase-3 (Gao et al. 2000; Wu et al. 2004). Activated caspase-3 also cleaves calpain inhibitor calpastatin, further upregulating calpain activity leading to apoptosis or necrosis (Blomgren et al. 2001; Ray et al. 2003).
Since different pathophysiological pathways are activated following SCI, treatment with one pharmacological agent may not be effective. A combination of pharmacological agents or one multi-active agent may be more effective. One such muti-active agent is estrogen (17β-estradiol) and we have recently shown that treatment with estrogen protected neurons and preserved axons during acute phase (48 h) following SCI (Sribnick et al. 2003; Sribnick et al. 2005; Sribnick et al. 2009a). Similar findings have also been noted in ischemia and TBI (Dubal et al. 1999; Roof and Hall 2000a; Roof and Hall 2000b; Dubal et al. 2001; Jover et al. 2002). A gender difference has also been found in animals with experimental TBI and female TBI patients recover better than males (Groswasser et al. 1998; Bayir et al. 2004). However, the neuroprotective effects of estrogen found in vivo have been confirmed in vitro studies in glia and neurons exposed to free radicals or glutamate excitotoxicity (Sur et al. 2003; Sribnick et al. 2004; Das et al. 2005; Sribnick et al. 2009b). The estrogen mediated neuroprotective effect may be due to the multi-action characteristics of estrogen. It is a potent anti-oxidant (Moosmann and Behl 1999) and anti-inflammatory agent (Dimayuga et al. 2005). It upregulates anti-apoptotic genes and reduces Ca2+ influx (Nilsen et al. 2002; Sribnick et al. 2009a), and reduces calpain activation and activity (Sur et al. 2003; Sribnick et al. 2004) and apoptosis (Linford and Dorsa 2002; Sribnick et al. 2007). The down regulation of Ca2+ influx by estrogen has been found to be due to modulation of voltage-gated Ca2+ channels (Sribnick et al. 2009a). Because of the many beneficial effects of estrogen, our studies were designed to use the possible neuroprotective potential of estrogen in experimental SCI. Our earlier studies on treatment of acute SCI with estrogen (4mg/kg) at 15 min and 24 h post-injury were correlated with varous neuroprotective measures (Sribnick et al. 2005; Sribnick et al. 2006a). The goal of this study was to assess whether the early neuroprotection observed in the moderately severe acute SCI (Perot et al. 1987) following estrogen treatment could translate into long-term functional benefits.
Our current data demonstrated significant increase in survival of the estrogen treated chronic SCI rats. Also, significant improvement in locomotor function was noted in estrogen trated rats and this continued from 3 days post-injury until sacrifice at 42 days post-injury. Results from our study suggest that estrogen treatment improved locomotor function in chronic SCI and implied that estrogen could be a promising therapy for treating chronic SCI in humans.
MATERIALS AND METHODS
General Animal Care and Surgical Preparation
Adult male Sprague-Dawley rats (n = 54, weight 250–300 g) were housed in individual cages and given food and water ad libitum. All care, surgery, and induction of SCI were conducted in accordance with the “Guide for the Care and Use of Laboratory Animals” of the US Department of Health and Humans Services (National Institutes of Health, Bethesda, MD, USA) and approved by the Institutional Animal Care and Use Committee (IACUC) at the Medical University of South Carolina (Charleston, SC). Injury was induced by a trained technician using the modified weight-drop method (Perot et al. 1987). Briefly, rats were anesthetized by intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg). Once rats were confirmed to be unconscious by toe-pinch, a laminectomy was performed at T10. The spine was immobilized stereotaxically and an impounder was gently placed onto the dura. A clinically relevant, moderately severe injury (40 g.cm force) was induced by dropping a 5 g weight from a height of 8 cm (Perot et al. 1987). Sham operated animals underwent laminectomy alone. Upon awakening, rats were evaluated neurologically and monitored for food and water uptake and urine output. Prophylactic antibiotics or analgesics were not used, in order to prevent any possible interaction with the experimental therapy.
Animal Care for Chronic SCI Studies
The animals used and the injury induced were the same as described above; however, animal care also consisted of monitoring the chronically injured rats. Until bladder function was regained, bladders were massaged three times daily. Animals that did not regain bladder function were sacrificed. At 42 days post-injury, rats were anesthetized and sacrificed by decapitation. Following decapitation, the original laminectomy was extended and three 1-cm segments of spinal cord were removed, representing the lesion segment, the rostral penumbra, and the caudal penumbra.
Drug Delivery
Rats were divided into three treatment groups. Estrogen was dissolved in the vehicle DMSO. Sham group animals received a laminectomy alone, vehicle group SCI rats received DMSO only, and estrogen group SCI animals received estrogen. At 15 min and 24 h post-injury, 4 mg/kg estrogen was delivered intravenously by tail-vein injection to SCI rats. Following these initial doses, five daily doses of 2 mg/kg estrogen were given intraperitoneally, and vehicle group SCI rats received equal volumes of DMSO at identical time-points.
Assessment of Locomotor Function
Beginning at 24 h post-injury and twice weekly for 6 weeks, locomotor function was assessed using the BBB open field locomotor scale, as described previously (Basso et al. 1995). Scoring was performed by two trained technicians who were blinded to treatments. BBB scores were reported using two methods: counting dead animals or animals that were sacrificed early at BBB score of 0 for the assessment days occurring after death or by simply excluding BBB scores on assessment days after death.
Luxol Fast Blue (LFB) Staining
For LFB staining, samples were harvested and fixed overnight in a 4% paraformaldehyde solution in phosphate-buffered saline (PBS, pH 7.4). Following fixation, samples were embedded in paraffin and then thinly sliced sections (10 μm) were mounted onto the slides. LFB staining was performed as reported previously (Tyor et al. 2002). Briefly, slides were stained overnight at 60°C in 0.1% LFB (Solvent Blue 38, Sigma-Aldrich, St. Louis, MO) in acidified 95% ethanol. Differentiation and counterstaining were performed with 0.01% Li2CO3 and incubation in nuclear fast red [0.1% in 5% Al2(SO4)3] for 5 min. Stained sections were viewed on an Olympus microscope (BH-2, Melville, NY) at 40x magnification. Images were captured with a Magna Fire SP CCD camera (Optronics, Goleta, CA) using Magna Fire SP 2.1 software (Optronics), and the image processing was done using Photoshop software (Adobe Systems, Seattle, WA).
Tissue was stained so that myelin appeared blue and grey matter counterstained red. Pathology in the cross sections was readily apparent: demyelinated tissue appeared blanched, areas of hemorrhage appeared dark green, and areas with myelin damage appeared darker than areas of normal myelin. Using Photoshop software, an automated system was used to determine the percentage of pathology within spinal cord samples. The total area of the spinal cord section was selected and the number of pixels determined. The area of pathology was then selected, and these pixels were quantified as previously described (Sribnick et al. 2005). Analysis was blinded by having one individual conduct the surgeries and another examine and analyze the samples.
Immunohistolabeling
Spinal cord tissues were collected, immediately placed into frozen tissue embedding media (Histo Prep, Fisher, Fairlawn, NJ), and stored at −70°C. Prior to cutting, samples were warmed to −18°C and thin sections (5 μm) were cut using a Reichart-Jung cryostat (Cryocut 1800, Leica, Wetzlar, Germany). Sections were fixed in 95% ethanol and stored in PBS. For immunohistolabeling, sections were blocked for 1 h in 2% horse and 2% goat sera (Sigma-Aldrich) in PBS. Sections were then incubated with a primary IgG antibody against ED-2 (BMA Biomedicals, Switzerland) at 1:100, OX-42 (Biosource, Camarillo, CA) at 1:100, glial fibrillary acidic protein (GFAP) at 1:400, NeuN at 1:100 (Chemicon), calpain (Banik et al. 1983) at 1:100, or SMI311 (Sternberger Monoclonals, Lutherville, MD) at 1:5000 for 2 h. We used SMI311 antibody for detection of dephosphorylated neurofilament protein (dNFP) in the spinal cord sections. Before analysis of dFNP, samples were placed in a 10 mM citrate buffer solution (pH 6.0), autoclaved for 5 minutes, cooled to room temperature, and then washed in PBS.
Following incubation with the primary antibody, sections were washed three times in PBS for 5 min and then incubated at a 1:100 dilution for 1 h with secondary antibody [(anti-mouse IgG conjugated with fluorescein isothiocyanate (FITC)] (Vector, Burlinghame, CA) for single labeling and also with anti-rabbit IgG conjugated with Texas Red for double labeling. Following incubation with the secondary antibody, sections were washed twice in PBS and once in distilled water for 5 min each. Further processing of samples and imaging were done as described previously (Sribnick et al. 2006a).
Western Blot Analysis
Spinal cord tissues were collected and stored at −70ºC until homogenization. Each tissue segment was placed in 1 ml of homogenizing buffer containing 50 mM Tris-HCl (pH 7.4), 1 mM phenylmethylsulfonyl fluoride (PMSF) (Bethesda Research Laboratories, Gaithersburg, MD) and 5 mM EGTA (Sigma-Aldrich) and homogenized with a Polytron batch homogenizer (Kinematica, Cincinnati, OH). The homogenized samples were then centrifuged in an Optima LE-80K Ultracentrifuge (Beckman Coulter, Fullerton, CA) for 1 h at 100,000x g to form a cytosolic supernatant and a pellet containing the nuclear fraction. Following centrifugation, protein concentration in each sample was determined using Coomassie Plus Reagent (Pierce, Rockford, IL) and spectrophotometric measurement at 595 nm (Spectronic, Rochester, NY). Samples were then diluted (1:1) in a sample buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 5 mM β-mercaptoethanol, 10% glycerol), boiled for 5 min, and stored at −20ºC. Samples were loaded onto 4–20% gels and electrophoresed at 200 V for 30 min. The resolved proteins were then transferred to nylon membrane (Millipore, Billerica, MA) using the transfer apparatus Genie (Idea Scientific, Minneapolis, MN). The nylon membranes were then blocked for 1 h in 5% non-fat milk in Tris/Tween buffer (20 mM Tris-HCl, pH 7.6, and 0.1% Tween 20 in saline). Membranes were incubated overnight with an appropriate dilution of a primary IgG antibody such as (1:5000) β-actin (clone AC-15) (Sigma Chemical, St. Louis, MO), (1:500) Bax, (1:500) Bcl-2 (Santa Cruz Biotechnology), (1:500) NF-κB, (1:500) IκB-α, (1:2000) caspase-3 (Santa Cruz Biotechnology, Santa Cruz, CA), (1:500) active calpain isoform (raised in our laboratory), or (1:5000) α-spectin (Affinity, Exeter, UK). Membranes were then incubated for 1 h with a horseradish peroxidase-conjugated secondary antibody (ICN Biomedicals, Aurora, OH) such as (1:2000) goat anti-mouse IgG for detecting monoclonal primary IgG antibody or (1:2000) goat anti-rabbit IgG for detecting polyclonal primary IgG antibody. Between each step, membranes were washed three times with Tris/Tween buffer. Membranes were then incubated with enhanced chemiluminescent (ECL) reagent (Amersham, Piscataway, NJ) and exposed to X-OMAT AR films (Kodak, Rochester, NY), as described before (Sribnick et al. 2006a; Sribnick et al. 2006b). Films were then scanned on a UMAX PowerLook Scanner using Photoshop software (Adobe Systems), and the optical density (OD) of each band was determined using Quantity One software (Bio-Rad).
Determination of NF-κB Translocation and IκB-α Degradation
Western blotting was also used to determine the levels of NF-κB and IκB-α in the cytosolic and nuclear fractions isolated from the spinal cord samples using a fractionation kit (BioVision, Mountain View, CA).
Colorimetric Assay for Cyclooxygenase-2 (COX-2) Activity
A COX-2 activity assay kit (Cayman Chemical, Ann Arbor, MI) was used according to the manufacturer’s protocol. Tissues were immediately removed and stored at −70ºC until homogenization. Each tissue segment was then placed in homogenizing buffer (0.1 M Tris-HCl, pH 7.8, 1 mM EDTA). A cytosolic supernatant was obtained by centrifugation at 10,000x g for 15 min. Approximately 100 μl of supernatant was mixed with 280 μl of assay buffer, 20 μl of heme, and 20 μl of the COX-1 inhibitor, SC-560, in 1.5 ml Eppendorf tubes. The total volume of each tube was then adjusted to 1 ml. Tubes were mixed by inversion and incubated for 10 min at 25ºC. Colorimetric substrate (40 μl) and 40 μl arachidonic acid solution were added sequentially, mixed by inversion, and incubated at 37ºC for 10 min. The absorbance for each sample was read at 590 nm using a spectrophotometer (Spectronic). All colorimetric assays for COX-2 activity were conducted in triplicate.
Statistical Analysis
Results obtained from different treatments were analyzed using StatView software (Abacus Concepts, Berkeley, CA). Data were expressed as mean ± standard error of the mean (SEM) of separate experiments (n≥3) and compared by one-way analysis of variance (ANOVA) followed by Fisher’s post hoc test. The difference between two treatments was considered significant at P ≤ 0.05.
RESULTS
Assessment of Locomotor Function
After SCI, locomotor function was assessed in animals at 1 day post-injury and then twice weekly until 42 days post-injury when the rats were sacrificed (Fig. 1). In general, improvements in motor function tended to occur in the 3rd week following injury with little change noted thereafter. In assessing the BBB scale by scoring dead animals as 0, significant improvements were noted in estrogen treated animals, when compared with vehicle treated animals. This significant difference was seen as early as 3 days post-injury (P = 0.024) and remained until the day the animals were sacrificed (P = 0.021). By 42 days, the final average scores were approximately 13 for estrogen treated rats and approximately 9 for vehicle treated rats. Functionally, these scores indicate that estrogen treated rats, on average, were supporting their own body weight, making weight-supported steps using the plantar surface of the hind paw, and coordinating hindlimb/forelimb stepping more often than not. Vehicle treated rats, on average, were able to use the plantar surface of the hind paw for weight-support but were not able to perform normal plantar stepping.
Fig. 1.
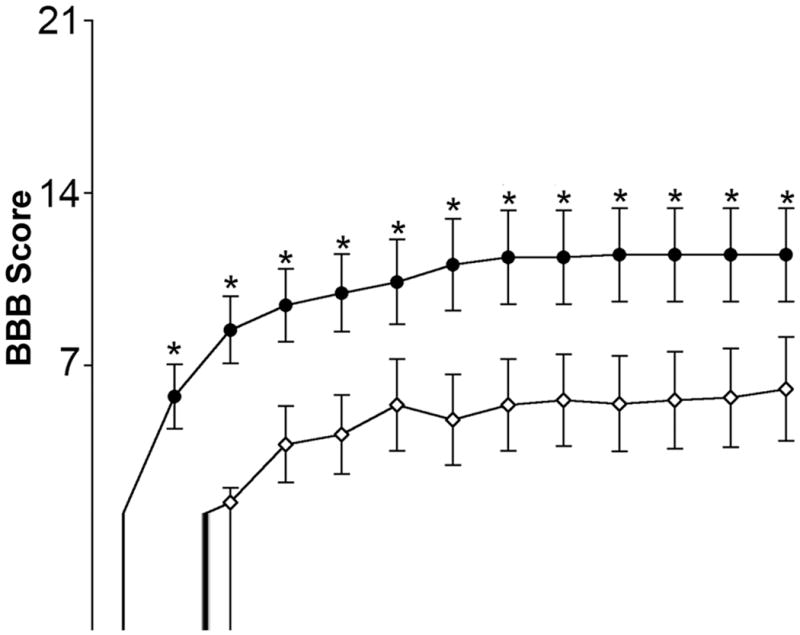
Assessment of locomotor function in chronic SCI. The BBB scale was used to examine locomotor function in estrogen treated animals (closed circles) and vehicle treated (open diamonds) SCI animals at day 1 and then twice a week thereafter. Sacrifice occurred on day 42. Significant difference between vehicle and estrogen was indicated by *P < 0.05 (n ≥ 20).
Post-Injury White Matter Integrity
In order to examine the histology of chronic SCI animals, tissue samples from the lesion and both penumbra were removed following sacrifice at 42 days post-injury, and all histological studies were conducted using 6-week post-injury tissue (Fig. 2). In the weeks following a contusion SCI (e.g. injury by weight drop), the cyst that forms is maximal at the lesion and narrows as it extends into the penumbra (Fig. 2A). This was seen in the current study when evaluating spinal cord stained with LFB. Damage in all sham segments was negligible (i.e. less than 0.05%), and any abnormalities in the sham tissue may be attributed to handling during dissection or the fixation/drying process for staining (Fig. 2B). Compared with sham, both vehicle treated and estrogen treated animals showed non-significant increases in tissue damage in the rostral and caudal penumbra, and the damaged tissue in these penumbra regions was less than 3%. At the lesion, damage was generally noted as a central cystic area surrounded by abnormal myelin. This abnormal myelin contained readily apparent spots, known as “microcysts” (Basso et al. 1996). Surrounding the abnormal myelin was a periphery of normal appearing myelin. While this general observation was seen at the lesion in samples from both vehicle treated and estrogen treated animals, the percentage of damaged spinal cord was significantly greater in the vehicle treated rats (P ≤ 0.0001 and P = 0.0011, respectively), when compared with sham or estrogen treated rats. A significant increase in spinal cord damage was also noted when comparing the lesion segment of estrogen treated animals with sham (P = 0.008).
Fig. 2.
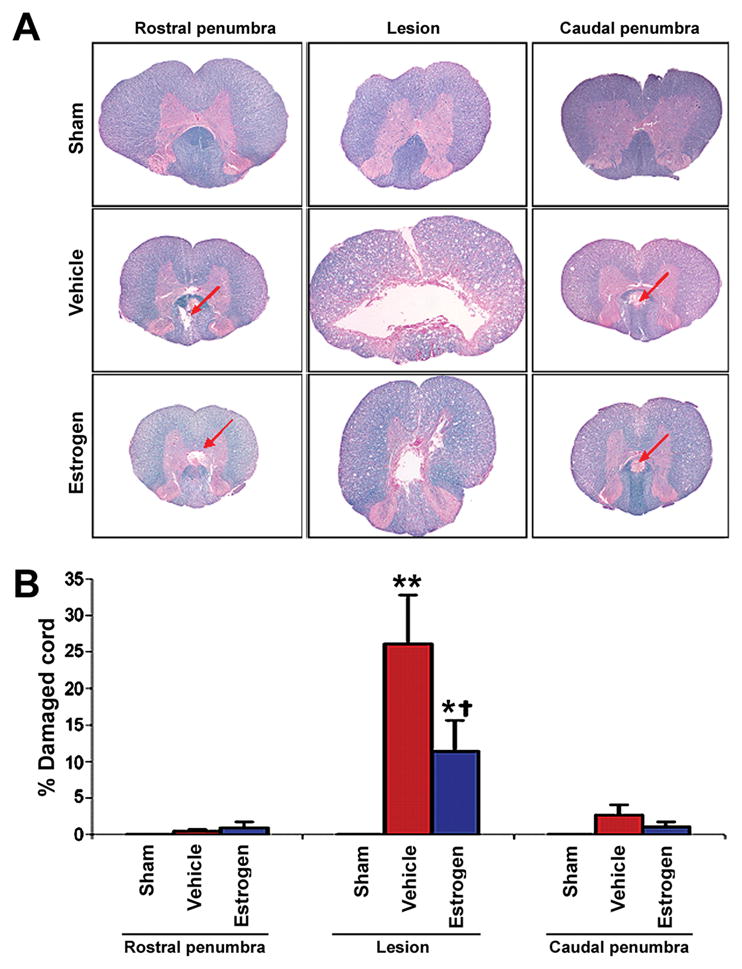
Assessment of myelin integrity using LFB staining. Thin (10 μm) sections were cut from paraffin-embedded spinal cord tissues. A: Representative samples are shown (at 40x magnification). Focal areas of pathology in the penumbra are indicated with red arrows. B: From each spinal cord segment, sections were taken at 500 μm intervals and were assessed for damage. Significant difference from sham values was indicated by *P <0.05 or **P < 0.0001. Significant difference between vehicle and estrogen was indicated by †P < 0.05 (n ≥ 3).
Estrogen Treatment Ameliorated Astroglial Reactivity in SCI
Astroglial reactivity was examined in chronic SCI tissue sections by immunostaining with GFAP antibody (Fig. 3). Increased astroglial reactivity was seen in the gray matter (ventral horn) as well as in the white matter of the lesion of SCI sections. Qualitatively, GFAP immunoreactivity was reduced by estrogen treatment in both gray matter and white matter of spinal cord (Fig. 3). The reduced GFAP immunoreactivity was seen in the lesion ventral horn and white matter following estrogen treatment, indicating restoration of normal morphology of the white matter (Fig. 3). No astroglial reactivity was seen in the caudal penumbra from SCI animals following estrogen treatment (data not shown), indicating that the inflammatory response was almost eliminated with estrogen treatment.
Fig. 3.
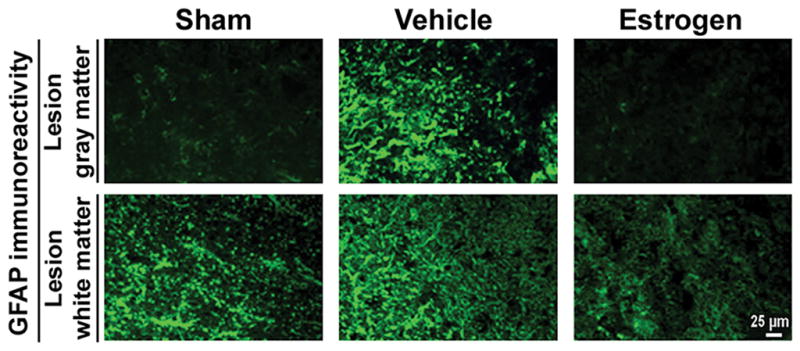
Examination of astrogliosis in chronic SCI. Thin frozen sections (5 μm) of the lesion and caudal penumbra spinal cord tissues (6 weeks post-injury) were stained with GFAP antibody as a marker for astrogliosis. Representative images from lesion spinal cord are presented (at 200x magnification). Caudal penumbra images are omitted because there were no significant differences among different groups after 6 weeks (n ≥ 3).
Estrogen Attenuated Macrophage and Microglial Activation in SCI
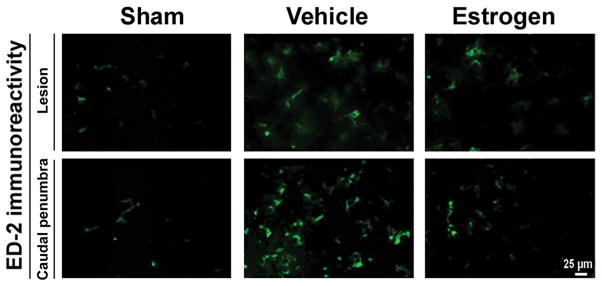
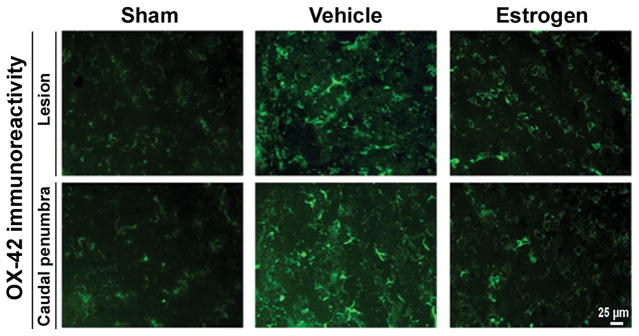
Activation of macrophages and microglia is a common feature in the inflammatory process following SCI. We examined the efficacy of estrogen in reducing the extent of activated macrophages and microglia by immunostaining of tissue sections with antibodies to OX-42 (Fig. 4) and ED-2 (Fig. 5), markers for microglia and activated macrophages, respectively. Our results indicated prominent increases in OX-42 and ED-2 immunoreactivity in the lesion and caudal penumbra of the chronic SCI animals. The extent of activation of microglia and macrophages was substantially reduced in both lesion and caudal penumbra of the spinal cord following treatment with estrogen.
Fig. 4.
Activated macrophage infiltration in chronic SCI tissues. Thin frozen sections (5 μm) of the lesion and caudal penumbra spinal cord tissues (6 weeks post-injury) were stained with ED-2 antibody as a marker for activated macrophages (at 200x magnification). Estrogen treatment attenuated the activated macrophage infiltration in chronic SCI tissues in rats (n≥3).
Fig. 5.
Examinantion of microgliosis in chronic SCI tissues. Thin frozen sections (5 μm) of the lesion and caudal penumbra spinal cord tissues (6 weeks post-injury) were stained with OX-42 antibody as a marker of microgliosis (at 200x magnification). Restored microglial morphology, similar to that seen in control, was noted in SCI spinal cords treated with estrogen (n ≥ 3).
Estrogen Attenuated Inflammatory COX-2 Activity in SCI
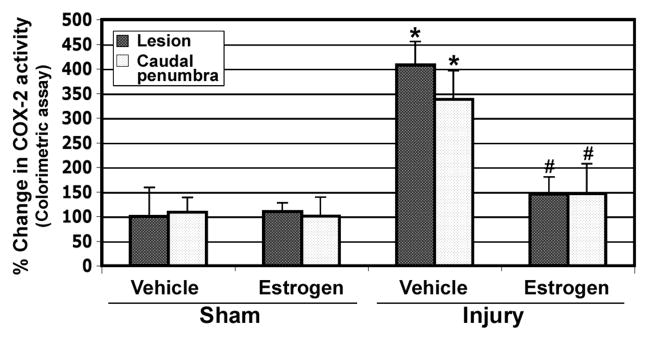
We colorimetrically determined the COX-2 activity in the lesion and penumbra of chronic SCI tissues from sham-vehicle and sham-estrogen as well as from injury-vehicle and injury-estrogen treated animals (Fig. 6). No changes were observed between sham-vehicle and sham-estrogen treated rats. In contrast, COX-2 activity was significantly increased in the lesion (4-fold) and penumbra (over 3-fold) in the injury-vehicle treated rats, compared with sham-vehicle animals. This increase in COX-2 activity in chronic SCI was markedly reduced following estrogen treatment, compared with untreated injury animals (Fig. 6).
Fig. 6.
Estrogen attenuated COX-2 activity in chronic SCI. The activity of the inflammatory enzyme COX-2 was determined by colorimetric assay in chronic SCI lesion and penumbra tissues. Treatment with estrogen significantly reduced COX-2 activity both in the lesion and caudal penumbra (n≥3).
Estrogen Attenuated NF-κB Translocation and IκB-α Degradation Following SCI
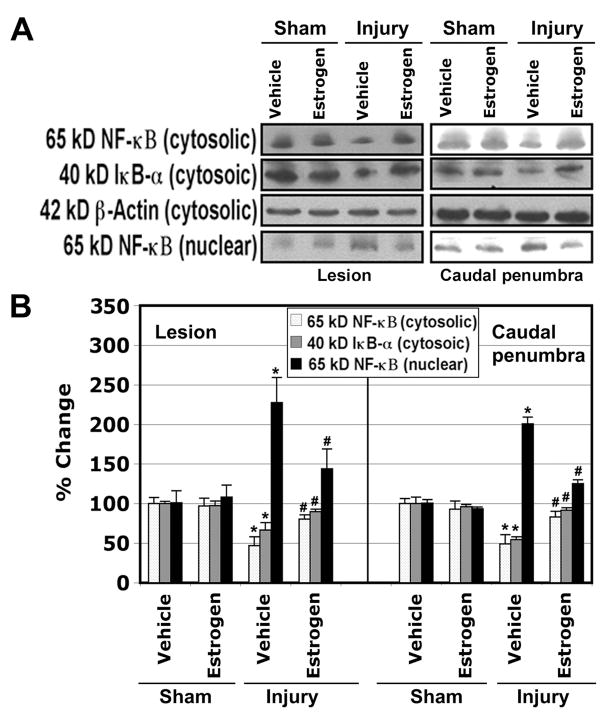
The levels of 65 kD NF-κB and 40 kD IκB-α in the lesion and caudal penumbra samples were determined in both cytosolic and nuclear fractions by Western blot analysis (Fig. 7). Experiments were conducted in triplicates and representative Western blots were shown (Fig. 7A). Our results indicated a significant 35% decline in cytosolic NF-κB in vehicle treated injury animals in the lesion as well as in caudal penumbra, compared with sham animals. This was reversed in the estrogen treated injury animals (Fig. 7B). The levels of NF-κB from nuclear fractions of both segments showed a significant 2-fold increase in the vehicle treated injury animals, compared with sham. Estrogen treated injury animals showed a significant reduction in nuclear NF-κB. Cytosolic IκB-α in the lesion and caudal penumbra samples from vehicle treated injury animals showed a significant (around 40%) decrease, compared with those samples from sham animals, and it was reversed in the estrogen treated injury animals. These results indicated that estrogen treatment attenuated NF-κB translocation and IκB-αdegradation in the lesion and caudal penumbra following chronic SCI.
Fig. 7.
Estrogen treatment attenuated translocation of NF-κB to nucleus in chronic SCI. Western blot analysis was used to assess the translocation of NF-κB from the cytosol to the nucleus and also the degradation of IκB-α in both lesion and caudal penumbra. A: Representative Western blots show NF-κB and IκB-α in the cytosolic fractions and NF-κB in the nuclear fractions.β-Actin was used as a loading control. B: Densitometry of the Western blots. Significant difference from sham and vehicle values was indicated by *P < 0.05 and significant difference between estrogen and vehicle was indicated by #P < 0.05.
Estrogen Maintained Neuron Density Following SCI
Neuron density was examined in SCI sections by immunohistolabeling using an antibody specific for neuronal nuclei (NeuN) and an antibody recognizing calpain (Fig. 8). Qualitatively, no apparent change in calpain expression was noted in different treatment groups or different spinal cord segments. Neuron density was decreased in both the lesion and caudal penumbra sections of vehicle treated animals, when compared with corresponding sections of sham animals. Also, cystic areas in the tissue were evident in vehicle treated animals. Neuron density in estrogen treated animals was comparable to sham animals; however, cystic areas were still noted in both the lesion and caudal penumbra.
Fig. 8.
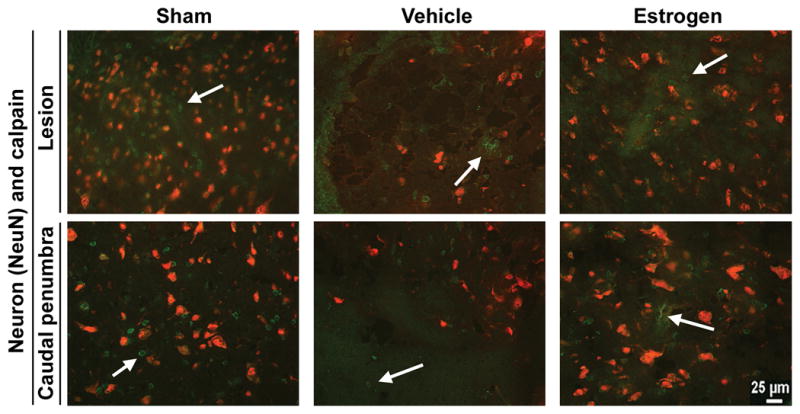
Immunofluorescent labeling for neurons and calpain. Thin frozen sections (5 μm) were obtained from the lesion and caudal penumbra spinal cord tissues (6 weeks post-injury). Sections were incubated with antibodies recognizing NeuN (a nuclear marker specific for neurons) and calpain. Representative images are shown (at 200x magnification) using the lesion and caudal penumbra (n ≥ 3). Due to the secondary antibodies used, neurons appeared red and calpain appeared green. Labeling of calpain expression was indicated by an arrow.
Estrogen Attenuated Increase in Bax:Bcl-2 Ratio in SCI
Activation of apoptotic process may be involved in decreasing neuron density in both the lesion and penumbra of tissue sections from SCI animals. Western blot analysis was carried out to determine the levels of expression of apoptosis regulatory proteins such as Bax (pro-apoptotic) and Bcl-2 (anti-apoptotic) in the vehicle treated and estrogen treated animals (Fig. 9). Increased expression of pro-apoptotic marker Bax was noted following injury in both the lesion and caudal penumbra, concomitant with a decrease in expression of anti-apoptotic marker Bcl-2 (Fig. 9A). There was an increase in Bax:Bcl-2 ratio in SCI tissues from animals treated with vehicle only (Fig. 9B), indicating activation of the apoptotic process. Estrogen was found to attenuate the increase in Bax:Bcl-2 ratio in SCI tissues (Fig. 9), suggesting that neuroprotective effect of estrogen was associated with prevention of apoptotic cell death.
Fig. 9.
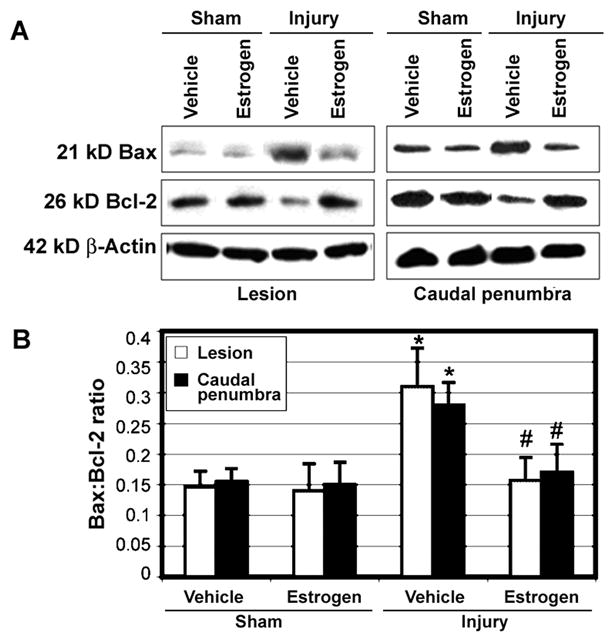
Western blotting of the apoptosis regulatory Bax and Bcl-2 proteins in chronic SCI tissues. A: The lesion and caudal penumbra spinal cord tissues were used in Western blotting of Bax and Bcl-2 proteins. β-Actin expression was monitored for equal loading. B: Quantification of OD of protein bands for determining Bax:Bcl-2 ratio (n ≥ 3). Significant difference was noted in Bax:Bcl-2 ratio between sham and injured animals (*P < 0.05). Significant difference between vehicle treated animals and estrogen treated animals was indicated by #P < 0.05.
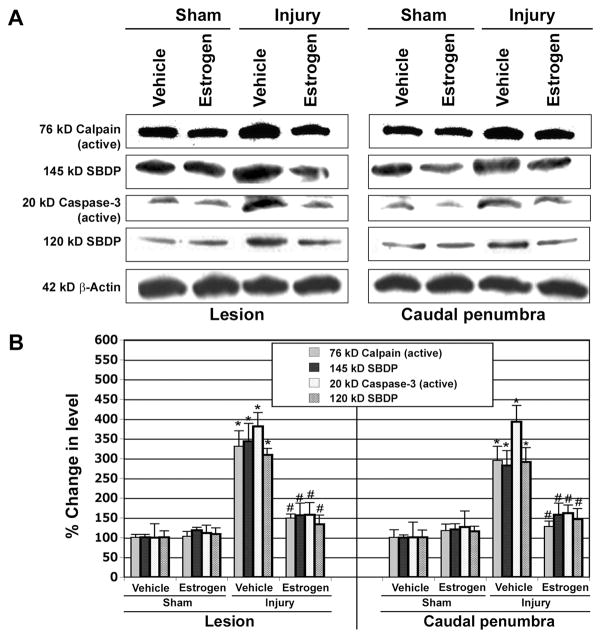
Estrogen Inhibited Activation and Activity of Calpain and Caspase-3 in SCI
Increases in cysteine protease activities such as calpain and caspase-3 have been implicated in the neurodegenerative mechanisms in SCI (Blomgren et al. 2001; Ray et al. 2003). The effect of estrogen treatment was examined on calpain and caspase-3 activity in the lesion and caudal penumbra tissue sections from sham and vehicle treated injury animals (Fig. 10). The degradation of α-spectrin to calpain-specific 145 kD spectrin breakdown product (SBDP) and caspase-3-specific 120 kD SBDP formation, as analyzed by Western blot, was taken as a measure of the activities of the respective enzymes (Fig. 10A). Analysis of Western blots showed significant increases in generation of active calpain and caspase-3 fragments as well as significant increases in the formation of calpain-specific 145 kD SBDP and caspase-3-specific 120 kD SBDP in the lesion and caudal penumbra tissues (Fig. 10B). The activation and activities of calpain and caspase-3 were significantly reduced in the estrogen treated SCI animals, compared with vehicle treated animals (Fig. 10B). Inhibition of these cysteine proteases by estrogen could contribute to survival of neurons in chronic SCI animals.
Fig. 10.
Western blotting to determine activation and activity of calpain and caspase-3 in chronic SCI tissues. A: The lesion and caudal penumbra spinal cord tissues were used in Western blotting to examine active forms and activities of calpain and caspase-3. β-Actin expression was monitored for equal loading. B: Quantification of OD of protein bands for determining levels of calpain and caspase-3 active forms and their proteolytic activities in generation of 145 kD SBDP and 120 kD SBDP, respectively (n≥3). Significant difference between sham and injured animals was indicated by *P < 0.05. Significant difference between estrogen treated animals and vehicle treated animals was indicated by #P < 0.05.
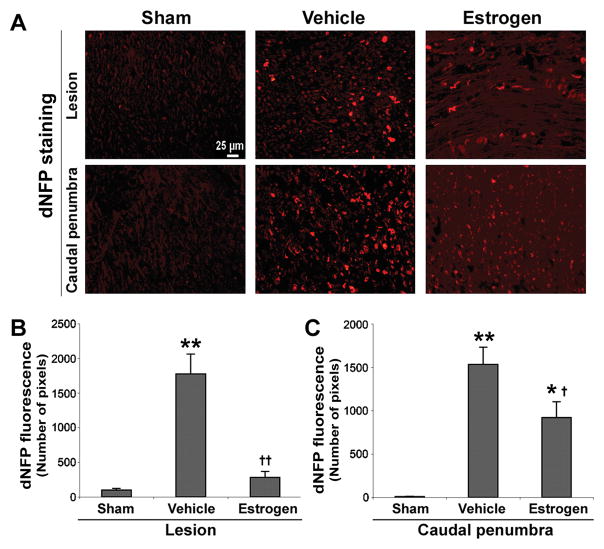
Examination of Axonal Integrity Using dNFP Antibody
In order to examine the integrity of axons in the white matter (Fig. 11), spinal cord sections were stained with an antibody that specifically binds dNFP (Sternberger and Sternberger 1983). Ventral and dorsal white matter was examined in the lesion and caudal penumbra spinal cord sections of the three treatment groups of animals (Fig. 11A). A basal level of dNFP fluorescence was noted in both the lesion and caudal penumbra of sham animals and, in general, dNFP levels were greater in the caudal penumbra than in the lesion of SCI animals. Quantitative analysis by NIH Image and pixel counting (Fig. 11B) showed that within the lesion, vehicle treated rats had significant increases in dNFP levels, compared with sham or estrogen treated rats (P ≤ 0.0001 for both), and there was no significant difference (P = 0.46) in dNFP fluoresecence between estrogen treated rats and sham animals. In caudal penumbra, dNFP fluorescence in vehicle treated rats was significantly greater than that seen in sham or estrogen treated animals (P ≤ 0.0001 and P = 0.0091, respectively). Estrogen treatment decreased dNFP in the lesion, indicating that estrogen partly preserved axonal integrity in chronic SCI rats.
Fig. 11.
Assessment of axonal integrity by immunohistolabeling for dNFP. Thin frozen sections (5 μm) were obtained from lesion and caudal penumbra spinal cord tissues (6 weeks post-injury). Sections were stained with antibody recognizing dNFP. A: Representative images are shown from the three treatment groups (at 200x magnification). B: Quantitation of dNFP fluorescence for determination of axonal damage (n≥3). Pixels were counted using the NIH Image software. Significant difference from control values was indicated by *P < 0.05 or **P < 0.0001, and significant difference between vehicle treated animals and estrogen treated rats was indicated by †P < 0.05 or ††P < 0.0001.
DISCUSSION
Estrogen treatment has been previously shown to be effective in reducing pathogenesis in TBI (Roof and Hall 2000b), ischemia (Dubal et al. 1999; Jover et al. 2002) and SCI models (Yune et al. 2004; Sribnick et al. 2005). While earlier work from our laboratory focused on estrogen in acute SCI, the current study involves a chronic SCI model and examines functional outcome in animals when the injury is comparatively static. In general, significant improvement in locomotor function occurred in the chronic SCI rats due to estrogen treatment. This investigation further demonstrated that estrogen treatment attenuated inflammation, protected neurons, and decreased axonal damage to promote functional recovery in chronic SCI.
Increased calpain activity has been implicated in many neurodegenerative processes, including damage to neurons and axons in SCI (Schaecher et al. 2004), experimental auto-immune enceahalomyelitis (EAE) (Guyton et al. 2005), TBI (Posmantur et al. 1997), ischemia (Bartus et al. 1994), and Parkinson’s disease (Samantaray et al. 2007). In SCI, infiltration of inflammatory cells in the lesion occurs following injury (Popovich et al. 1997; Carlson et al. 1998), and these cells have been found to produce and release chemokines, cytokines, ROS, COX-2, and others factors involved in the inflammatory process (Miyamoto et al. 1999; Aronica et al. 2004). Compared with vehicle treated rats, estrogen treated acute SCI rats showed significantly less edema and markedly fewer macrophages and microglia in the lesion and penumbra (Sribnick et al. 2006b). Similar changes were found in chronic SCI following estrogen treatment, as marked attenuation of astroglial reactivity was seen in ventral horn and white matter in the lesion and caudal penumbra. Activated microglia/macrophages in the lesion and penumbra were also reduced in chronic SCI following estrogen treatment, suggesting that estrogen showed anti-inflammatory effects. Such effects were also demonstrated in the decrease in COX-2 activity in the estrogen treated animals, compared with vehicle treated chronic SCI rats.
The activation of NF-κB, a pro-inflammatory transcription factor, is an important step in the development of inflammation, and estrogen treatment has been found to block activation of NF-κB in vitro in thymocytes (Xie et al. 2002) as well as in acute SCI (Sribnick et al. 2005). Calpain has an important role in NF-κB activation: IκB, the endogenous inhibitor of NF-κB, is a calpain substrate (Schaecher et al. 2004) and the cleavage of IκB by calpain facilitates translocation of NF-κB from cytosol to nucleus. Activation of NF-κB has been previously demonstrated in SCI (Bethea et al. 1998) and estrogen treatment significantly attenuated NF-κB activation and decreased the level of nuclear NF-κB in acute SCI (Sribnick et al. 2005) as well as in chronic, as reported in the present study. The prevention of activation or translocation of NF-κB has been shown to be neuroprotective in ischemia in vivo (Schneider et al. 1999). In agreement with our SCI studies, estrogen treatment of rats following ischemia was found to inactivate NF-κB (Wen et al. 2004). These in vivo findings of decreased NF-κB activation by estrogen have been complemented by in vitro studies (Dodel et al. 1999; Xie et al. 2002). Whether the inhibition of NF-κB activation is estrogen receptor mediated or not is unclear, but upregulation of both ERα and ERβ in the penumbra of estrogen treated acute SCI suggests a possible link (Sribnick et al. 2006b). This has been recently confirmed in our studies with low dose estrogen treatment of acute and chronic SCI (Samantaray et al. 2009). Thus, there is a possibility that such a mechanism may exist in estrogen mediated attenuation of inflammation in chronic SCI. Nonetheless, the supraphysiologic dose of estrogen that we used in this study suggests that the neuroprotection may also have been due to anti-oxidant effects. Estrogen receptors have been found to be both anti-inflammatory and neuroprotective following estrogen treatment in ischemia, TBI, and EAE, and multiple sclerosis (Dubal et al. 2001; Vegeto et al. 2003; Morales et al. 2006). In the in vitro studies using microglia, estrogen treatment has been shown to upregulate both estrogen receptors and estrogen receptor agonists can reverse their levels (Smith et al. 2009).
Estrogen treatment has been correlated with changes in calpain content and activity in acute SCI (Sribnick et al. 2006a). The finding that estrogen prevents post-traumatic increases in calpain activity was also replicated in the in vitro studies. In C6 cells treated with H2O2 (Sur et al. 2003) or glutamate (Sribnick et al. 2006b), estrogen treatment significantly attenuated increases in formation of 145 kD calpain-specific SBDP. In the acute SCI rats, estrogen treatment significantly decreased the formation of 145 kD calpain-specific SBDP in caudal penumbra (Sribnick et al. 2006b). Calpain expression in vehicle treated chronic SCI rats was decreased in the lesion and penumbra. This might be due to phagocytosis of cells that died due to apoptosis and necrosis and were removed during this long time following injury. Estrogen treatment protected neurons in the lesion and caudal penumbra of chronic SCI rats. This is supported by significant decrease in Bax:Bcl-2 ratio, which is a commitment to cell death, following estrogen treatment. Neuronal death in chronic SCI could be associated with increases in activation and activity of calpain and caspase-3, which were significantly down regulated due to estrogen treatment. Also, axonal degeneration was prevented following estrogen treatment. The attenuation of post-traumatic neuronal death and the preservation of axons and myelin may explain the overall improvements seen in tissue histology and locomotor function.
The LFB staining indicated that while there were some changes in the caudal penumbra of SCI animals at 6-weeks post-injury, these were relatively small, compared with the damage seen at the lesion epicenter. When comparing damage seen in acute phase (i.e. 2 days post-injury) with that seen chronic phase (i.e. 42 days post-injury), the striking difference is that while pathology in the penumbra becomes negligible over time, pathology at the lesion center is reduced (by approximately 10%) but still present. The LFB staining results from the chronic SCI also correlate well with the TUNEL staining from the acute SCI (Sribnick et al. 2005). These findings suggest that areas with high levels of TUNEL staining days after the injury are more likely to be damaged at 42 days post-injury.
Neuron density at 6 weeks post-injury also correlated with LFB staining. This finding suggests that, while there was little co-staining of NeuN and TUNEL in the acute injury (Sribnick et al. 2006a), neurons are dying as the injury evolves. Axonal integrity was examined by immunohistolabeling of dNFP. Dephosphorylation accelerates calpain mediated degradation of NFP (Pant 1988). Past studies showed that dNFP was rapidly degraded following SCI, and calpain inhibitors limited this degradation (Schumacher et al. 2000). The current study showing substantially higher level of dNFP in the caudal penumbra than that in the lesion is puzzling; however, there are possible explanations for this finding. If calpain activity in the lesion is elevated, compared with caudal penumbra, there will be increased calpain mediated degradation of NFP in the lesion, causing decreases in dNFP content as well. In support of this explanation, at 6 weeks post-injury, prominent damage to the white matter was noted in the LFB staining of the lesion, whereas only areas of focal pathology were noted in the caudal penumbra. In general, the estrogen treated rats showed significant recovery starting at 3 days post-injury, as assessed by locomotor scoring. Estrogen treatment significantly increased the locomotor function in the injured animals over the 42 day post-injury period, compared with vehicle treated chronic SCI rats.
In conclusion, estrogen treatment protected neurons and axons and also attenuated inflammation in chronic SCI so as to significantly improve locomotor function and survival of the animals. Although the focus of this study was to investigate the efficacy of high dose estrogen in chronic SCI, studies with low or physiologic doses of estrogen would be ideal and clinically relevant. Such studies are in progress in our laboratory and preliminary results indicate that low dose estrogen can be promising for treating acute as well as chronic SCI (Samantaray et al. 2009). All in all, estrogen is a promising therapeutic agent for treatment of chronic SCI.
Acknowledgments
Grant support: This work was funded in part by the grants from the NIH (NS-31622 and NS-45967) and also by a grant from the State of South Carolina Spinal Cord Injury Research Fund (SCIRF-1205).
References
- Agrawal SK, Fehlings MG. Mechanisms of secondary injury to spinal cord axons in vitro: role of Na+, Na+-K+-ATPase, the Na+-H+ exchanger, and the Na+-Ca2+ exchanger. J Neurosci. 1996;16:545–552. doi: 10.1523/JNEUROSCI.16-02-00545.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal SK, Nashmi R, Fehlings MG. Role of L- and N-type calcium channels in the pathophysiology of traumatic spinal cord white matter injury. Neuroscience. 2000;99:179–188. doi: 10.1016/s0306-4522(00)00165-2. [DOI] [PubMed] [Google Scholar]
- Aronica SM, Fanti P, Kaminskaya K, Gibbs K, Raiber L, Nazareth M, Bucelli R, Mineo M, Grzybek K, Kumin M, Poppenberg K, Schwach C, Janis K. Estrogen disrupts chemokine-mediated chemokine release from mammary cells: implications for the interplay between estrogen and IP-10 in the regulation of mammary tumor formation. Breast Cancer Res Treat. 2004;84:235–245. doi: 10.1023/B:BREA.0000019961.59306.f6. [DOI] [PubMed] [Google Scholar]
- Banik NL, Hogan EL, Jenkins MG, McDonald JK, McAlhaney WW, Sostek MB. Purification of a calcium-activated neutral proteinase from bovine brain. Neurochem Res. 1983;8:1389–1405. doi: 10.1007/BF00964996. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Hayward NJ, Elliott PJ, Sawyer SD, Baker KL, Dean RL, Akiyama A, Straub JA, Harbeson SL, Li Z, et al. Calpain inhibitor AK295 protects neurons from focal brain ischemia. Effects of postocclusion intra-arterial administration. Stroke. 1994;25:2265–2270. doi: 10.1161/01.str.25.11.2265. [DOI] [PubMed] [Google Scholar]
- Barut S, Canbolat A, Bilge T, Aydin Y, Cokneseli B, Kaya U. Lipid peroxidation in experimental spinal cord injury: time-level relationship. Neurosurg Rev. 1993;16:53–59. doi: 10.1007/BF00308614. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Bayir H, Marion DW, Puccio AM, Wisniewski SR, Janesko KL, Clark RS, Kochanek PM. Marked gender effect on lipid peroxidation after severe traumatic brain injury in adult patients. J Neurotrauma. 2004;21:1–8. doi: 10.1089/089771504772695896. [DOI] [PubMed] [Google Scholar]
- Bethea JR, Castro M, Keane RW, Lee TT, Dietrich WD, Yezierski RP. Traumatic spinal cord injury induces nuclear factor-κB activation. J Neurosci. 1998;18:3251–3260. doi: 10.1523/JNEUROSCI.18-09-03251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomgren K, Zhu C, Wang X, Karlsson JO, Leverin AL, Bahr BA, Mallard C, Hagberg H. Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia: a mechanism of "pathological apoptosis"? J Biol Chem. 2001;276:10191–10198. doi: 10.1074/jbc.M007807200. [DOI] [PubMed] [Google Scholar]
- Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, Fehlings MG, Herr DL, Hitchon PW, Marshall LF, Nockels RP, Pascale V, Perot PL, Jr, Piepmeier J, Sonntag VK, Wagner F, Wilberger JE, Winn HR, Young W. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up. Results of the third National Acute Spinal Cord Injury randomized controlled trial. J Neurosurg. 1998;89:699–706. doi: 10.3171/jns.1998.89.5.0699. [DOI] [PubMed] [Google Scholar]
- Carlson SL, Parrish ME, Springer JE, Doty K, Dossett L. Acute inflammatory response in spinal cord following impact injury. Exp Neurol. 1998;151:77–88. doi: 10.1006/exnr.1998.6785. [DOI] [PubMed] [Google Scholar]
- Das A, Sribnick EA, Wingrave JM, Del Re AM, Woodward JJ, Appel SH, Banik NL, Ray SK. Calpain activation in apoptosis of ventral spinal cord 4.1 (VSC4.1) motoneurons exposed to glutamate: calpain inhibition provides functional neuroprotection. J Neurosci Res. 2005;81:551–562. doi: 10.1002/jnr.20581. [DOI] [PubMed] [Google Scholar]
- Dhillon HS, Carman HM, Prasad RM. Regional activities of phospholipase C after experimental brain injury in the rat. Neurochem Res. 1999;24:751–755. doi: 10.1023/a:1020779413122. [DOI] [PubMed] [Google Scholar]
- Dimayuga FO, Reed JL, Carnero GA, Wang C, Dimayuga ER, Dimayuga VM, Perger A, Wilson ME, Keller JN, Bruce-Keller AJ. Estrogen and brain inflammation: effects on microglial expression of MHC, costimulatory molecules and cytokines. J Neuroimmunol. 2005;161:123–136. doi: 10.1016/j.jneuroim.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Dodel RC, Du Y, Bales KR, Gao F, Paul SM. Sodium salicylate and 17beta-estradiol attenuate nuclear transcription factor NF-κB translocation in cultured rat astroglial cultures following exposure to amyloid Aβ (1–40) and lipopolysaccharides. J Neurochem. 1999;73:1453–1460. doi: 10.1046/j.1471-4159.1999.0731453.x. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Shughrue PJ, Wilson ME, Merchenthaler I, Wise PM. Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. J Neurosci. 1999;19:6385–6393. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptorα, notβ, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci USA. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao MX, Li K, Dong J, Liege S, Jiang B, Neveu PJ. Strain-dependent association between lateralization and lipopolysaccharide- induced IL-1β and IL-6 production in mice. Neuroimmunomodulation. 2000;8:78–82. doi: 10.1159/000026456. [DOI] [PubMed] [Google Scholar]
- Groswasser Z, Cohen M, Keren O. Female TBI patients recover better than males. Brain Inj. 1998;12:805–808. doi: 10.1080/026990598122197. [DOI] [PubMed] [Google Scholar]
- Guyton MK, Wingrave JM, Yallapragada AV, Wilford GG, Sribnick EA, Matzelle DD, Tyor WR, Ray SK, Banik NL. Upregulation of calpain correlates with increased neurodegeneration in acute experimental auto-immune encephalomyelitis. J Neurosci Res. 2005;81:53–61. doi: 10.1002/jnr.20470. [DOI] [PubMed] [Google Scholar]
- Hurlbert RJ. Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J Neurosurg. 2000;93 (1 Suppl):1–7. doi: 10.3171/spi.2000.93.1.0001. [DOI] [PubMed] [Google Scholar]
- Jover T, Tanaka H, Calderone A, Oguro K, Bennett MV, Etgen AM, Zukin RS. Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1. J Neurosci. 2002;22:2115–2124. doi: 10.1523/JNEUROSCI.22-06-02115.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Jiang Q, Stys PK. Important role of reverse Na+-Ca2+ exchange in spinal cord white matter injury at physiological temperature. J Neurophysiol. 2000;84:1116–1119. doi: 10.1152/jn.2000.84.2.1116. [DOI] [PubMed] [Google Scholar]
- Linford NJ, Dorsa DM. 17β-Estradiol and the phytoestrogen genistein attenuate neuronal apoptosis induced by the endoplasmic reticulum calcium-ATPase inhibitor thapsigargin. Steroids. 2002;67:1029–1040. doi: 10.1016/s0039-128x(02)00062-4. [DOI] [PubMed] [Google Scholar]
- Mills CD, Xu GY, Johnson KM, McAdoo DJ, Hulsebosch CE. AIDA reduces glutamate release and attenuates mechanical allodynia after spinal cord injury. Neuroreport. 2000;11:3067–3070. doi: 10.1097/00001756-200009280-00007. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Mandai M, Suzuma I, Suzuma K, Kobayashi K, Honda Y. Estrogen protects against cellular infiltration by reducing the expressions of E-selectin and IL-6 in endotoxin-induced uveitis. J Immunol. 1999;163:374–379. [PubMed] [Google Scholar]
- Moosmann B, Behl C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc Natl Acad Sci USA. 1999;96:8867–8872. doi: 10.1073/pnas.96.16.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales LB, Loo KK, Liu HB, Peterson C, Tiwari-Woodruff S, Voskuhl RR. Treatment with an estrogen receptor α ligand is neuroprotective in experimental autoimmune encephalomyelitis. J Neurosci. 2006;26:6823–6833. doi: 10.1523/JNEUROSCI.0453-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath R, Raser KJ, Stafford D, Hajimohammadreza I, Posner A, Allen H, Talanian RV, Yuen P, Gilbertsen RB, Wang KK. Non-erythroid α-spectrin breakdown by calpain and interleukin 1 beta-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem J. 1996;319:683–690. doi: 10.1042/bj3190683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J, Chen S, Brinton RD. Dual action of estrogen on glutamate-induced calcium signaling: mechanisms requiring interaction between estrogen receptors and Src/mitogen activated protein kinase pathway. Brain Res. 2002;930:216–234. doi: 10.1016/s0006-8993(02)02254-0. [DOI] [PubMed] [Google Scholar]
- Pang Z, Bondada V, Sengoku T, Siman R, Geddes JW. Calpain facilitates the neuron death induced by 3-nitropropionic acid and contributes to the necrotic morphology. J Neuropathol Exp Neurol. 2003;62:633–643. doi: 10.1093/jnen/62.6.633. [DOI] [PubMed] [Google Scholar]
- Pant HC. Dephosphorylation of neurofilament proteins enhances their susceptibility to degradation by calpain. Biochem J. 1988;256:665–668. doi: 10.1042/bj2560665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perot PL, Jr, Lee WA, Hsu CY, Hogan EL, Cox RD, Gross AJ. Therapeutic model for experimental spinal cord injury in the rat: I. Mortality and motor deficit. Cent Nerv Syst Trauma. 1987;4:149–159. doi: 10.1089/cns.1987.4.149. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Posmantur R, Kampfl A, Siman R, Liu J, Zhao X, Clifton GL, Hayes RL. A calpain inhibitor attenuates cortical cytoskeletal protein loss after experimental traumatic brain injury in the rat. Neuroscience. 1997;77:875–888. doi: 10.1016/s0306-4522(96)00483-6. [DOI] [PubMed] [Google Scholar]
- Ray SK, Banik NL. Calpain. In: Collins G, editor. The Encyclopedia of Molecular Medicine. New York: John Wiley and Sons, Inc; 2002. pp. 435–440. [Google Scholar]
- Ray SK, Hogan EL, Banik NL. Calpain in the pathophysiology of spinal cord injury: neuroprotection with calpain inhibitors. Brain Res Rev. 2003;42:169–185. doi: 10.1016/s0165-0173(03)00152-8. [DOI] [PubMed] [Google Scholar]
- Roof RL, Hall ED. Estrogen-related gender difference in survival rate and cortical blood flow after impact-acceleration head injury in rats. J Neurotrauma. 2000a;17:1155–1169. doi: 10.1089/neu.2000.17.1155. [DOI] [PubMed] [Google Scholar]
- Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J Neurotrauma. 2000b;17:367–388. doi: 10.1089/neu.2000.17.367. [DOI] [PubMed] [Google Scholar]
- Samantaray S, Das A, Sribnick EA, Matzelle DD, Yu SP, Ray SK, Wei L, Banik NL. Neuroprotective efficacy of estrogen in acute and chronic spinal cord injury in rats. J Neurochem. 2009;108(S1):105. (PSM107–110) [Google Scholar]
- Samantaray S, Knaryan VH, Guyton MK, Matzelle DD, Ray SK, Banik NL. The parkinsonian neurotoxin rotenone activates calpain and caspase-3 leading to motoneuron degeneration in spinal cord of Lewis rats. Neuroscience. 2007;146:741–755. doi: 10.1016/j.neuroscience.2007.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaecher K, Goust JM, Banik NL. The effects of calpain inhibition on IkBα degradation after activation of PBMCs: identification of the calpain cleavage sites. Neurochem Res. 2004;29:1443–1451. doi: 10.1023/b:nere.0000026410.56000.dd. [DOI] [PubMed] [Google Scholar]
- Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med. 1999;5:554–559. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- Schumacher PA, Siman RG, Fehlings MG. Pretreatment with calpain inhibitor CEP-4143 inhibits calpain I activation and cytoskeletal degradation, improves neurological function, and enhances axonal survival after traumatic spinal cord injury. J Neurochem. 2000;74:1646–1655. doi: 10.1046/j.1471-4159.2000.0741646.x. [DOI] [PubMed] [Google Scholar]
- Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine. 2001;26 (24 Suppl):S2–12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- Smith JA, Das A, Butler JT, Ray SK, Banik NL. Estrogen receptor agonist or estrogen inhibits lipopolysaccharide induced microglial death. J Neurochem. 108(S1):137. doi: 10.1007/s11064-010-0336-7. (PTW104–107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sribnick EA, Del Re AM, Ray SK, Woodward JJ, Banik NL. Estrogen attenuates glutamate-induced cell death by inhibiting Ca2+ influx through L-type voltage-gated Ca2+ channels. Brain Res. 2009a;1276:159–170. doi: 10.1016/j.brainres.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sribnick EA, Matzelle DD, Banik NL, Ray SK. Direct evidence for calpain involvement in apoptotic death of neurons in spinal cord injury in rats and neuroprotection with calpain inhibitor. Neurochem Res. 2007;32:2210–2216. doi: 10.1007/s11064-007-9433-7. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Matzelle DD, Ray SK, Banik NL. Estrogen treatment of spinal cord injury attenuates calpain activation and apoptosis. J Neurosci Res. 2006a;84:1064–1075. doi: 10.1002/jnr.21016. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Matzelle DD, Ray SK, Banik NL. Estrogen as a Prospective Multi-active Agent for the Treatment of Spinal Cord Injury. In: Banik NL, Ray SK, editors. Brain and Spinal Cord Trauma. 3. New York: Springer Publisher; 2009b. pp. 581–598. [Google Scholar]
- Sribnick EA, Ray SK, Banik NL. Estrogen as a multi-active neuroprotective agent in traumatic injuries. Neurochem Res. 2004;29:2007–2014. doi: 10.1007/s11064-004-6874-0. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Ray SK, Banik NL. Estrogen prevents glutamate-induced apoptosis in C6 glioma cells by a receptor-mediated mechanism. Neuroscience. 2006b;137:197–209. doi: 10.1016/j.neuroscience.2005.08.074. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Wingrave JM, Matzelle DD, Ray SK, Banik NL. Estrogen as a neuroprotective agent in the treatment of spinal cord injury. Ann N Y Acad Sci. 2003;993:125–133. doi: 10.1111/j.1749-6632.2003.tb07521.x. discussion 159–160. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Wingrave JM, Matzelle DD, Wilford GG, Ray SK, Banik NL. Estrogen attenuated markers of inflammation and decreased lesion volume in acute spinal cord injury in rats. J Neurosci Res. 2005;82:283–293. doi: 10.1002/jnr.20622. [DOI] [PubMed] [Google Scholar]
- Sternberger LA, Sternberger NH. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci USA. 1983;80:6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur P, Sribnick EA, Wingrave JM, Nowak MW, Ray SK, Banik NL. Estrogen attenuates oxidative stress-induced apoptosis in C6 glial cells. Brain Res. 2003;971:178–188. doi: 10.1016/s0006-8993(03)02349-7. [DOI] [PubMed] [Google Scholar]
- Tyor WR, Avgeropoulos N, Ohlandt G, Hogan EL. Treatment of spinal cord impact injury in the rat with transforming growth factor-β. J Neurol Sci. 2002;200:33–41. doi: 10.1016/s0022-510x(02)00113-2. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, Krust A, Dupont S, Ciana P, Chambon P, Maggi A. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci USA. 2003;100:9614–9619. doi: 10.1073/pnas.1531957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Yang S, Liu R, Perez E, Yi KD, Koulen P, Simpkins JW. Estrogen attenuates nuclear factor-kappa B activation induced by transient cerebral ischemia. Brain Res. 2004;1008:147–154. doi: 10.1016/j.brainres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Wingrave JM, Sribnick EA, Wilford GG, Matzelle DD, Mou JA, Ray SK, Hogan EL, Banik NL. Relatively low levels of calpain expression in juvenile rat correlate with less neuronal apoptosis after spinal cord injury. Exp Neurol. 2004;187:529–532. doi: 10.1016/j.expneurol.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Wu HY, Tomizawa K, Oda Y, Wei FY, Lu YF, Matsushita M, Li ST, Moriwaki A, Matsui H. Critical role of calpain-mediated cleavage of calcineurin in excitotoxic neurodegeneration. J Biol Chem. 2004;279:4929–4940. doi: 10.1074/jbc.M309767200. [DOI] [PubMed] [Google Scholar]
- Xie LP, Fu WX, Jin C, Dong XY, Chen WF. Negative regulation of monocyte chemoattractant protein-1 gene expression by a mouse estrogen-enhanced transcript. Eur J Immunol. 2002;32:2837–2846. doi: 10.1002/1521-4141(2002010)32:10<2837::AID-IMMU2837>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Yune TY, Kim SJ, Lee SM, Lee YK, Oh YJ, Kim YC, Markelonis GJ, Oh TH. Systemic administration of 17β-estradiol reduces apoptotic cell death and improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 2004;21:293–306. doi: 10.1089/089771504322972086. [DOI] [PubMed] [Google Scholar]