Abstract
A protective role for estrogen against neurodegeneration and neurotrauma has received enormous attention in recent years, unraveling multiple facets and thus establishing this steroid as a multi-active neuroprotectant. The present study briefly reports our findings on the neuroprotective efficacy of physiologically relevant low doses of estrogen in experimental spinal cord injury (SCI) in rats. The current finding further corroborates our earlier results on efficacy of pharmacological/supraphysiological levels of estrogen in SCI and adds to the significance of conducting preclinical studies on estrogen efficacy in SCI.
Keywords: estrogen, neuroprotection, spinal cord injury
Introduction
Spinal cord injury (SCI) is a complex assault with varying degrees of neurological deficit and loss of function depending upon the severity of the injury. Although methylprednisolone is administered in acute SCI, it remains controversial and the actual benefits of the steroid have lately been questioned.[1, 2] Consequently, there is an urgent need for development of efficient drug for treatment of SCI. Our laboratory has long explored the diverse mechanisms of tissue destruction following SCI in rats and is currently involved in testing the efficacy of different neuroprotective drugs for treatment of SCI. A major and well-acknowledged issue in such research is formulation of a combination therapy or a multi-active drug as a choice for promoting therapeutic benefits in the complex multi-faceted process of tissue destruction during SCI. Estrogen is such a candidate drug that has been reported by us in a series of in vitro and in vivo studies using supraphysiological doses of estrogen. [3–8] However, considering the various hormonal properties of estrogen that may be limited in its beneficial effects in the predominantly male SCI population, we are currently focused on testing the lower physiological doses of estrogen for its efficacy in SCI. The readers are advised to look at the large array of neuroprotective efficacy of estrogen especially in SCI in a review article published in this volume, where we have succinctly presented the efficacy of estrogen in the field. Here, we briefly report that physiological low doses of estrogen can protect neurons in the spinal cord after moderately severe injury in rats.
Materials and Methods
Induction of SCI in Rats and Treatment with Estrogen
SCI was induced in adult male Sprague-Dawley rats using the modified weight-drop method. [9] Animal care, surgery, and induction of SCI were conducted in accordance with the “Guide for the Care and Use of Laboratory Animals” of the US Department of Health and Human Services (National Institutes of Health, Bethesda, MD) and were approved by the Institutional Animal Care and Use Committee (IACUC) at the Medical University of South Carolina (Charleston, SC). Laminectomy was performed at T10; the spine was immobilized, and an impounder was gently placed onto the dura. Dropping a 5 g weight from a height of 8 cm induced a moderately severe 40 g.cm force injury. Sham operated animals underwent laminectomy alone. Low dose estrogen (10 μg/kg/day and 200 μg/kg/day) was delivered by osmotic pumps implanted subcutaneously via treatment bolus at 15 min plus osmotic pump continuously delivering estrogen until sacrifice at 48 hours. At 48 hours post-injury, rats were anesthetized and sacrificed by decapitation. Following decapitation, the original laminectomy was extended and three 1-cm segments of SC were removed, representing the lesion segment, the rostral penumbra, and the caudal penumbra.
Collection of SC Samples and Immunofluorescent Stainings
Harvested SC samples were immediately frozen in tissue embedding media (HistoPrep) at −70°C and processed for immunofluorescent stainings. TUNEL assay for cell death was done according to our previously reported method [10] followed by double immunofluoresecent stainings with the neuronal marker anti-NeuN antibody (Chemicon, 1:100). TUNEL-positive neurons were tracked with rhodamine-conjugated anti-digoxigenin IgG primary antibody raised in sheep (Roche) and fluorescein conjugated anti-mouse IgG secondary antibody raised in horse (Vector, Burlingame, CA) as reported earlier. [11] Sections were finally mounted with the anti-fade mounting medium Vectashield (Vector) and images were captured with a fluorescence non-confocal microscope using ImagePro Plus software.
Results
Assessment of neuronal integrity was carried out in different treatment groups following acute SCI (Figure 1). Representative photomicrographs from both the dorsal horn and ventral horn containing sensory neurons and motoneurons, respectively, showed significant number of them to be TUNEL-positive in vehicle SCI as compared to the uninjured sham SC. Estrogen (200 μg) treatment alone did not show any discernable effects compared to sham, however, estrogen treatment (10 and 200 μg) in acute SCI rats significantly attenuated the number of TUNEL-positive neurons in the caudal penumbra, as demonstrated by reduced co-localization of TUNEL with NeuN staining (Figure 1).
Figure 1.
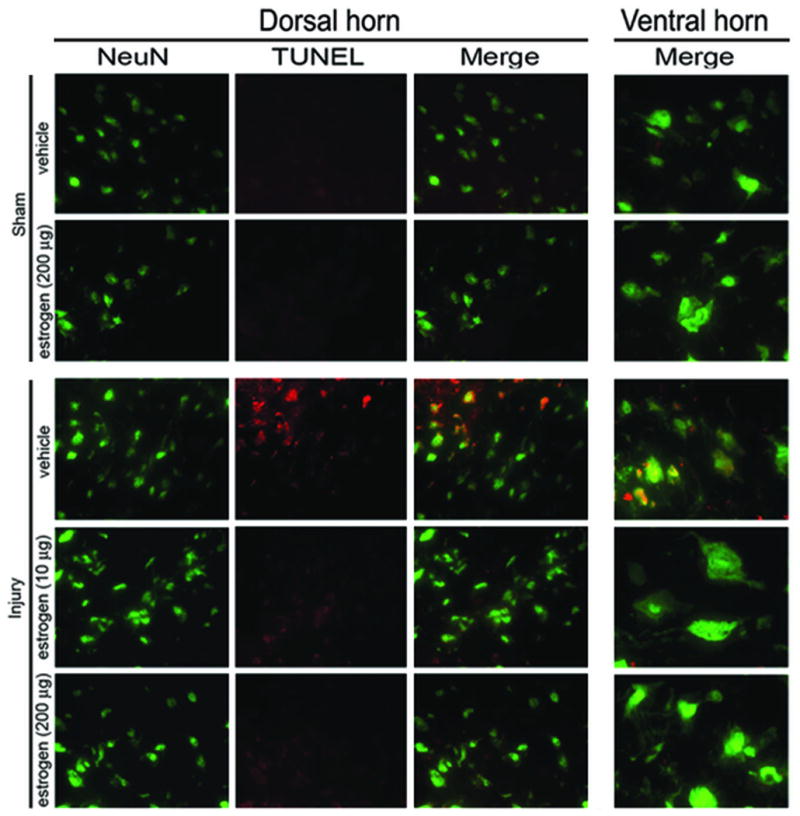
Double immunofluorescent stainings showed that low dose estrogen rendered neuroprotection in acute SCI in rats. Coronal slices (5–7 μm) of SC from caudal penumbra were double stained with neuronal marker NeuN (as green) and TUNEL (as red) to assess the neuronal death. SC from sham-vehicle animals showed healthy neurons - smaller sensory neurons in dorsal horn and larger motoneurons in ventral horn. SC slices from sham animals receiving estrogen (200 μg) were similar to sham vehicle and none of the groups showed any TUNEL-positive neurons. However, TUNEL staining in SC of injury-vehicle animals depicted significant neuronal death (yellow merge), which was protected upon estrogen treatment (10–200 μg). Panels of individual stains are shown in dorsal horn, whereas the merged panel alone is represented for ventral horn.
Discussion
The present study supports our earlier reports [5, 7] on the beneficial effects of estrogen after SCI. In this study, our primary goal was to define a physiological low dose of the steroid that could still render neuroprotective effects. Present study showed that both 10 and 200 μg doses were neuroprotective after acute SCI. Estrogen administration was beneficial to neurons in the caudal penumbra where the neurons die largely by apoptotic mechanisms. [12] Both the sensory neurons in dorsal horn as well as the motoneurons in the ventral horn were protected, thus suggesting that some common protective mechanisms are activated by estrogen regardless of the neuronal subtype specificity. SCI is due to mechanical injury in vast majority of the cases and revival of functional loss implies recovery of the total neuronal population in the dorsal and ventral horn. Present study is clinically highly relevant since estrogen effects on both the sensory and motoneuron were assessed and found to be protected. Furthermore, our study showed neuroprotective effects with both the doses, a lower 10 μg and a higher 200 μg, thus suggesting that receptor-mediated mechanisms are operative. It also narrows down the required dose to a physiological low profile thus, limiting the undesired side reactions of the steroid. It would be worthwhile to emphasize that present study used 17β-estradiol, the endogenous format of the steroid, and that it was applied post-injury thus, making it more reasonable to expect translational benefits of the study to human SCI. The neuroprotective efficacy of estrogen in the present study may certainly be explained as result of multiple mechanisms that are triggered by the low dose of estrogen that renders modification of the micro-environment in caudal penumbra by multiple other aspects. Ongoing research in our laboratory is addressing the molecular mechanisms of such protection and exploring different administration paradigm in both acute and chronic SCI regimen to validate estrogen in preclinical testing following SCI.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Miller SM. Methylprednisolone in acute spinal cord injury: a tarnished standard. Journal of neurosurgical anesthesiology. 2008;20:140–142. doi: 10.1097/01.ana.0000314442.40952.0d. [DOI] [PubMed] [Google Scholar]
- 2.Rozet I. Methylprednisolone in acute spinal cord injury: is there any other ethical choice? Journal of neurosurgical anesthesiology. 2008;20:137–139. doi: 10.1097/01.ana.0000314441.63823.b0. [DOI] [PubMed] [Google Scholar]
- 3.Sur P, et al. Estrogen attenuates oxidative stress-induced apoptosis in C6 glial cells. Brain research. 2003;971:178–188. doi: 10.1016/s0006-8993(03)02349-7. [DOI] [PubMed] [Google Scholar]
- 4.Sribnick EA, et al. Estrogen improves locomotor function following spinal cord injury. International Society for Neurochemistry. 2007;102(S1):203. [Google Scholar]; Journal of Neurochemistry. Cancum, Mexico: [Google Scholar]
- 5.Sribnick EA, et al. Estrogen treatment of spinal cord injury attenuates calpain activation and apoptosis. Journal of neuroscience research. 2006;84:1064–1075. doi: 10.1002/jnr.21016. [DOI] [PubMed] [Google Scholar]
- 6.Sribnick EA, et al. 17beta-estradiol attenuates glutamate-induced apoptosis and preserves electrophysiologic function in primary cortical neurons. Journal of neuroscience research. 2004;76:688–696. doi: 10.1002/jnr.20124. [DOI] [PubMed] [Google Scholar]
- 7.Sribnick EA, et al. Estrogen attenuated markers of inflammation and decreased lesion volume in acute spinal cord injury in rats. Journal of neuroscience research. 2005;82:283–293. doi: 10.1002/jnr.20622. [DOI] [PubMed] [Google Scholar]
- 8.Sribnick EA, Ray SK, Banik NL. Estrogen prevents glutamate-induced apoptosis in C6 glioma cells by a receptor-mediated mechanism. Neuroscience. 2006;137:197–209. doi: 10.1016/j.neuroscience.2005.08.074. [DOI] [PubMed] [Google Scholar]
- 9.Perot PL, Jr, et al. Therapeutic model for experimental spinal cord injury in the rat: I. Mortality and motor deficit. Cent Nerv Syst Trauma. 1987;4:149–159. doi: 10.1089/cns.1987.4.149. [DOI] [PubMed] [Google Scholar]
- 10.Ray SK, et al. Combined TUNEL and double immunofluorescent labeling for detection of apoptotic mononuclear phagocytes in autoimmune demyelinating disease. Brain Res Brain Res Protoc. 2000;5:305–311. doi: 10.1016/s1385-299x(00)00027-1. [DOI] [PubMed] [Google Scholar]
- 11.Samantaray S, et al. Melatonin attenuates calpain upregulation, axonal damage and neuronal death in spinal cord injury in rats. Journal of pineal research. 2008;44:348–357. doi: 10.1111/j.1600-079X.2007.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banik EASDDMSKRNL. Estrogen as a Promising Multi-Active Agent for the Treatment of Spinal Cord Injury. Handbook of neurochemistry and molecular neurobiology. In: Lajtha NLBSRA, editor. Brain and spinal cord trauma. Vol. 24. New York, London: Springer; 2008. [Google Scholar]


