Abstract
Spliceosome assembly has been characterized as the ordered association of the snRNP particles U1, U2, and U4/U6·U5 onto pre-mRNA. We have used an in vitro trans-splicing/cross-linking system in Saccharomyces cerevisiae nuclear extracts to examine the first step of this process, 5′ splice site recognition. This trans-splicing reaction has ATP, Mg2+, and splice-site sequence requirements similar to those of cis-splicing reactions. Using this system, we identified and characterized a novel U4–5′ splice site interaction that is ATP-dependent, but does not require the branch point, the 3′ splice site, or the 5′ end of the U1 snRNA. Additionally, we identified several ATP-dependent U6 cross-links at the 5′ splice site, indicating that different regions of U6 sample it before a U6–5′ splice site interaction is stabilized that persists through the first step of splicing. This work provides evidence for ATP-dependent U4/U6 association with the 5′ splice site independent of ATP-mediated U2 association with the branch point. Furthermore, it defines specific nucleotides in U4 and U6 that interact with the 5′ splice site at this early stage, even in the absence of base-pairing with the U1 snRNA.
Keywords: RNA cross-linking, trans-splicing, Saccharomyces cerevisiae
Messenger RNA splicing is a critical step in the process of eukaryotic gene expression. In splicing, the introns are removed from newly synthesized RNA by the complex molecular machinery of the spliceosome. Assembly of the spliceosome is driven by a series of interactions between the pre-mRNA, five small nuclear ribonucleoprotein particles, and a host of trans-acting, non-snRNP proteins (Will and Lührmann 1997; Burge et al. 1999; Stevens and Abelson 1999). Extensive biochemical and genetic analyses have been carried out to identify the steps in assembly as well as the specific RNA–RNA and RNA–protein contacts that are involved.
The first step of splicing is the accurate recognition of the pre-mRNA at the correct splice sites. These events are thought to be initiated when, in the absence of ATP, the U1-snRNP particle associates with the 5′ splice site via base-pairing with the 5′ end of the U1 snRNA (Mount 1982; Zhuang and Weiner 1986; Seraphin et al. 1988; Siliciano and Guthrie 1988) and association of U1-snRNP proteins with sequences around the 5′ splice site (Liu et al. 1998; Zhang and Rosbash 1999). In what has been thought to be the first energy-requiring step, U2 snRNA base-pairs with the pre-mRNA branch-point sequence (Cheng and Abelson 1987; Parker et al. 1987; Krämer 1988; Wu and Manley 1989; Zhuang and Weiner 1989; Pruzan et al. 1990; Michaud and Reed 1991; Liao et al. 1992). Once U1 and U2 are bound to the pre-mRNA, U4/U6·U5 associates with the 5′ splice site region of the pre-mRNA (Ruby and Abelson 1988; Liao et al. 1992), and the U5 snRNP is positioned such that the invariant loop 1 is aligned with exon sequences near the 5′ and 3′ splice sites (Wyatt et al. 1992; Sontheimer and Steitz 1993; Newman et al. 1995). In turn, U6 associates with the intron nucleotides at the 5′ splice site, replacing U1 interactions. Although neither U1 nor U4 appears to be involved in catalysis, it is not clear when they leave the spliceosome.
The description of the interactions that take place during spliceosome assembly is based largely on analysis of splicing complexes using nondenaturing gels. This model has been extremely useful in defining the general sequence of interactions involved in the splicing reaction, but accumulating data from metazoan systems indicate that there may be more flexibility in the order of events surrounding splice-site recognition by the splicing machinery than the model predicts. Splicing of the SV40 large T-antigen intron is insensitive to removal of the 5′ end of U1 (Black and Steitz 1986), although it is inhibited by anti-U1 snRNP antibody (Fradin et al. 1984; van Santen and Spritz 1986). The requirement for U1 in 5′ splice site choice can also be bypassed by high concentrations of SR proteins or specific cis-acting sequence elements near the 5′ splice site and branch point (Crispino et al. 1994, 1996; Tarn and Steitz 1995; Crispino and Sharp 1995). These results challenge the prevailing view of spliceosome assembly that describes U1 binding as the prerequisite step for 5′ splice site recognition.
Furthermore, in a series of experiments using an 11-nucleotide RNA molecule, Konarska and coworkers demonstrated that, in mammalian extracts, the U4/U6·U5 snRNP could associate with the 5′ splice site RNA oligonucleotide independently of U1 snRNP. This oligonucleotide could participate in both steps of splicing even more efficiently in the absence of U1 snRNP than when intact U1 was in the extract (Konforti and Konarska 1995). Additionally, using an in vitro cis/trans-splicing competition assay with both HeLa and nematode splicing extracts, Nilsen and coworkers demonstrated that early 5′ splice site recognition by U4/U6·U5 tri-snRNP did not require prior U2 binding (Maroney et al. 2000). These results challenge the assumption that U1 and U2 must preassemble on the pre-mRNA as a prerequisite for triple snRNP binding.
Even the timing of the ATP-dependent steps in splicing appears to be more complicated than the working model of spliceosome assembly predicts. Although the first ATP-dependent step is predicted to be U2 association with the branch-point sequences, U2 and U1 both appear to associate with the pre-mRNA in an ATP-independent fashion, although U2 appears to be weakly associated in this complex (Das et al. 2000; Perriman and Ares 2000). Furthermore, U2 can bind at the consensus branch point of a minimal substrate containing the 3′ half of the pre-mRNA and lacking a 5′ splice site in an ATP-independent manner (Query et al. 1997). Hence, ATP is most likely used in a subsequent step to induce a conformational change that stabilizes the ATP-independent interaction with the branch-point sequence. These results support a model in which ATP is required to modulate relatively unstable, preformed interactions between particular snRNPs and the pre-mRNA.
Although the precise order of events that leads to the U6 interaction with the 5′ splice site remains unclear, it is quite apparent that the U4 and U6 snRNAs are at the center of the dramatic structural rearrangements as the spliceosome is assembled into its catalytic form. U4 and U6 are associated via an extensive ∼20-nucleotide base-pairing. In a remarkable set of structural rearrangements, U6 associates with the 5′ splice site, and the base-paired interaction with U4 is replaced by base-pairing with U2, forming U2–U6 helices I and II and a U6 intramolecular stem loop (Hausner et al. 1990; Datta and Weiner 1991; Madhani and Guthrie 1992; Fortner et al. 1994; Brow and Vidaver 1995). The U2/U6 helix I is highly conserved and is proposed to be a component of the active site (Brow and Guthrie 1989; Fabrizio and Abelson 1990; Madhani and Guthrie 1992; Valadkhan and Manley 2000). U4 and U6 are completely unwound before the first step of splicing (Yean and Lin 1991), although genetic evidence indicates that U4 remains associated with U6 after recognition of the 5′ splice site (Li and Brow 1996).
U6 specifically interacts with the 5′ splice site of the pre-mRNA via its conserved ACAGAG sequence (Sawa and Abelson 1992; Sawa and Shimura 1992; Wassarman and Steitz 1992; Lesser and Guthrie 1993; Sontheimer and Steitz 1993; Kandels-Lewis and Seraphin 1994; Wolff et al. 1994; Kim and Abelson 1996). Mutation of the ACAGAG box hampers the ability of U6 to bind to the 5′ splice site and cripples spliceosome assembly in vitro (Fabrizio and Abelson 1990). In vivo, mutations in any of these nucleotides result in temperature sensitivity or lethality (Madhani et al. 1990). Additionally, the U6 ACAGAG sequence–5′ splice site interaction is thought to be a prerequisite for U4/U6 separation (Li and Brow 1996), for subsequent U2–U6 interaction, and therefore for activation of the spliceosome. Recent studies of the U12-dependent spliceosome suggest that, at least in that system, U4atac and U6atac may still be partially base-paired (via stem II) even after U12 and U6atac association (via stem Ia). Quite interestingly, this U12/U6atac helix Ia can form even without U6atac replacement of U11 at the 5′ splice site (Frilander and Steitz 2001).
To further define the interactions that occur at the 5′ splice site during spliceosome assembly and catalysis, we are using an in vitro trans-splicing system with nuclear extracts from the yeast Saccharomyces cerevisiae. In these reactions, the 5′ splice site is contained on a 20-nucleotide, biotinylated, synthetic RNA, and the 3′ acceptor containing the branch point and the 3′ splice site is prepared by in vitro transcription (Ghetti and Abelson 1995). The trans-splicing reaction occurs accurately, and has the same requirements for ATP, Mg2+, and splice-site consensus sequences as does cis-splicing.
Using this approach, we are able to substitute photoreactive, cross-linkable groups within the 5′ splice site-containing RNA to identify the RNA–RNA interactions that occur during splicing. We have found and further characterized a previously identified cross-link between the branched intron pre-mRNA intermediate and U6 snRNA. Additionally, we have identified a novel interaction between U4 snRNA and the +2 position of the 5′ splice site. This interaction is ATP-dependent and does not require the 3′ acceptor or the 5′ end of U1, but it does require a wild-type 5′ splice site sequence. We have also identified a novel ATP-dependent, acceptor-independent U6 cross-link to the 5′ splice site. These results provide evidence of an early, ATP-dependent U4/U6 association with the 5′ splice site in the absence of the ATP-mediated association of U2 with the branch point, suggesting that there is remarkable flexibility in the order of events in spliceosome assembly.
Results
In vitro trans-splicing with biotinylated substrates recapitulates cis-splicing
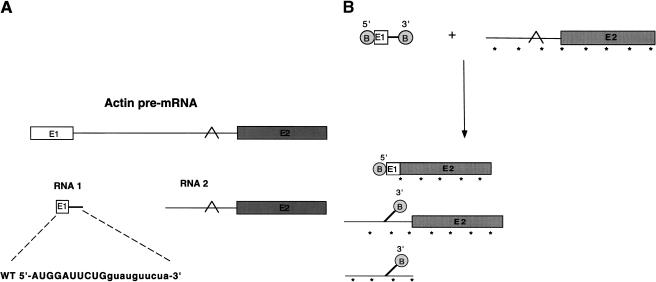
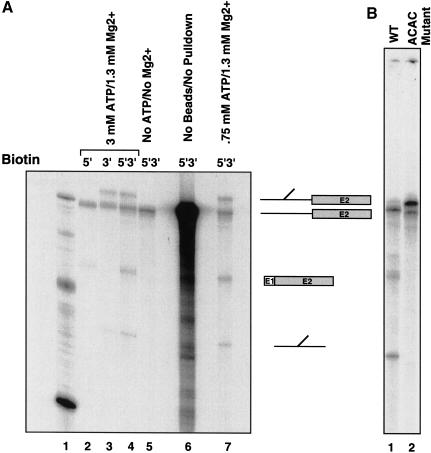
To analyze RNA–RNA interactions at the 5′ splice site, we have developed an in vitro trans-splicing system using a biotinylated 5′ splice site-containing RNA oligonucleotide. The 5′ splice site RNA is a 20-nucleotide synthetic RNA, based upon the actin pre-mRNA sequence (Fig. 1A). Biotinylation of the oligonucleotide substrate allows efficient isolation of the splicing products and intermediates. The 5′ splice site donor molecule and the 3′ acceptor are added to a final concentration of 10 μM and 40 nM, respectively. The products of the trans-splicing reaction are purified using streptavidin-coated magnetic beads (Fig. 1B), and, depending on whether the biotin is placed at the 5′ end (Fig. 2A, lane 2), the 3′ end (Fig. 2A, lane 3), or both ends (Fig. 2A, lanes 4 and 7), the specific splicing products and intermediates can be purified. This approach provides a significant purification over the background (Fig. 2A, cf. lanes 4 and 6) and allows monitoring of the trans-splicing reaction, despite its relative inefficiency. Previously published results and our own experiments indicate that, at most, 18% of the 3′ acceptor precursor is spliced. Some acceptor RNA nonspecifically associates with the beads even after washing (<1%); however, this nonspecific association does not affect the efficiency of biotinylated oligonucleotide binding.
Figure 1.
Trans-splicing RNA substrates. (A) The 20-nucleotide 5′ splice site oligonucleotide sequence and the 3′ acceptor sequence are derived from the actin pre-mRNA sequence. Exon sequences are shown in capital letters, and intron sequences are shown in lowercase letters. (E1) Exon 1; (E2) exon 2. The branch point is indicated by a caret. (B) Trans-splicing products and intermediates are isolated by biotinylation (B in circle) of the 5′ and 3′ ends of the 5′ splice site oligonucleotides. Asterisks indicate internal incorporation of radiolabel.
Figure 2.
A 20-nucleotide, biotinylated RNA can serve as a 5′ splice site donor for easy isolation of splicing products. (A) Mg2+ and ATP requirements. The 5′ splice site RNA was biotinylated at the 5′ end (lane 2), the 3′ end (lane 3), or both ends (lanes 4–7) and added to reactions to a final concentration of 10 μM. The ATP and Mg2+ concentrations are indicated. Splicing reactions were performed for 2 h at 16°C. Lane 6 shows the splicing reactions without isolation of splicing products. An RNA marker is shown in lane 1. The products are depicted to the right of the gel. (B) An ACAC 3′-splice-site mutant blocks splicing after the first step.
Previous experiments indicate that the trans-splicing reaction has similar requirements to cis-splicing. Mutation of the conserved G+1 inhibits the reaction (Ghetti and Abelson 1995; data not shown), as does depletion of ATP or the absence of Mg2+ (Fig. 2, lane 5). For the experiments that follow, the concentration of ATP was optimized to 1.5 mM. When the 3′ splice site is mutated from AGAG to ACAC, splicing is blocked after the first step, and hence the intron intermediate accumulates, but no splicing products are generated (Fig. 2B, lane 2). In these experiments, the ACAC mutant RNA is 6 nucleotides shorter, as a cryptic branch point has been removed (Vijayraghavan et al. 1986), and, therefore, the RNA runs at a slightly faster mobility. This result recapitulates the second step block observed when the 3′ splice site is mutated in cis-splicing reactions (Vijayraghavan et al. 1986). However, when 3′ acceptors containing branch-point mutations are used in the splicing reaction, splicing is inhibited (data not shown). The results indicate that functional spliceosomes with the same sequence requirements form in the trans-splicing system, and splicing can proceed through both catalytic steps.
U6 cross-links to the intron in the trans-splicing system
This minimal RNA substrate facilitates the generation of RNAs that are chemically modified to detect RNA–RNA or RNA–protein interactions. To this end, the 5′ leader was synthesized with 4-thiouridine at positions at and around the 5′ splice site. Upon photoactivation with low-energy UV light (365 nm), 4-thiouridine forms a covalent linkage to proximal RNA and protein. In the experiments described here, 4-thiouridine was incorporated at the conserved intron position U + 2 to look for RNA cross-links at this site. The 4-thiouridine itself has no effect on splicing (data not shown; see also Kim and Abelson 1996).
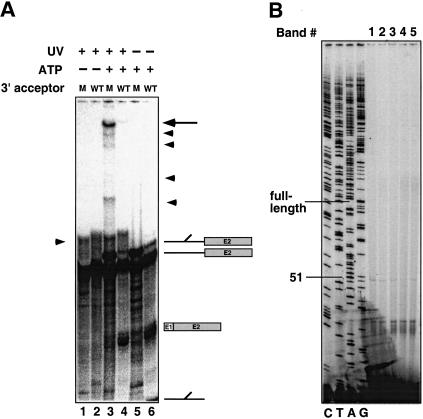
The trans-splicing reactions were carried out using both the wild-type and the ACAC 3′ splice site mutant substrate. After the samples were UV-irradiated, the proteins were removed, and the splicing products and intermediates were isolated using streptavidin-coated magnetic beads. The reactions shown in Figure 3A were scaled up fivefold to visualize low-abundance cross-links. Similar to the results shown in Figure 2, in the presence of ATP, the predicted splicing products and intermediates are detectable using the wild-type substrate (Fig. 3A, lane 6), whereas the mutant substrate that blocks splicing after the first step accumulates the branched intron intermediate (Fig. 3A, lane 5). Upon UV irradiation, several high-molecular-weight species are detected. A faint band is visible using the wild-type splicing substrate, which accumulates when the ACAC mutant substrate is used (Fig. 3A, lanes 3 and 4, bands indicated by arrows). The cross-links are UV- and ATP-dependent (Fig. 3A, lanes 1, 2, 5, and 6), as well as 4-thioU-dependent (data not shown). To allow better separation of the cross-linked products the gel was run longer, and the branched intron ran off the gel (Fig. 3A, lane 4). However, as is shown in Figure 2, the mutant 3′ splice site completely blocks splicing after the first step, so that no branched intron is generated in the reactions shown in lanes 3 or 5.
Figure 3.
(A) An ATP-dependent cross-link at intron position U+2 accumulates with the ACAC mutant. Trans-splicing reactions were carried out with a 4-thioU+2-substituted 5′ splice site oligonucleotide and a body-labeled acceptor RNA containing either a wild-type actin sequence (even-numbered lanes) or an ACAC mutation at the 3′ splice site (odd-numbered lanes). The products of the trans-splicing reaction in the absence of UV irradiation are shown in lanes 5 and 6, and the products of the trans-splicing reaction after UV irradiation are shown in lanes 1–4. Lanes 1 and 2 show reactions in which the extracts were depleted of ATP. The top right arrow indicates the primary cross-link. The arrowheads at the right indicate other minor cross-links. The arrowhead on the left is a nonspecific ATP- and extract-independent 5′ splice site–3′-acceptor cross-link. (B) The 5′ splice site cross-link to the intron intermediate maps to the U6 ACAGAG sequence of U6 snRNA. The primary cross-link and the minor cross-links (indicated by arrows in A) were extracted from the gel and mapped by primer extension using oligonucleotides complementary to the 3′ end of U6. Extension products of the bands are labeled from top to bottom 1–5, respectively. A U6 dideoxy sequencing ladder is shown to the left of the mapped cross-link.
Because the 32P label is supplied only by the 3′ acceptor, the cross-link must contain at least a portion of this molecule (see Fig. 1), indicating that the RNA species represented by these cross-links are products of the first step of splicing (after which splicing is blocked). From this it can be deduced that the bands represent cross-links to the branched intermediate. To identify which snRNA is cross-linked to the intermediate, the band is excised from the gel, and the cross-linked RNA is incubated with DNA oligonucleotides complementary to each of the snRNAs and RNase H. Only the oligonucleotide complementary to the U6 snRNA renders the cross-link sensitive to digestion (data not shown).
It is possible to map the site of cross-linking by primer extension (Sontheimer and Steitz 1993). The cross-link blocks primer extension at a position one nucleotide 3′ to the site where cross-linking occurs. A DNA primer complementary to the 3′ end of U6 was annealed to each of the gel-purified RNAs, and this was followed by extension with reverse transcriptase and gel electrophoresis (Fig. 3B). The site where primer extension stopped was determined by comparison with a dideoxy sequencing ladder of a U6 plasmid using the same primer. The primer-extension block occurs at position 51 or, if the band is running at slightly slower mobility (as the faint read-through product is), position 52, indicating that the cross-link is at position 50 or 51 within the conserved ACAGAG sequence of the U6 snRNA (Fig. 3B). The lower-abundance cross-links that accumulate with the ACAC mutant also map between positions 51 and 52 and appear to be degradation products of the primary cross-link. These results are in agreement with previous biochemical results that indicate that U6 associates with the intron sequences of the 5′ splice site (Sontheimer and Steitz 1993; Kim and Abelson 1996). Experiments using extracts that block splicing just prior to the first step illustrate that the U6–intron interaction occurs prior to catalysis (Kim and Abelson 1996). Therefore, the cross-link described here probably occurs prior to the first step, between the 5′ splice site-containing oligonucleotide and U6, and this cross-linked species is then a substrate for the trans-splicing reaction. These data provide further evidence that the interactions seen in the trans-splicing reaction recapitulate the interactions that have previously been identified in both yeast and mammalian splicing systems.
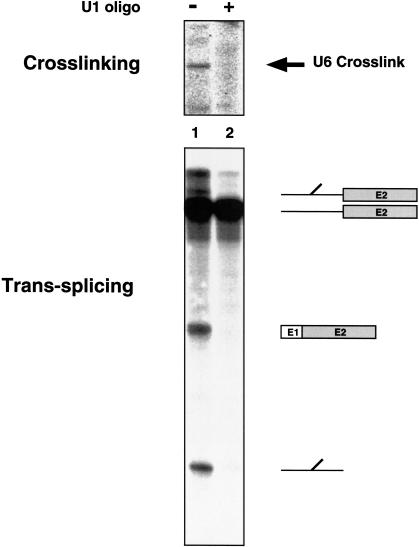
The trans-splicing reaction requires an intact 5′ end of U1
One of the intriguing findings in the mammalian trans-splicing system is that when the 5′ end of U1 is blocked or removed, or when the U1 snRNP is depleted, the short 5′ splice site oligonucleotide can participate in both steps of splicing (Hall and Konarska 1992; Konforti et al. 1993; Konforti and Konarska 1995). A similar analysis was carried out in this yeast trans-splicing system. In the presence of an oligonucleotide complementary to the 5′ end of U1, endogenous RNase H degrades the 5′ end of the U1 snRNA (Kretzner et al. 1987), which was confirmed by a primer-extension assay of the oligonucleotide-treated extract (data not shown). Unlike the mammalian system, however, the trans-splicing reaction is dependent on intact U1 snRNA, as the splicing products, intermediates, and cross-links fail to accumulate in the oligonucleotide-treated sample (Fig. 4, lane 2). Based on the results from other systems in which the U1 requirement is bypassed (Crispino et al. 1994; Tarn and Steitz 1994; Crispino and Sharp 1995), it is possible that protein factors such as SR proteins (which are abundant in higher eukaryotes but not yeast) are involved in the U1 bypass mechanism in the mammalian trans-splicing system. Certainly, the mammalian results illustrate that events that take place at the 5′ splice site are more complicated than a simple prerequisite base-pairing of the pre-mRNA with U1. Hence, even though in this yeast system U1 snRNA was required for splicing, it was of interest to examine the ability of factors other than the 5′ end of U1 to mediate early 5′ splice site recognition.
Figure 4.
The trans-splicing reaction and the U6 cross-link are dependent on the 5′ end of U1. The 5′ end of U1 was depleted by preincubating the extract with a DNA oligonucleotide complementary to the snRNA or mock depleted by adding water. After mock depletion (lane 1) or oligonucleotide-mediated depletion (lane 2), the splicing reaction and cross-linking were carried out as described in Figure 3A.
Early RNA cross-links to the 5′ splice site
To examine other interactions at the 5′ splice site that occur prior to the first catalytic step, a method of detecting the 5′ leader was required. However, when the 5′ splice site oligonucleotide was radioactively labeled at either the 3′ or 5′ end, the label was susceptible to removal by either nuclease or phosphatase activity in the extract. To overcome this problem, a nonradioactive method of detecting the biotin on the 5′ leader was developed. After electrophoresis on a 6% denaturing gel, the gel was transferred to a membrane, where it was incubated with alkaline phosphatase-conjugated streptavidin and a substrate that, when cleaved by alkaline phosphatase, emitted light. The chemiluminescent signal was then visualized by autoradiography.
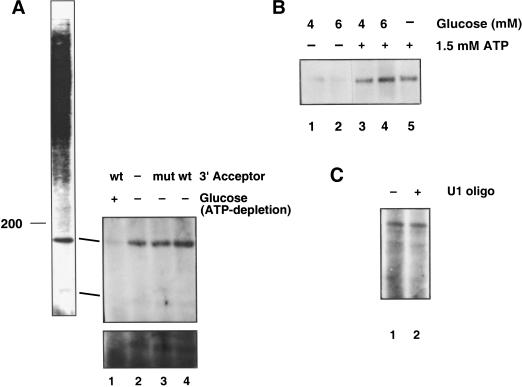
Using this method, interactions at the +2 intron position were probed in reactions containing either a wild-type 3′ acceptor or the ACAC mutant 3′ acceptor, or in the absence of any added acceptor. Two cross-links that migrate with apparent molecular weights between 100 and 180 were detected (Fig. 5A). These molecular weights were assigned by comparison with 32P-labeled size markers. In the absence of extract, 4-thioU, or UV-irradiation, no cross-links are generated (data not shown). In extracts that are depleted of ATP, the cross-links are inhibited (Fig. 5A, lane 1). The lower band is shown overexposed (bottom panel) to confirm that the band is not present in the absence of ATP.
Figure 5.
ATP-dependent, acceptor-independent cross-links to the 5′ splice site oligonucleotide. (A) The trans-splicing and cross-linking reactions were performed as described above, followed by chemiluminescence and detection by autoradiography. The splicing reactions were then carried out in the presence of a wild-type 3′ acceptor (lane 4), in the presence of a 3′ acceptor containing the ACAC 3′-splice-site mutation (mut, lane 3), in the absence of the 3′ acceptor (lane 2), or after glucose depletion of ATP (lane 1). The bottom panel shows an overexposure of the lower cross-link. (B) Glucose was added to the extracts to a final concentration of 4 mM (lanes 1 and 3) or 6 mM (lanes 2 and 4), and ATP was added back to the reactions shown in lanes 3 and 4 to a final concentration of 1.5 mM. The reaction shown in lane 5 was mock depleted, followed by addition of 1.5 mM ATP. After depletion and add-back, the RNAs and salts were added, and the reactions were carried out as shown in A. (C) The 5′ end of U1 was degraded by the endogenous RNase H activity and a complementary DNA oligonucleotide (lane 2). As a control, the reaction was incubated in the absence of the U1 DNA oligonucleotide (lane 1).
Further analysis of the higher molecular weight band reinforces that ATP is required to generate this cross-link. As is shown in Figure 5B, when ATP is added to extracts that have been ATP-depleted using glucose, cross-linking is restored. The depletion reactions were carried out using two different final concentrations of glucose, 4 mM (Fig. 5B, lanes 1 and 3) and 6 mM (Fig. 5B, lanes 2 and 4). ATP was added back to the splicing reactions shown in lanes 3 and 4. As a control, extract was mock depleted, and the splicing reaction was carried out in the presence of ATP (Fig. 5B, lane 5). Figure 5B, like Figure 5A, shows that the cross-link is ATP-dependent (Fig. 5B, cf. lane 1 with 3 and 2 with 4).
However, the cross-links are not dependent on the presence of a 3′ acceptor (Fig. 5A, cf. lanes 3 and 4 with 2). Since these cross-links are generated even in the absence of the 3′ acceptor, U2 addition to the branch point is not a required prerequisite for ATP-mediated snRNP association with the 5′ splice site.
When the 5′ end of U1 is depleted using oligonucleotide-directed RNase H digestion, the cross-links are also present (Fig. 5C). In multiple experiments with efficient U1 knockout, both bands are visible. Because the lower-molecular-weight cross-link is always less efficient, the intensity of the signal from experiment to experiment can be variable. Even though U1 is required for yeast trans-splicing, therefore, the ATP-mediated interaction with the 5′ splice site can occur even in the absence of U1, implying that U1 is required for some function beyond 5′ splice site recognition.
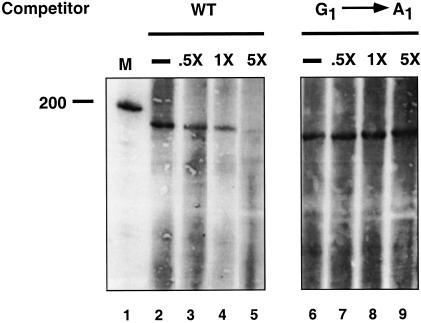
To determine the specificity of the 5′ splice site cross-links, trans-splicing and cross-linking were examined in the presence of RNA oligonucleotide competitors lacking 4-thioU with either a wild-type 5′ splice site sequence or a G1 → A1 mutation. The latter mutation inhibits in vitro splicing both in cis-splicing and in trans-splicing assays (data not shown) (Vijayraghavan et al. 1986; Ghetti and Abelson 1995). The wild-type RNA oligonucleotide was able to efficiently compete away the 5′ splice site interactions, but the RNAs containing the mutation could not (Fig. 6). Additionally, a 5′ splice site oligonucleotide with a mutation in the −5 exon position, which has no effect on splicing (Vijayraghavan et al. 1986; Ghetti and Abelson 1995), could also efficiently compete away the 5′ splice site interactions, and a U2 → A2 mutation could not (data not shown). These results indicate that the observed interactions are sequence specific.
Figure 6.
Oligonucleotides with mutations in conserved intron sequences cannot compete for the interactions. The trans-splicing reaction was carried out with 8 μM of the 4-thioU-containing RNA and 0 μM, 4 μM, 8 μM, or 40 μM of competitor oligonucleotide lacking 4-thioU with either the wild-type sequence (lanes 1–5) or with a G1 → A1 mutation. After cross-linking, the products were detected by chemiluminescence.
U4 cross-links to the 5′ splice site RNA oligonucleotide
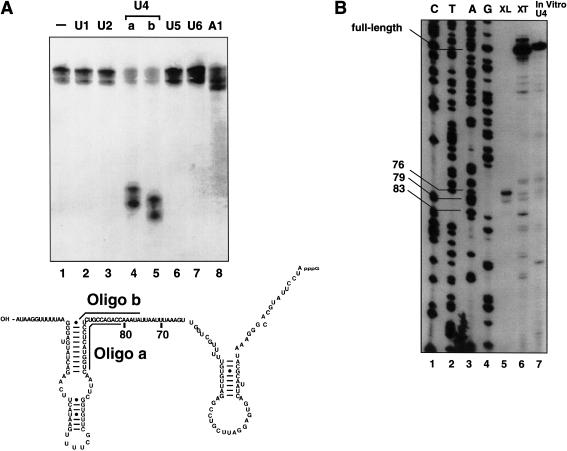
The first focus was to identify the more efficient, higher molecular weight cross-link. To determine the identity of the cross-linked RNA, oligonucleotide-directed RNase H digestion was performed. The trans-splicing reaction was carried out, the region of the gel corresponding to the cross-link was excised, and the RNA was eluted from the gel slice. DNA oligonucleotides complementary to each of the 5 snRNAs were annealed to the eluted RNA, and the samples were treated with RNase H (Fig. 7A).
Figure 7.
(A) The 5′ splice site RNA cross-links to U4 snRNA. The trans-splicing experiment was carried out in duplicate and two gels were run. One was transferred to a membrane for chemiluminescence, and, after detection of the bands, the comparable region was cut from the second gel. DNA oligonucleotides complementary to each of the snRNAs or to the intron sequence of the 5′ splice site RNA (A1) were annealed to the cross-linked RNA, and the samples were treated with RNase H. The sequence of U4 is shown, and the regions where the two U4 oligonucleotides anneal are indicated (a and b). After electrophoresis, chemiluminescence was carried out as described above. (B) The high-molecular-weight cross-link maps to positions in the central domain of U4. After the cross-link bands were cut from the gel, the RNA was eluted and analyzed by primer extension (XL) using an oligonucleotide complementary to the 3′ end of U4. For comparison, extract was phenol-extracted, and the RNA was precipitated and used for primer extension with the U4 oligonucleotide (XT) or in vitro synthesized U4 RNA (In vitro U4).
The higher molecular weight species was sensitive to two different oligonucleotides directed against the U4 snRNA (Fig. 7A, lanes 4 and 5), indicating that the 5′ splice site oligonucleotide was cross-linked to the U4 snRNA. In some experiments the cross-link runs as a doublet, although both bands are sensitive to the U4 oligonucleotide.
U4 cross-linking was also observed when 6-thioguanosine was placed at the +1 position of the intron. In all, RNAs with 4-thiouridine or 6-thioguanosine at positions −3, −2, −1, +1, +2, and +4 were examined, and the U4 cross-link was detected only at the +1 and +2 positions (data not shown).
The precise site of cross-linking was mapped by primer extension. A DNA primer complementary to the 3′ end of U4 was annealed to the gel-purified RNA, followed by extension with reverse transcriptase and gel electrophoresis. The site where primer extension stopped was determined by comparison with a dideoxy sequencing ladder of a U4 plasmid using the same primer (Fig. 7B). Primer extension was also carried out using in vitro synthesized U4 RNA (lane 7) and RNA from extract that had been UV-irradiated and deproteinized (lane 6). In the lanes containing the 4-thioU-induced cross-link (lane 5), two strong stops and one weak stop are detectable at positions 76, 79, and 83, respectively. These cross-links fall within the “central domain” of U4. There is no detectable difference when the 3′ acceptor is left out of the splicing reaction (data not shown). Notably, in mammalian extracts when this region of U4 is cleaved using RNase H digestion, splicing is eliminated (Black and Steitz 1986). Additionally, when the region is digested, an ATP-dependent cross-link between the 5′ splice site and the highly conserved Prp8 protein is eliminated (Maroney et al. 2000).
The 5′ splice site RNA cross-links to U6
Based on the molecular weight of the second band that was detected by chemiluminescence shown in Figure 5A, it seemed likely that the 5′ splice site was cross-linked to the U6 snRNA, which is 112 nucleotides long. This was tested by RNase H digestion of the RNA with the same set of DNA oligonucleotides as above. The only oligonucleotide that rendered the cross-link sensitive to nuclease digestion was the one complementary to U6 (data not shown).
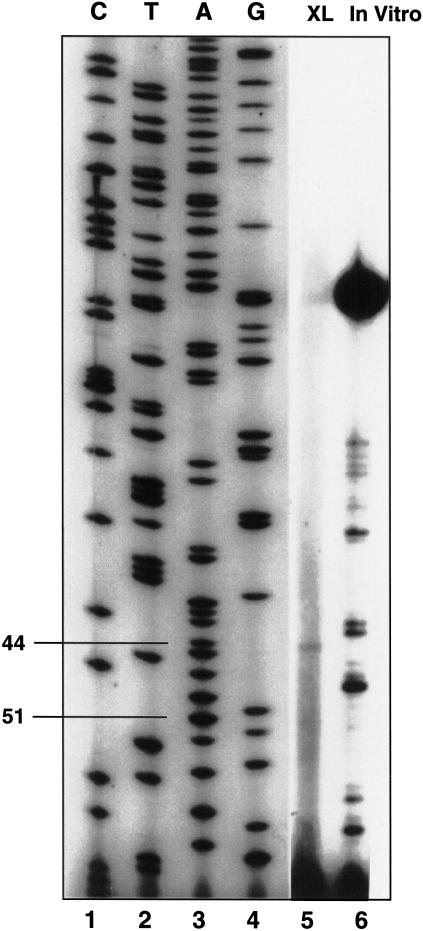
The RNAs were eluted from the gel to map the precise location of the cross-link by primer-extension analysis (Fig. 8). To map the cross-link, the reaction was carried out in the presence of the 3′ acceptor, and the cross-link was mapped. There was a strong block to primer extension at position 44, indicating a cross-link at position 43.
Figure 8.
U6 cross-link mapping. The trans-splicing experiment was carried out in duplicate and two gels were run. One was transferred to a membrane for chemiluminescence, and, after detection of the bands, the comparable region was cut from the second gel and the RNA was eluted for primer extension (XL). In vitro synthesized U6 was analyzed by primer extension for comparison (In vitro). Dideoxy sequencing with the same U6 was carried out for comparison (lanes 1–4).
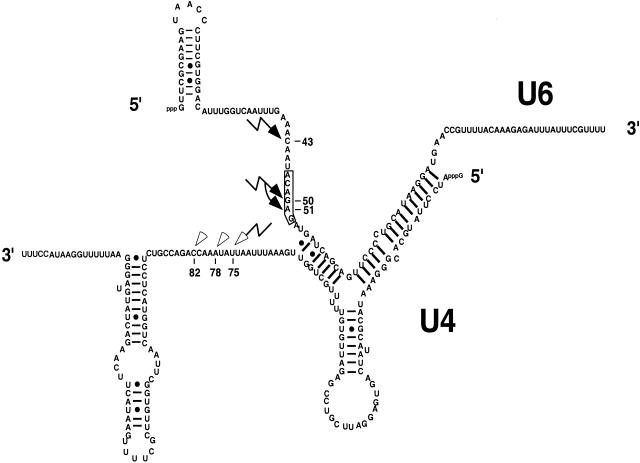
When the location of this cross-link is compared to the U4 cross-link in the context of U4/U6, the cross-links are almost directly across from each other in the single-stranded regions of the RNAs that flank the intermolecular stem II (Fig. 9). It therefore seems likely that the U4 and U6 cross-links represent the same interaction between U4/U6 and the 5′ splice site.
Figure 9.
Summary of U4 and U6 cross-links. Shown is the predicted secondary structure of the U4/U6 duplex. The U6 ACAGAG sequence is boxed. The primary U6 cross-links are indicated by solid lightning bolts. The primary U4 cross-link is indicated by an open lightning bolt, and the open arrowheads indicate the weaker cross-links.
Additionally, under these conditions there is consistently a weaker primer-extension stop detected at position 51. The cross-link at position 51 quite likely persists through the first step of splicing such that we are able to detect the U6 cross-link to the intermediate (Fig. 3). The results are consistent with a model in which, after initial ATP-dependent recognition of the 5′ splice site by U6 snRNA, other events are required to modulate the interaction such that U6 is associated with the pre-mRNA in its “catalytically active” form.
Discussion
To analyze RNA–RNA interactions at the 5′ splice site, we have further developed an in vitro trans-splicing system using yeast splicing extracts. A precursor to this system was first described by Ghetti and Abelson (1995); however, the utility of the system was limited by the relative inefficiency of the reaction. The problem of reaction inefficiency has been overcome by biotinylating the 5′ splice site-containing RNA and purifying the reaction products and intermediates using streptavidin-coated magnetic beads. This in vitro trans-splicing reaction has the same Mg2+, ATP, and sequence requirements as the cis-splicing reaction. We also have demonstrated that a well-characterized interaction between intron nucleotides just downstream of the 5′ splice site and the conserved ACAGAG sequence of U6 (Lesser and Guthrie 1993; Sontheimer and Steitz 1993; Kandels-Lewis and Seraphin 1994; Kim and Abelson 1996) is detectable in this trans-splicing system. Using site-specific cross-linking, we are able to map spliceosome-dependent interactions that have not been detected by other methods.
Here we report several unexpected features of 5′ splice site recognition. First, the U4 and U6 snRNAs both interact with the 5′ splice site in an ATP-dependent fashion. However, this interaction does not require the 3′ splice site or branch point sequences, challenging a canonical view of splicing that describes stable U2 association with the spliceosome to be the first ATP-requiring step in spliceosome assembly. Second, these data indicate that multiple regions of U6 interact with the 5′ splice site, interactions that may be important for 5′ splice site recognition. Our results also support a collection of data that suggests that U1-snRNA–5′ splice site base pairing is not absolutely required for snRNP interactions with the 5′ splice site, although the U1 snRNA is required for some subsequent step (Konforti et al. 1993; Crispino et al. 1994, 1996; Konforti and Konarska 1994, 1995; Tarn and Steitz 1994; Crispino and Sharp 1995; Du and Rosbash 2001).
We describe several novel cross-links between the 5′ splice site and U4 and U6 snRNA. Because 4-thioU is a short-arm cross-linker, it is clear that U4 is in very close proximity to the 5′ splice site at the earliest stages of interaction between the spliceosome and the pre-mRNA. Importantly, the U4 and U6 cross-links that we detect—nucleotide 75 within the central domain region of U4 and to nucleotide 43 in U6—lie almost directly across from each other if the single-stranded regions of the RNAs that flank the intermolecular stem II are aligned (Fig. 9). It seems quite likely, then, that the interactions we see between U4 and the 5′ splice site are an indication of U4/U6 interaction with the 5′ splice site, where the role of U4 may be to align U6 with the 5′ splice site.
These results support other data that indicate that U4 plays an important role in aligning U6 to the 5′ splice site during spliceosome assembly. Recently, Nilsen and coworkers (Maroney et al. 2000) demonstrated that U4/U6·U5 could associate with pre-mRNA independently of U2-snRNP association. By UV cross-linking, they further demonstrated that the highly conserved splicing protein Prp8 specifically mediated this interaction in a 5′ splice site sequence-specific manner. Interestingly, this cross-link was specifically abolished when U4 snRNA was degraded by oligonucleotide-directed RNase H digestion using a DNA oligonucleotide complementary to mammalian U4 at positions 64–83, the central domain of U4. This cross-link was also abolished when mammalian U6 was blocked at positions 33–47 (equivalent to positions 39–53 in yeast U6) using a 2‘-o-methyl oligonucleotide. This U6 oligonucleotide also inhibited splicing (Maroney et al. 2000; see also Black and Steitz 1986).
Importantly, a second overlapping oligonucleotide complementary to U6 at positions 42–60 (equivalent to yeast 48–66) did not inhibit the Prp8 cross-link. This second oligonucleotide included position 45 (mammalian U6), where the well-characterized interaction between U6 ACAGAG and the 5′ splice site occurs (Sontheimer and Steitz 1993; Kim and Abelson 1996), the G50 (or A51) interaction described here. These results support our findings that U6 sequences upstream of the ACAGAG play an important role in 5′ splice site interactions with the spliceosome—a role that appears to be mediated by Prp8. Furthermore, these results support our conclusion that this region of U6 has a function separable from the function of the downstream ACAGAG.
Studies using reconstituted U6 snRNA in nematode splicing extracts also demonstrated that the upstream region of U6 is involved in 5′ splice site recognition. In these studies, when a region of U6 upstream of the U6 ACAGAG was mutated, the branch point attacked U6 rather than the 5′ splice site, indicating that these mutations prevented the correct positioning of the 5′ splice site. The sites of attack on U6 also mapped to a region upstream of the ACAGAG sequence (Yu et al. 1993).
In a set of yeast genetic experiments, the effect of a mutation in U4 that extended the U4/U6 stem I interaction was examined (Li and Brow 1996). The mutation produced a cold-sensitive phenotype that blocked the splicing pathway after U4/U6 complex formation, but before the first catalytic step of splicing. The mutation was suppressed when the hyperstabilized base-pairing was disrupted, indicating that accessibility of the conserved ACAGAG was essential for splicing. One class of U6 mutants that suppressed U4-cs1 included two mutations at positions 42 and 43 of U6, both of which may weaken potential U6–5′ splice site interactions with these nucleotides. One possibility is that when the interaction of the downstream ACAGAG sequence is inhibited by stem I hyperstabilization, the complex gets “stuck” at the U6–5′ splice site interactions at the upstream nucleotides. Weakening these early 5′ splice site interactions might allow the 5′ splice site to progress to interactions with the downstream, catalytically crucial ACAGAG sequence.
Interestingly, one of the suppressors of the U4-cs1 mutation is Prp8-201 (Kuhn et al. 1999). This and the results by Maroney et al. (2000) are consistent with Prp8 interacting either directly or indirectly with regions of U6 upstream of the U6 ACAGAG sequence as well as the ACAGAG region itself, where genetic and cross-linking interactions with Prp8 have been identified (Collins and Guthrie 1999; Vidal et al. 1999). Prp8 interactions with U6 sequences upstream of the ACAGAG are also critical for ATP-dependent Prp8 cross-linking with the 5′ splice site (Maroney et al. 2000).
Taken together with the results presented here, it seems likely that part of the function of Prp8 is to mediate at least two different interactions between U6 and the 5′ splice site and at least one interaction between U4 and the 5′ splice site. Because Prp8 is not known to be directly involved in ATP hydrolysis, some factor or factors must act in concert with Prp8 to use ATP and mediate these interactions. There are several RNA-dependent ATPases that may be candidates for this. One such candidate is Prp28, which mediates the U1–U6 switch at the 5′ splice site (Staley and Guthrie 1999) and shows a genetic interaction with Prp8 (Strauss and Guthrie 1991). Another is Brr2, which is involved in U4/U6 unwinding (Laggerbauer et al. 1998; Raghunathan and Guthrie 1998; Kim and Rossi 1999). Interestingly, the U4-cs1 mutation, which is suppressed by Prp8-201, is exacerbated by Brr2 mutations (Kuhn and Brow 2000), and Brr2 has been shown to interact with Prp8 both biochemically and genetically (Achsel et al. 1998; van Nues and Beggs 2001). This suggests that it may act in concert with Prp8 to mediate U4/U6 unwinding and spliceosome activation. Our own preliminary data indicate that the U4 cross-link that we observe is mediated by the activity of Brr2 (T.L. Johnson and J. Abelson, in prep.).
The results presented in this paper as well as the genetic and biochemical results described above indicate an important role for nucleotides just upstream of the conserved ACAGAG in splicing and, specifically, 5′ splice site recognition. It may be that the A42C43A44–5′ splice siteUGU interaction is an early intermediate in the U6–5′ splice site interactions. The interaction with the region upstream of the conserved ACAGAG may even be a checkpoint along the pathway to the catalytically important interaction at the ACAGAG. Based on the predicted structure of U4/U6, C43 and G50 (and A51) are upstream of stem I and should both be accessible to interact with the 5′ splice site. It is possible that interactions at the ACAGAG may be blocked by some factor (or factors) in order to ensure a sequenced order of events. Therefore, the interactions detected by our yeast trans-splicing system may be an intermediate that transiently forms until the downstream ACAGAG is made available by subsequent events.
When the 5′ end of U1 snRNA is deleted using oligonucleotide-directed digestion by endogenous RNase H, the 5′ splice site is still recognized by U4/U6. Nonetheless, splicing is inhibited. It therefore appears that, in this system, 5′ splice site recognition is not dependent on the 5′ end of U1, although some other activity is.
These results support a growing body of work that reevaluates the role of 5′ splice site–U1-snRNA base-pairing in pre-mRNA recognition. Recent work using S. cerevisiae splicing extracts demonstrates that the 5′ end of U1 snRNA is dispensable for U1-snRNP–pre-mRNA complex formation and supports a role for several U1 snRNP proteins in mediating this interaction. The authors suggest that base-pairing, although not absolutely required for complex formation, probably contributes to complex stability (Du and Rosbash 2001).
Notably, in mammalian splicing systems, the 5′ end of U1 snRNA and the entire U1 snRNP are dispensable for stable complex formation and even splicing. In some cases addition of excess SR protein is required (Tarn and Steitz 1994, 1995; Crispino et al. 1994; Crispino and Sharp 1995). However, in other cases, no addition of SR protein is necessary (Konforti and Konarska 1995; Crispino et al. 1996). In fact, in the absence of U1 or excess SR protein, 5′ splice site–U6 interactions occur in regions of U6 that are consistent with the U6–5′ splice site cross-links that we report here (Konforti and Konarska 1994). These results indicate that, at least in the mammalian system, non-U1 snRNP components can direct subsequent snRNP interactions with the 5′ splice site.
The finding that the 5′ end of the U1 snRNA is required for the splicing reaction described here suggests that it is required for some event downstream of initial 5′ splice site recognition. Similarly, although U1 snRNP interactions occur even in the absence of the 5′ end of U1, the efficiency of splicing is dramatically reduced in its absence (Du and Rosbash 2001). Apparently, this other role for the 5′ end of U1 can be bypassed in the mammalian in vitro cis- and trans-splicing reactions. Nevertheless, there is evidence both in yeast and in mammalian splicing systems that U1 has multiple roles, including enhancement of U2 binding to the branch point (Seraphin et al. 1988; Barabino et al. 1990; Seiwert and Steitz 1993; Ruby and Abelson 1998).
Taken together, the results reported here suggest that there are a number of possible 5′ splice site recognition events. In fact, it is intriguing to hypothesize that these multiple interactions may serve a proofreading function to ensure accurate splice-site recognition.
The in vitro trans-splicing system allows us to capture some of the rapid and transient events that occur even at the earliest steps of splicing. We are continuing to exploit this approach to explore other interactions involved in the splicing reaction.
Materials and methods
Extract preparation
Yeast extracts were prepared as described previously using strain EJ101 (Lin et al. 1985).
RNA oligonucleotides
The 5′ splice site RNA oligonucleotides described in the text were synthesized using an Applied Biosystems synthesizer. The 4-thioU-containing oligonucleotide was synthesized and purified in Peter Stockley’s laboratory at the University of Leeds (Adams et al. 1994).
Plasmid constructs and in vitro transcription
The plasmids for both the wild-type 3′ acceptor and the ACAC mutant 3′ acceptor were generated as described in Vijayraghavan et al. (1986) and Ghetti and Abelson (1995). For RNA synthesis, the plasmid was linearized with EcoRI and used as a template for in vitro transcription using T7 RNA polymerase. All of the RNAs were isolated by gel electrophoresis and eluted from the gel in elution buffer (0.5 M NaCl, 50 mM Tris-Cl at pH 7.5, 1 mM EDTA at pH 8.0, and 0.1% SDS).
Trans-splicing analysis and site-specific cross-linking
Trans-splicing reactions were carried out in a final volume of 10 μL using 10 μM biotinylated 5′ leader, 40 nM 3′ acceptor, 60% extract, and 1× Splicing Buffer containing 55 mM KPO4 (pH 7.3), 1.3 mM MgCl2, 1.5 mM ATP, 2.5% Peg 8000, 1 mM spermidine, 1 mM DTT, and 0.5 units/μL RNasin (Promega). The reactions were carried out for 2 h at 16°C. For the cross-linking, the reactions were carried out in individual wells on a microtiter dish on ice and irradiated for 15 min with a BLAK-RAY longwave UV lamp (at >300 nM). After splicing, the reaction was stopped with proteinase K-containing splicing stop buffer (Lin et al. 1985), phenol-extracted, and ethanol-precipitated.
The products of the trans-splicing reaction were incubated in 1× SSC with (for a 10-μL splicing reaction) 0.25 μg of streptavidin-coated magnetic beads (Promega). After a 30-min incubation at room temperature, the tubes were placed in a magnetic stand and the supernatant was removed. Then the beads were washed with a buffer containing 2× SSC and 20% ethanol. To dissociate the RNA from the beads, the samples were heated for 5 min at 90°C in sample loading buffer (80% formamide, 1× TBE, 0.1% xylene cyanol, and 0.1% bromophenol blue). The reaction products were separated on a 6% (29:1 acrylamide:bis) polyacrylamide gel.
U1 knockouts were carried out as described in Ruby and Abelson (1988), except the sequence of the U1 oligonucleotide was 5′-TCTTAAGGTAAGTAT-3′.
ATP depletion was carried out as described in Horowitz and Abelson (1993) with the modification that 6 μL of extract was incubated with glucose for a final glucose concentration in the splicing reaction of 4 mM or 6 mM.
Chemiluminescent detection
After electrophoresis, the gels were transferred to Zeta-Probe Blotting Membrane (BioRad) using the semi-dry transfer apparatus (BioRad). The membranes were UV cross-linked, blocked with I-Block blocking reagent (Tropix), and incubated with Avidx-AP (Tropix). After washing the membranes several times with 1× PBS/0.5% SDS, the membranes were treated with CDP-Star (Tropix) and visualized by autoradiography.
RNase H and primer-extension analysis
The following DNA oligonucleotides were synthesized for the RNase H experiments: U1, 5′-GAATGGAAACGTCAGCAAA CAC-3′; U2, 5′-GTTTTTTACACATAAC-3′; U4a, 5′-ACCAT GAGGAGACGGTCTGG-3′; U4b, 5′-AGACGGTCTGGTTT AT-3′; U5, 5′-TTCGTTATAAGTTCTATAGGC-3′; and U6, 5′-AGGGGAACTGCTGATC-3′.
The oligonucleotide was annealed to the gel-purified RNA and treated with RNase H (TaKaRa) according to the manufacturer's conditions. Then 7 M urea loading buffer was added, and the samples were loaded on a 6% polyacrylamide gel. Where appropriate, the gels were then transferred and detected by chemiluminescence.
For U4 primer-extension experiments, the DNA oligonucleotide complementary to the 3′ end of U4 with the sequence 5′-AGGTATTCCAAAAATTCCCT-3′ was annealed to the RNA samples and reverse transcribed with Superscript II (GIBCO BRL) reverse transcriptase at 40°C. After extension, the RNA was degraded by incubation with NaOH at 65°C, and the samples were run on an 8% sequencing gel. The U6 primer extension was carried out similarly, and the DNA oligonucleotide used was complementary to the 3′ end of U6 with the sequence 5′-AAACGAAATAAATCTCTTTG-3′.
Acknowledgments
We are grateful to Chris Adams, James Murray, and Peter Stockley for synthesis and purification of the 4-thioU and 6-thioG RNAs; Christine Guthrie, Alexander Hoffmann, Alan Kutach, Andreas Kuhn, Stephen Rader, Daniel Ryan, and Eric Sontheimer for helpful discussions and critical comments on the manuscript; and Rozalie Jackson for excellent technical assistance. T.J. was supported by the Jane Coffin Childs Medical Research Fund.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL abelsonj@starbase1.caltech.edu; FAX (626) 796-7066.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.895601.
References
- Achsel T, Ahrens K, Brahms H, Teigelkamp S, Lührmann R. The human U5-220kD protein (hPrp8) forms a stable RNA-free complex with several U5-specific proteins, including an RNA unwindase, a homologue of ribosomal elongation factor EF-2, and a novel WD-40 protein. Mol Cell Biol. 1998;18:6756–6766. doi: 10.1128/mcb.18.11.6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams C, Murray J, Arnold J, Stockley P. A convenient synthesis of s-cyanoethyl-protected 4-thiouridine and its incorporation into oligoribonucleotides. Tetrahedron Lett. 1994;35:765–768. [Google Scholar]
- Barabino SML, Blencowe BJ, Ryder U, Sproat BS, Lamond AI. Targeted snRNP depletion reveals an additional role for mammalian U1 snRNP in spliceosome assembly. Cell. 1990;63:293–302. doi: 10.1016/0092-8674(90)90162-8. [DOI] [PubMed] [Google Scholar]
- Black D, Steitz J. Pre-mRNA splicing in vitro requires intact U4/U6 small nuclear ribonucleoprotein. Cell. 1986;46:697–704. doi: 10.1016/0092-8674(86)90345-4. [DOI] [PubMed] [Google Scholar]
- Brow DA, Guthrie C. Spliceosomal RNA U6 is remarkably conserved from yeast to mammals. Nature. 1989;337:14–15. doi: 10.1038/334213a0. [DOI] [PubMed] [Google Scholar]
- Brow DA, Vidaver RM. An element in human U6 RNA destabilizes the U4/U6 spliceosomal RNA complex. RNA. 1995;1:122–131. [PMC free article] [PubMed] [Google Scholar]
- Burge CB, Tuschl T, Sharp PA. Splicing of precursors to mRNAs by the splicesosome. In: Gesteland RR, Cech TR, Atkins JF, editors. The RNA world II. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1999. pp. 525–560. [Google Scholar]
- Cheng SC, Abelson J. Spliceosome assembly in yeast. Genes & Dev. 1987;1:1014–1027. doi: 10.1101/gad.1.9.1014. [DOI] [PubMed] [Google Scholar]
- Collins CA, Guthrie C. Allele-specific genetic interactions between Prp8 and RNA active site residues suggest a function for Prp8 at the catalytic core of the spliceosome. Genes & Dev. 1999;13:1970–1982. doi: 10.1101/gad.13.15.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispino JD, Sharp PA. A U6 snRNA:pre-mRNA interaction can be rate-limiting for U1 independent splicing. Genes & Dev. 1995;9:2314–2323. doi: 10.1101/gad.9.18.2314. [DOI] [PubMed] [Google Scholar]
- Crispino JD, Blencowe BJ, Sharp PA. Complementation by SR proteins of pre-mRNA splicing reactions depleted of U1 snRNP. Science. 1994;265:1866–1869. doi: 10.1126/science.8091213. [DOI] [PubMed] [Google Scholar]
- Crispino JD, Mermoud JE, Lamond AI, Sharp PA. Cis-acting elements distinct from the 5′ splice site promote U1-independent pre-mRNA splicing. RNA. 1996;2:664–673. [PMC free article] [PubMed] [Google Scholar]
- Das R, Zhou Z, Reed R. Functional association of U2 snRNP with the ATP-independent spliceosomal complex E. Mol Cell. 2000;5:779–787. doi: 10.1016/s1097-2765(00)80318-4. [DOI] [PubMed] [Google Scholar]
- Datta B, Weiner AM. Genetic evidence for base pairing between U2 and U6 snRNA in mammalian mRNA splicing. Nature. 1991;352:821–824. doi: 10.1038/352821a0. [DOI] [PubMed] [Google Scholar]
- Du H, Rosbash M. Yeast U1 snRNP–pre-mRNA complex formation without U1 snRNA–pre-mRNA base pairing. RNA. 2001;7:133–142. doi: 10.1017/s1355838201001844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio P, Abelson J. Two domains of yeast U6 small nuclear RNA required for both steps of nuclear precursor messenger RNA splicing. Science. 1990;250:404–409. doi: 10.1126/science.2145630. [DOI] [PubMed] [Google Scholar]
- Fortner DM, Troy RG, Brow DA. A stem/loop in U6 snRNA defines a conformational switch required for pre-mRNA splicing. Genes & Dev. 1994;8:221–233. doi: 10.1101/gad.8.2.221. [DOI] [PubMed] [Google Scholar]
- Fradin A, Jove R, Hemenway C, Keiser HD, Manley JL, Prives C. Splicing pathways of SV40 mRNAs in X. laevis oocytes differ in their requirements for snRNPs. Cell. 1984;37:927–936. doi: 10.1016/0092-8674(84)90427-6. [DOI] [PubMed] [Google Scholar]
- Frilander MJ, Steitz JA. Dynamic exchanges of RNA interactions leading to catalytic core formation in the U12-dependent spliceosome. Mol Cell. 2001;7:217–226. doi: 10.1016/s1097-2765(01)00169-1. [DOI] [PubMed] [Google Scholar]
- Ghetti A, Abelson J. In vitro trans-splicing in Saccharomyces cerevisiae. Proc Natl Acad Sci. 1995;92:11461–11464. doi: 10.1073/pnas.92.25.11461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall KB, Konarska MM. The 5′ splice site consensus RNA oligonucleotide induces assembly of U2/U4/U5/U6 small nuclear ribonucleoprotein complexes. Proc Natl Acad Sci. 1992;89:10969–10973. doi: 10.1073/pnas.89.22.10969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausner TP, Giglio LM, Weiner AM. Evidence for base-pairing between mammalian U2 and U6 small nuclear ribonucleoprotein particles. Genes & Dev. 1990;4:2146–2156. doi: 10.1101/gad.4.12a.2146. [DOI] [PubMed] [Google Scholar]
- Horowitz DS, Abelson J. Stages in the second reaction of pre-mRNA splicing: The final step is ATP independent. Genes & Dev. 1993;7:320–329. doi: 10.1101/gad.7.2.320. [DOI] [PubMed] [Google Scholar]
- Kandels-Lewis S, Seraphin B. Involvement of U6 snRNA in 5′ splice site selection. Science. 1994;262:2035–2039. doi: 10.1126/science.8266100. [DOI] [PubMed] [Google Scholar]
- Kim CH, Abelson J. Site-specific crosslinks of yeast U6 snRNA to the pre-mRNA near the 5′ splice site. RNA. 1996;2:995–1010. [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Rossi JJ. The first ATPase domain of the yeast 246-kDa protein is required for in vivo unwinding of the U4/U6 duplex. RNA. 1999;5:959–971. doi: 10.1017/s135583829999012x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konforti BB, Konarska MM. U4/U5/U6 snRNP recognizes the 5′ splice site in the absence of U2 SnRNP. Genes & Dev. 1994;8:1962–1973. doi: 10.1101/gad.8.16.1962. [DOI] [PubMed] [Google Scholar]
- ————— A short 5′ splice site RNA oligo can participate in both steps of splicing in mammalian extracts. RNA. 1995;1:815–827. [PMC free article] [PubMed] [Google Scholar]
- Konforti BB, Koziolkiewicz MJ, Konarska MM. Disruption of base pairing between the 5′ splice site and the 5′ end of U1 snRNA is required for spliceosome assembly. Cell. 1993;75:863–873. doi: 10.1016/0092-8674(93)90531-t. [DOI] [PubMed] [Google Scholar]
- Krämer A. Presplicing complex formation requires two proteins and U2 snRNP. Genes & Dev. 1988;2:1155–1167. doi: 10.1101/gad.2.9.1155. [DOI] [PubMed] [Google Scholar]
- Kretzner L, Rymond BC, Rosbash M. S. cerevisiae U1 RNA is large and has limited primary sequence homology to metazoan U1 snRNA. Cell. 1987;50:593–602. doi: 10.1016/0092-8674(87)90032-8. [DOI] [PubMed] [Google Scholar]
- Kuhn AN, Brow DA. Suppressors of a cold-sensitive mutation in yeast U4 RNA define five domains in the splicing factor Prp8 that influence spliceosome activation. Genetics. 2000;155:1667–1682. doi: 10.1093/genetics/155.4.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn AN, Li Z, Brow DA. Splicing factor Prp8 governs U4/U6 RNA unwinding during activation of the spliceosome. Mol Cell. 1999;3:65–75. doi: 10.1016/s1097-2765(00)80175-6. [DOI] [PubMed] [Google Scholar]
- Laggerbauer B, Achsel T, Lührmann R. The human U5-200kD DEXH-box protein unwinds U4/U6 RNA duplices in vitro. Proc Natl Acad Sci. 1998;95:4188–4192. doi: 10.1073/pnas.95.8.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser CF, Guthrie C. Mutations in U6 that alter splice site specificity: Implications for the active site. Science. 1993;262:1982–1988. doi: 10.1126/science.8266093. [DOI] [PubMed] [Google Scholar]
- Li Z, Brow DA. A spontaneous duplication in U6 spliceosomal RNA uncouples the early and late functions of the ACAGA element in vivo. RNA. 1996;2:879–894. [PMC free article] [PubMed] [Google Scholar]
- Liao X, Colot HV, Wang Y, Rosbash M. Requirements for the U2 snRNP addition to yeast pre-mRNA. Nucleic Acids Res. 1992;20:4237–4245. doi: 10.1093/nar/20.16.4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RJ, Newman AJ, Cheng S-C, Abelson J. Yeast mRNA splicing in vitro. J Biol Chem. 1985;260:14780–14792. [PubMed] [Google Scholar]
- Liu ZR, Sargueil B, Smith C. Detection of a novel ATP-dependent cross-linked protein at the 5′ splice site–U1 small nuclear RNA duplex by methylene blue-mediated photo-cross-linking. Mol Cell Biol. 1998;18:6910–6920. doi: 10.1128/mcb.18.12.6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhani HD, Guthrie C. A novel base pairing interaction between U2 and U6 snRNAs suggests a mechanism for the catalytic activation of the spliceosome. Cell. 1992;71:803–817. doi: 10.1016/0092-8674(92)90556-r. [DOI] [PubMed] [Google Scholar]
- Madhani HD, Bordonné R, Guthrie C. Multiple roles for U6 snRNA in the splicing pathway. Genes & Dev. 1990;4:2264–2277. doi: 10.1101/gad.4.12b.2264. [DOI] [PubMed] [Google Scholar]
- Maroney PA, Romfo CM, Nilsen TW. Functional recognition of the 5′ splice site by U4/U6·U5 tri-snRNP defines a novel ATP-dependent step in early spliceosome assembly. Mol Cell. 2000;6:317–328. doi: 10.1016/s1097-2765(00)00032-0. [DOI] [PubMed] [Google Scholar]
- Michaud S, Reed R. An ATP-independent complex commits pre-mRNA to the mammalian spliceosome assembly pathway. Genes & Dev. 1991;5:2534–2546. doi: 10.1101/gad.5.12b.2534. [DOI] [PubMed] [Google Scholar]
- Mount SM. A catalogue of splice junction sequences. Nucleic Acids Res. 1982;10:459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AJ, Teigelkamp S, Beggs JD. snRNA interactions at the 5′ and 3′ splice sites monitored by photoactivated cross-linking in yeast spliceosomes. RNA. 1995;1:968–980. [PMC free article] [PubMed] [Google Scholar]
- Parker R, Siliciano PG, Guthrie C. Recognition of the TACTAAC box during mRNA splicing in yeast involves base pairing to the U2-like snRNA. Cell. 1987;49:229–239. doi: 10.1016/0092-8674(87)90564-2. [DOI] [PubMed] [Google Scholar]
- Perriman R, Ares M. ATP can be dispensable for prespliceosome formation in yeast. Genes & Dev. 2000;14:97–107. [PMC free article] [PubMed] [Google Scholar]
- Pruzan R, Furneaux H, Lassota P, Hong GY, Hurwitz J. Assemblage of the prespliceosome complex with separated fractions isolated from HeLa cells. J Biol Chem. 1990;265:2804–2813. [PubMed] [Google Scholar]
- Query CC, McCaw PS, Sharp PA. A minimal spliceosomal complex A recognizes the branch site and polypyrimidine tract. Mol Cell Biol. 1997;17:2944–2953. doi: 10.1128/mcb.17.5.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunathan PL, Guthrie C. RNA unwinding in U4/U6 snRNPs requires ATP hydrolysis and the DEIH-box splicing factor Brr2. Curr Biol. 1998;8:847–855. doi: 10.1016/s0960-9822(07)00345-4. [DOI] [PubMed] [Google Scholar]
- Ruby SW, Abelson J. An early hierarchic role of U1 small nuclear ribonucleoprotein in spliceosome assembly. Science. 1988;242:1028–1035. doi: 10.1126/science.2973660. [DOI] [PubMed] [Google Scholar]
- Sawa H, Abelson J. Evidence for a base-pairing interaction between U6 small nuclear RNA and 5′ splice site during the splicing reaction in yeast. Proc Natl Acad Sci. 1992;89:11269–11273. doi: 10.1073/pnas.89.23.11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa H, Shimura Y. Association of U6 snRNA with the 5′-splice site region of pre-mRNA in the spliceosome. Genes & Dev. 1992;6:244–254. doi: 10.1101/gad.6.2.244. [DOI] [PubMed] [Google Scholar]
- Seiwert SD, Steitz JA. Uncoupling two functions of the U1 small nuclear ribonucleoprotein particle during in vitro splicing. Mol Cell Biol. 1993;13:3135–3145. doi: 10.1128/mcb.13.6.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seraphin B, Kretzner L, Rosbash M. A U1 snRNA: pre-mRNA base pairing interaction is required early in yeast spliceosome assembly but does not uniquely define the 5′ cleavage site. EMBO J. 1988;7:2533–2538. doi: 10.1002/j.1460-2075.1988.tb03101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siliciano PG, Guthrie C. 5′ splice site selection in yeast: Genetic alterations in base-pairing with U1 reveal additional requirements. Genes & Dev. 1988;2:1258–1267. doi: 10.1101/gad.2.10.1258. [DOI] [PubMed] [Google Scholar]
- Sontheimer EJ, Steitz JA. The U5 and U6 small nuclear RNAs as active site components of the spliceosome. Science. 1993;626:1989–1996. doi: 10.1126/science.8266094. [DOI] [PubMed] [Google Scholar]
- Staley JP, Guthrie C. An RNA switch at the 5′ splice site requires ATP and the DEAD box protein Prp28p. Mol Cell. 1999;3:55–64. doi: 10.1016/s1097-2765(00)80174-4. [DOI] [PubMed] [Google Scholar]
- Stevens SW, Abelson J. Purification of the yeast U4/U6·U5 small nuclear ribonucleoprotein particle and identification of its proteins. Proc Natl Acad Sci. 1999;96:7226–7231. doi: 10.1073/pnas.96.13.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss EJ, Guthrie C. A cold-sensitive mRNA splicing mutant is a member of the RNA helicase gene family. Genes & Dev. 1991;5:629–641. doi: 10.1101/gad.5.4.629. [DOI] [PubMed] [Google Scholar]
- Tarn WY, Steitz JA. SR proteins can compensate for the loss of U1 snRNP functions in vitro. Genes & Dev. 1994;8:2704–2717. doi: 10.1101/gad.8.22.2704. [DOI] [PubMed] [Google Scholar]
- Tarn WY, Steitz JA. Modulation of a 5′ splice site choice in pre-messenger RNA by two distinct steps. Proc Natl Acad Sci. 1995;92:2504–2508. doi: 10.1073/pnas.92.7.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadkhan S, Manley JL. A tertiary interaction detected in human U2–U6 snRNA complex assembled in vitro resembles a genetically proven interaction in yeast. RNA. 2000;6:206–219. doi: 10.1017/s1355838200992197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nues RW, Beggs JD. Functional contacts with a range of splicing proteins suggest a central role for Brr2p in the dynamic control of the order of events in spliceosomes of Saccharomyces cerevisiae. Genetics. 2001;157:1451–1467. doi: 10.1093/genetics/157.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Santen VL, Spritz RA. Alternative splicing of SV40 early pre-mRNA in vitro. Nucleic Acids Res. 1986;14:9911–9926. doi: 10.1093/nar/14.24.9911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal V, Verdone L, Mayes AE, Beggs JD. Characterization of U6 snRNA–protein interactions. RNA. 1999;5:1470–1481. doi: 10.1017/s1355838299991355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan U, Parker R, Tamm J, Iimura Y, Rossi J, Abelson J, Guthrie C. Mutations in conserved intron sequences affect multiple steps in the yeast splicing pathway, particularly assembly of the spliceosome. EMBO J. 1986;5:1683–1695. doi: 10.1002/j.1460-2075.1986.tb04412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman DA, Steitz JA. Interactions of small nuclear RNA's with precursor messenger RNA during in vitro splicing. Science. 1992;257:1918–1925. doi: 10.1126/science.1411506. [DOI] [PubMed] [Google Scholar]
- Will CL, Lührmann R. Protein functions in pre-mRNA splicing. Curr Opin Cell Biol. 1997;9:320–328. doi: 10.1016/s0955-0674(97)80003-8. [DOI] [PubMed] [Google Scholar]
- Wolff T, Menssen R, Hammel J, Bindereif A. Splicing function of mammalian U6 small nuclear RNA: Conserved positions in central domain and helix I are essential during the first and second step of pre-mRNA splicing. Proc Natl Acad Sci. 1994;91:903–907. doi: 10.1073/pnas.91.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Manley JL. Mammalian pre-mRNA branch site selection by U2 snRNP involves base pairing. Genes & Dev. 1989;3:1553–1561. doi: 10.1101/gad.3.10.1553. [DOI] [PubMed] [Google Scholar]
- Wyatt JR, Sontheimer EJ, Steitz JA. Site-specific cross-linking of mammalian U5 snRNP to the 5′ splice site before the first step of pre-mRNA splicing. Genes & Dev. 1992;257:1918–1925. doi: 10.1101/gad.6.12b.2542. [DOI] [PubMed] [Google Scholar]
- Yean SL, Lin RJ. U4 small nuclear RNA dissociates from the yeast spliceosome and does not participate in the subsequent splicing reaction. Mol Cell Biol. 1991;11:5571–5577. doi: 10.1128/mcb.11.11.5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YT, Maroney PA, Nilsen TW. Functional reconstitution of U6 snRNA in nematode cis- and trans-splicing: U6 can serve as both a branch acceptor and a 5′ exon. Cell. 1993;75:1049–1059. doi: 10.1016/0092-8674(93)90315-h. [DOI] [PubMed] [Google Scholar]
- Zhang D, Rosbash M. Identification of eight proteins that cross-link to pre-mRNA in the yeast commitment complex. Genes & Dev. 1999;13:581–592. doi: 10.1101/gad.13.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y, Weiner AM. A compensatory base change in U1 snRNA suppresses a 5′ splice site mutation. Cell. 1986;46:827–835. doi: 10.1016/0092-8674(86)90064-4. [DOI] [PubMed] [Google Scholar]
- ————— A compensatory base change in human U2 snRNA can suppress a branch site mutation. Genes & Dev. 1989;3:1545–1552. doi: 10.1101/gad.3.10.1545. [DOI] [PubMed] [Google Scholar]