Abstract
Most cancer vaccines induce cytotoxic T lymphocyte (CTL) responses to tumor-associated antigens (TAA). Killing of tumor cells occurs through TAA-specific CTL-mediated cytolysis. Here we show that one preventive followed by two therapeutic immunizations with an attenuated Listeria monocytogenes (LM)-based vaccine eradicates all metastases and almost the entire primary tumor in the syngeneic, aggressive mouse breast tumor model 4T1. We provide strong evidence that this is due to the combined result of direct kill by Listeria infecting the tumors cells, and by CTL responses against Listeria antigens. We demonstrated by electron microscopy (EM) that LM expressing truncated lysteriolysinO (LLO) and amino acid fragment 311-660 of TAA Mage-b (LM-LLO-Mage-b311-660) and the control strain LM-LLO infect tumor cells in vitro and in vivo. In vitro data indicate that tumor cell death occurs through activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and increased intracellular Ca2+ levels, both resulting in the production of high ROS levels. Since both LM-LLO and LM-LLO-Mage-b311-660 showed equally strong efficacies in vivo we concluded that LM-LLO was crucial and Mage-b of less importance. We found strong CTL responses to LM-LLO in the spleen, and depletion of CD8 T cells in vivo resulted in significant tumor re-growth (52%) in LM-LLO-vaccinated mice, indicating that LM-LLO-specific CTL indeed partially contributed to tumor cell kill in vivo. This dual mode of action of a Listeria-based vaccine has not been described before and may provide new directions in the development of more effective vaccines against metastatic breast cancer.
Keywords: Listeria monocytogenes, LysteriolysinO, NADPH oxidase, ROS, metastases, 4T1 model
INTRODUCTION
Breast cancer is the most common cancer among women around the world (1), and 30–40% of the women diagnosed with breast cancer will progress to metastatic disease (2). Current treatment options for metastatic cancer includes surgery followed by chemotherapy or radiation, and/or adjuvant therapy (3). Although first-line endocrine therapy with tamoxifen or the newer third generation aromatases is promising (4), the cure rate of metastatic breast cancer is low (5). In previous studies we found evidence that vaccination with Mage-b DNA was effective against metastases in various metastatic mouse breast tumor models (6, 7). Mage is an attractive tumor-associated antigen (TAA) since it is expressed in more than 90% of all breast cancers but not in normal cells (8). To further improve the vaccine efficacy of Mage-b, we used an attenuated Listeria monocytogenes (LM) as DNA delivery system. LM is an intracellular pathogen that delivers the vaccine antigen directly into APC such as macrophages with high efficiency (9). Cell entrance of macrophages by LM occurs through active phagocytosis, and the LM escape into the host cytosol by perforating the phagosomal membrane through the action of a cytolysin, listeriolysin O (LLO) (10, 11).. Once in the cytosol, the vaccine antigen produced by the LM is processed and presented as short peptides via the MHC class I and II pathways, generating both CD4 and CD8 T cell responses (12). Killing of tumor cells occurs through CD8 T cells. Previous studies have shown that TAA Her2/neu, expressed by an attenuated LM as fusion protein with LLO, is effective against primary tumors in a syngeneic mouse breast tumor model NT-2 (13). LLO, required for the establishment of intracellular infections (14, 15), also improves immunogenicity of poor immunogenic antigens(13).
In the study presented here we demonstrate that one preventive followed by two therapeutic immunizations with LM-LLO-Mage-b311-660 completely eradicates the metastases and reduces the primary tumors by 90% in a poorly immunogenic metastatic mouse breast tumor model 4T1. Our in vitro and in vivo data strongly suggest that this is due to infection of the tumor cells with Listeria bacteria, resulting in tumor cell kill by high ROS levels and by Listeria-specific CTL. These results point the way towards novel approaches in the use of Listeria vaccines as anti-tumor agents.
MATERIALS AND METHODS
Mice
Normal female Balb/c mice (3 months old) were obtained from Jackson Laboratories (Bar Harbor, Maine) and maintained in the animal husbandry facility of the Pacific Medical Center Research Institute (CPMCRI) according to the Association and Accreditation of Laboratory Animal Care (AACAC) guidelines.
Plasmids and Listeria monocytogenes
The LM-LLO-Mage-b311-660 was developed in our laboratory (16). The Listerial pGG-34 plasmid, expressing the positive regulatory factor A (prfA) and LLO, was developed in the laboratory of Yvonne Paterson, University of Pennsylvania, PA (17, 18). The LM-LLO used in this study is attenuated, i.e., the coding region for the C-terminal part of the LLO (cytolytic domain that binds cholesterol in the membranes) protein has been deleted, but the PEST sequence is still present. Mutations have been introduced into the prfA gene (expressed by the pGG34 vector), which reduced the pathogenicity of the LM (13). pcDNA3.1-Mage-b/V5 was developed in our laboratory (6) Mouse GM-CSF plasmid (CMV1-GM-CSF) was provided by Dr. Stephen Johnston (The Center for Innovations in Medicine, The Biodesign Institute at Arizona State University)(19).
Cells and cell culture
The 4T1 cell line, derived from a spontaneous mammary carcinoma in a BALB/c mouse (20), was cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1 mM mixed nonessential amino acids, 2 mM L-glutamine, insulin (0.5 HSP units/ml) penicillin (100units/ml) and streptomycin (100 μg/ml).
Immunization and tumor challenge
Three different immunization protocols have been tested in this study: (1) Three vaccinations given one week apart (days 1, 8, and 15), with tumor challenge (105 cells injected into a mammary fat pad) at ten days after the last immunization (day 25); or (2) one preventive vaccination, followed by tumor challenge at day 5, with two additional vaccinations at days 8 and 15; or (3) three therapeutic vaccinations given three days after tumor challenge. Injections of 0.1× LD50 (107 bacteria) of LM-LLO-Mage-b311-660 or the control vector LM-LLO, or saline was given ip. Fourteen to eighteen days after tumor challenge the mice were euthanized and analyzed for tumor weight, frequency and location of metastases, as well as for immunological responses in the spleen. Primary tumors extend to the chest cavity lining and metastases (visible by the naked eye as nodules) predominantly to the mesenteric lymph nodes (MLN), and less frequently to the diaphragm, portal liver, spleen, and kidneys (16).
CD8 depletion in vivo
CD8 T cells were depleted in 4T1-tumor-bearing mice with 0.5 mg of anti-CD8 antibodies 2.43 (18) on days 3, 7, 8, 9, 14, 15, and 16 while one preventive and two therapeutic immunizations with LM-LLO were given at days 1, 8, and 15, and tumor challenge at day 5. Mice were euthanized and analyzed for tumor weight and frequency of metastases 14 days after tumor challenge. The antibodies were purchased from BioXCell (West Lebanon, NH). This antibody has been shown by many investigators to specifically deplete only CD8 T cells by >95% (13). As control, isotype-matched rat antibodies against HRPN were used (13, 17).
In vitro analysis of immune responses against Mage-b and Listeria
Spleen cells were isolated from vaccinated and control mice with 4T1 tumors and metastases. To detect Mage-b-specific immune responses, 2×105 cells from spleens were re-stimulated with 5×104 autologous bone marrow (BM) cells (transfected with pcDNA3.1-Mage-b plasmid DNA and pCMV-GM-CSF plasmid DNA) (1 μg of each plasmid DNA per 5×106 BM cells), using the Nucleofector kit of AMAXA (Gaithersburg, MD). To detect Listeria-induced immunes response, 5×104 autologous BM cells were infected with 103 LM-LLO for 1 hour, and subsequently treated with gentamycin. Two days later, the frequency of IFNγ-producing cells was determined by ELISPOT for both re-stimulation assays according to standard protocols (Pharmingen, San Diego, CA), using an ELISPOT reader (CTL Immunospot S4 analyzer, Cellular Technology Ltd, Cleveland, OH). Spleen cells were depleted for CD8 T cells using magnetic bead depletion techniques according to the manufacturer’s instructions (Miltenyi Biotec Inc, Auburn, CA). FACS analysis demonstrated that ≥90% of all CD8 T cells were depleted.
Infection of tumor cells in vitro
The infectivity rate of the tumor cell lines was assessed in vitro. 5 × 105 cells/ml were infected with 108 (per well) of LM-LLO, or LM-LLO-Mage b311-660, for 1 hr at 37°C in culture medium as described above. After incubation with gentamicin (50 μg/ml) for 1 hr (killing all extracellular Listeria bacteria), cells were washed with PBS, lysed in sterile water, and serial dilutions were plated onto LB agar to determine the infection rate the next day, or analyzed by EM using a Philips Tecnai 10 Electron Microscope (Eindhoven, The Netherlands)(21, 22).
Infection of tumor cells in vivo
Balb/C mice were injected with 105 4T1 tumor cells in a mammary fat pad, and 10 days later injected ip with the usual immunization dose, i.e., 0.1×LD50 (107 CFU) of LM-LLO-Mage-b311-660. Next day, mice were euthanized and tumors and metastases were analyzed by EM for the presence of Listeria bacteria.
Evaluation of Cell Death
Death of tumor or normal cells induced by LM-LLO, or LM-LLO-Mage b311-660 was determined in vitro as follows. 3×103 of 4T1 or MCF7 cells plated on 96 well plates were infected with 106 (per well) of LM-LLO or LM-LLO-Mage b311-660, for 3 hrs at 37°C. Gentamycin (50 μg/ml) was added until live and dead cells were counted using Trypan blue staining on the next day. 100 ng/ml of recombinant Listeriolysin-O (LLO), or LLOΔPEST (LLO without PEST sequence) were added in 4T1 cell cultures. Cell death was evaluated as described above. Trolox (100 μM), Apocynin (500 μM), DPI (50 nM), or membrane permeable Ca2+-chelator BAPTA (2 μM) was concomitantly cultured with the Listeria bacteria.
Immunostaining
Immunostaining was performed in 4T1 cultures grown on glass bottom dishes with or without 108 bacteria of LM-LLO or LM-LLO-Mage b311-660, for 2hrs. Subcellular localization of the p47phox subunit of NADPH oxidase was evaluated in cultures fixed with 4% paraformaldehyde for 20 minutes on ice. After preincubation in blocking buffer (0.01% PBS, 2% goat serum, 0.2% Triton-X, 0.1% BSA) for 30 minutes, the cultures were incubated with a 1:500 dilution of mouse anti-p47phox antibody (BD Biosciences, Transduction Laboratories). Primary antibody binding was visualized with a 1:400 dilution of Alexa Fluor 555–conjugated anti-mouse IgG (Molecular Probes; Invitrogen). Fluorescence microscopy was performed on a TE2000 Nikon (Tokyo, Japan) inverted microscope with a Photometrics (Tucson, AZ) and MetaMorph software (MolecularDevices, Downingtown, PA).
Detection of Listeria- and Mage-b proteins in infected tumor cells
4T1 tumor cells (5×105) were infected with LM-LLO or LM-LLO-Mage-b311-660 (108 bacteria), then treated with gentamycin (50 μg/ml) for 2 hrs, washed three times with PBS and analyzed by western blotting. LLO (containing PEST motif) and LLO fused with Mage-b311-660 were detected with anti-PEST antibodies (dilution 1:1000).
Imaging of ROS and Ca2+
2×105/ml 4T1 or MCF7 tumor cells grown on glass bottom dishes were infected with 106 of LM. Two hours later, cells were loaded with 5 μM 5- (and -6)-carboxy-2′, 7′-difluorodihydrofluorescein diacetate (carboxy-H2DFFDA) for intracellular ROS, 100 nM Mitotracker Red CM-H2XROS for mitochondrial ROS, or 10 μM Calcium Green-1 (Molecular Probes; Invitrogen) for intracellular free Ca2+ in Hank’s balanced salt solution (HBSS) for 25 minutes at 37°C and washed three times with HBSS. The signals of ROS or Ca2+ were observed at room temperature on the stage of a TE2000 Nikon inverted microscope with a Photometrics and Coolsnap HQ CCD camera controlled by MetaMorph software (Molecular Devices, Downingtown, PA).
RESULTS
Combined preventive and therapeutic immunization completely eradicates metastases
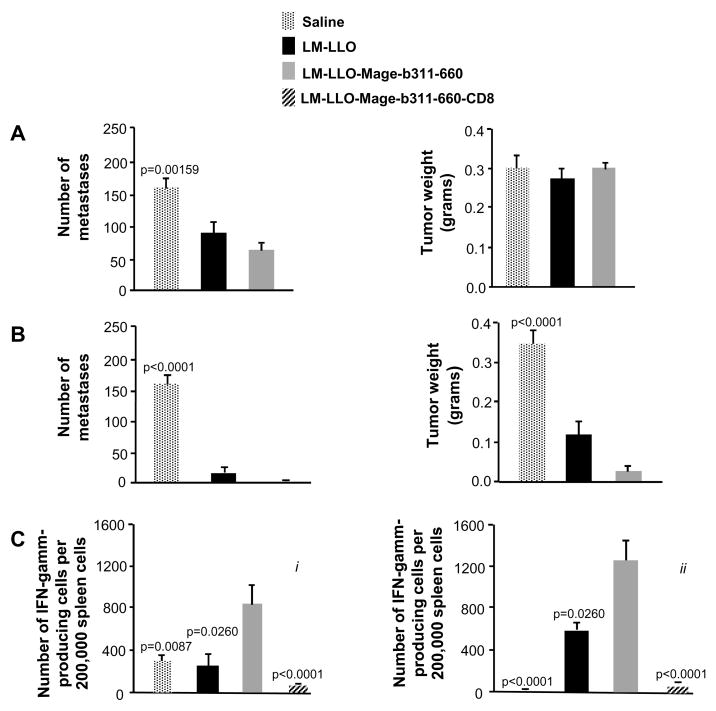
In a previous study we developed a LM-LLO-Mage-b311-660 vaccine (16). After the combination of two preventive and one therapeutic immunization, a significant effect was observed on the metastases but not on the primary tumors (16). In the current study we initially aimed to further improve our vaccination protocol. We reasoned that since the vaccine effect on the tumor was likely due to immune responses, preventive immunizations only (tumor challenge at 10 days after the last immunization) should increase the vaccine efficacy, but this was not the case. A moderate but significant effect was observed on the metastases by the LM-LLO-Mage-b311-660 vaccine, but again no effect was observed on the primary tumors (Fig. 1A). A similarly modest effect was also observed after three therapeutic immunizations (Supplementary Fig. S1). However, one preventive immunization followed by two therapeutic immunizations completely eradicated the metastases and the growth of primary tumors was reduced by almost 90% (Fig. 1B). Moreover, inexplicably, the control strain LM-LLO was almost as effective as LM-LLO-Mage-b311-660.
Figure 1.
Effect of LM-LLO and LM-LLO-Mage-b311-660 in vivo and in vitro. The frequency of metastases and tumor weight was determined after three preventive immunizations (A) and after a combination of one preventive and two therapeutic immunizations (B). Also Mage-b-specific immune responses (C) were analyzed after three preventive immunizations (i) and after the combination of one preventive and two therapeutic immunizations (ii) by ELISPOT. For this purpose, spleen cells were pooled per group and restimulated with bone marrow cells (transfected with pcDNA3.1-Mage-b and pCMV1-GM-CSF), and analyzed for the production of IFNγ 72 hrs later. The involvement of CD8 T cells was determined by negative depletion, using magnetic beads with anti-CD8, antibodies. Controls such as BM cells transfected with pcDNA3.1-Mage-b or pCMV1-GM-CSF, or non-transfected BM cells did not produce IFNγ (data not shown). The results shown in this figure are the average of two-three independent experiments. n=5 mice per group. The error bars in all graphs represent the standard error of the mean (SEM). Significant differences between Mage-b and other groups were analyzed using Mann-Whitney test. p<0.05 is significant.
In order to find an explanation for this difference in vaccine efficacy, we compared Mage-b-specific CTL responses after the three preventive immunizations with the more successful combination of one preventive and two therapeutic immunizations. Re-stimulation of spleen cells of vaccinated and control mice, bearing 4T1 tumors and metastases, with autologous bone marrow cells expressing Mage-b, showed strong Mage-b-specific CD8 T cell responses after both vaccination strategies (Fig. 1C, i and ii), suggesting that CTL-mediated tumor cell kill was not the only cause of this strong in vivo effect in the combination strategy (one preventive followed by two therapeutic immunizations). As expected, three therapeutic immunizations showed reduced Mage-b-specific immune responses in the spleen (Supplementary Fig. S1), likely induced by the primary tumors. However, the similar in vivo efficacies of the control and vaccine strain in the combined immunization suggested that the Listeria bacteria itself may have an effect on the tumor cells, a hypothesis that we further pursued as described below.
Listeria infects and kills tumor cells
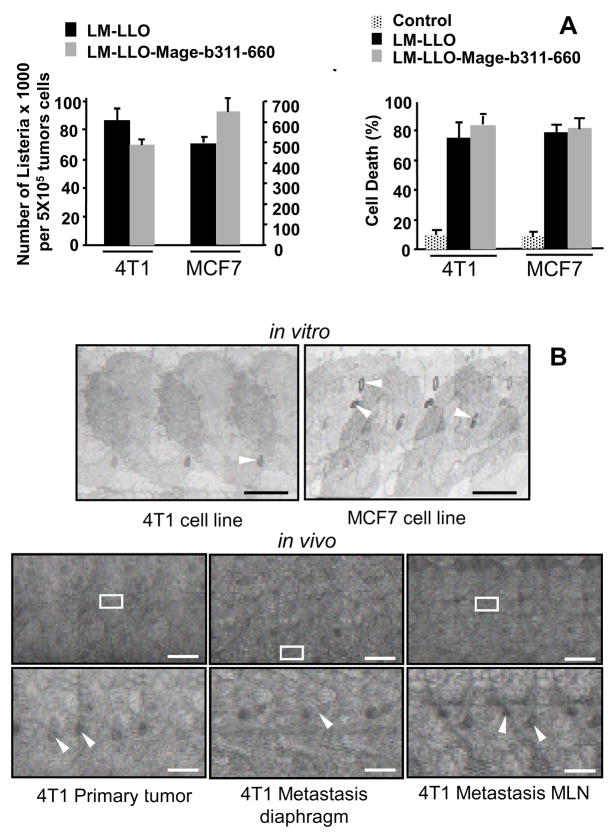
To analyze whether the Listeria bacteria could have a direct effect on tumor cells, 4T1 tumor cells were cultured with the LM-LLO-Mage-b311-660 or with the control strain LM-LLO in vitro. LM-LLO-Mage-b311-660 and LM-LLO not only infects but also kills 4T1 tumor cells with high efficiency in the complete absence of immune cells (Fig. 2A). A similar result was observed with a human breast tumor cell line MCF7 (Fig. 2A). Although the infection rate of MCF7 was five times higher than of 4T1 tumor cells, i.e., after one hour of incubation, 20% of the 4T1 and 100% of the MCF7 tumor cells were infected (Fig. 2A), after 2–3 hours of incubation the infection rate of both tumor cell lines was 100% (data not shown). Interestingly, LM-LLO and LM-LLO-Mage-b311-660 killed the tumor cells (4T1 and MCF7) with the same efficacy. Therefore, we concluded that LM-LLO and not Mage-b311-660 mediated the direct tumor cell kill in vitro.
Figure 2.
Listeria infects and kills tumor cells. A, Both LM-LLO and LM-LLO-Mage-b311-660, infected 4T1 and MCF7 with high efficiency after one hour of infection. Experiments were performed in triplicates, and repeated three times. The results presented in this figure are the average of three infection experiments. The error bars represent the SEM. B, Both LM-LLO and LM-LLO-mage-b311-660 killed 4T1 and MCF7 with the same efficiency (Unpaired t test p>0.05). In this experiment, 4T1 tumor cells were incubated with the Listeria bacteria for 3 hours and then treated with gentamycin. Next day, dead and alive tumor cells were determined with trypan blue. From each sample, nine fields were blinded analyzed by two different investigators. Experiments were performed in triplicates, and repeated three times. The results presented in this figure are the average of three experiments. The error bars represent SEM. C, EM analysis shows the Listeria bacteria (LM-LLO-Mage-b311-660) inside 4T1 and MCF7 tumor cells after one-hour infection in vitro. Bar=200μ. The Listeria bacteria also infected 4T1 primary tumor and metastases in vivo with high efficiency, as shown here after one immunization with LM-LLO-mage-b311-660. Bar=400μ (top), Bar=50μ (bottom). In this experiment tumors and metastases of three different mice that received LM-LLO-Mage-b311-660, were analyzed. The photographs shown here are representative for the three different mice analyzed. The Listeria bacteria are marked with white arrows.
Using electron microscopy (EM), we demonstrated the presence of Listeria bacteria in the tumor cells both in vitro (cultured 4T1 and MCF7 tumor cell lines) and in vivo (4T1 metastases and primary tumor) (Fig. 2B).
Mechanism(s) of tumor cell kill by Listeria
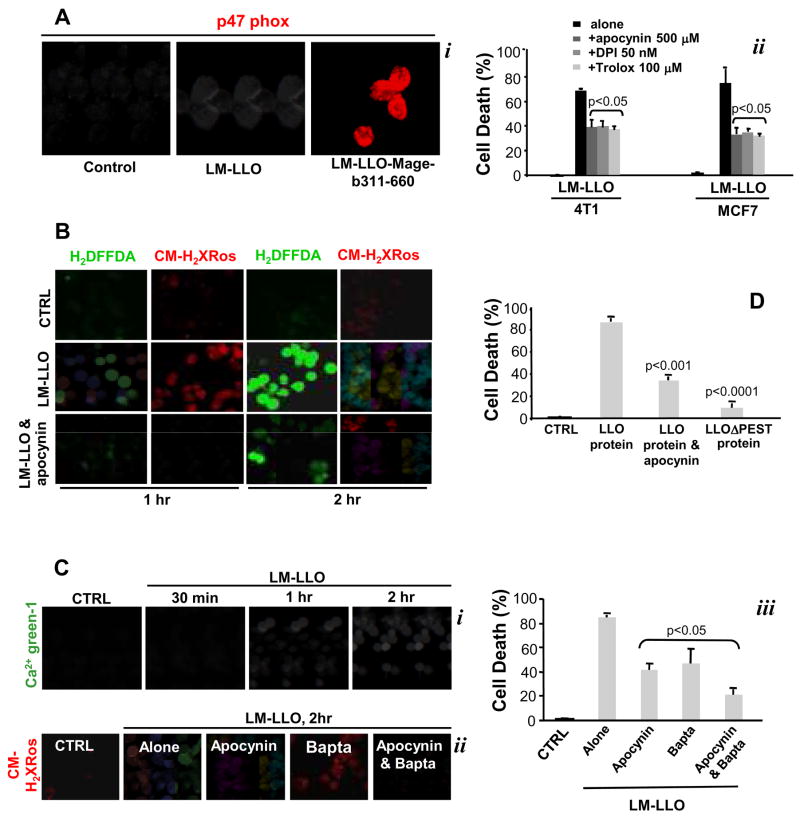
We then asked which pathways could be triggered by LM-LLO and LM-LLO-Mage-b311-660 resulting in tumor cell death. Bacteria can trigger apoptosis through a large variety of mechanisms that include the secretion of protein synthesis inhibitors, pore forming proteins, or molecules responsible for the activation of the endogenous death machinery in infected cells (23). It is known that LM activates NADPH oxidase in macrophages and neutrophils (24–26). Here we show that LM-LLO and LM-LLO-Mage-b311-660 induce the death of 4T1 and MCF7 tumor cells through the activation of NADPH oxidase and subsequent production of ROS (Fig. 3A, i). Trolox, a scavenger of OH• radicals, and apocynin or diphenylene iodonium (DPI), both selective inhibitors of NADPH oxidase, significantly decreased LM-LLO- and LM-LLO-Mage-b311-660-induced cell death of 4T1 or MCF7 tumor cells (Fig. 3A, ii), which demonstrates the involvement of NADPH oxidase-mediated ROS in tumor cell death. Accordingly, we examined whether ROS were produced in tumor cells upon Listeria infection. Live cell microscopy with H2DFFDA or CM-H2XRos revealed that cytosolic ROS were produced through activated NADPH oxidase, and that mitochondrial ROS were produced as well (Fig. 3B). Our findings collectively imply that NADPH oxidase-mediated ROS production and subsequent mitochondrial dysfunction contribute to LM-LLO or LM-LLO-Mage-b311-660-induced tumor cell death.
Figure 3.
Mechanism(s) of tumor cell kill by LM-LLO-Mage-b311-660 and LM-LLO. A, Listeria-induced tumor cell death by activation of NADPH oxidase and production of ROS. 4T1 tumor cells were incubated with LM-LLO or LM-LLO-Mage-b311-660 for two hours, and analyzed for activation of NADPH oxidase using antibodies to p47phox (i). NADPH oxidase-induced cell death was prevented by apocynin, while ROS-induced cell death was prevented by trolox and DPI (ii). Significant differences were found between 4T1 tumor cells treated with inhibitors compared to untreated 4T1. Unpaired t test: P<0.05 is significant. A similar result was observed with MCF7. B, Prevention of Listeria-induced ROS with apocynin. 4T1 tumor cells were incubated with LM-LLO, in the presence or absence of apocynin, and showed decreased production of ROS with H2DFFA (cROS and mROS), or CM-H2XRos (mROS). C, LM-LLO also induced increase in intracellular Ca2+ levels in 4T1 tumor cells as shown with Ca2+-green-1 (i). LM-LLO-induced mitochondrial disruption (ii) in 4T1 tumor cells and subsequent tumor cell death (iii), could be prevented with apocynin an/or BAPTA. While both apocynin and BAPTA were able to prevent tumor cell death by 50% when added separately, 80–90% of tumor cell death could be prevented when added combined to infected tumor cell cultures (iii). Significant differences were found between 4T1 tumor cells treated with inhibitors compared to untreated 4T1. Unpaired t test: p<0.05 is significant. D, The PEST motif in LLO of the Listeria bacteria activates NADPH oxidase and is involved in tumor cell death. This could be prevented with apocynin. LLO protein (LLO) with or without the PEST motif (LLOΔPEST) was added to 4T1 cultured, and cell death was determined the next day. Significant differences between LLO protein and other groups were analyzed. Unpaired t test: p<0.05 is significant. Experiments were performed in triplicates, and repeated two times. The results presented here are the average (Aii, Ciii, D) of or a representative (Ai, B, Ci, Cii) of two experiments performed. The error bars represent SEM.
It was obvious that trolox and/or apocynin could only prevent 50% of the LM-LLO-induced cell death (Fig. 3A, ii). This suggests that in addition to NADPH oxidase-mediated ROS, other pathway (s) are involved in tumor cell death. It has been shown by others that LLO is involved in the rapid increase in intracellular Ca2+ levels in a macrophage cell line, J774 (27). Therefore, we analyzed intracellular Ca2+ levels in 4T1 cells after the addition of LM-LLO. Indeed, LM-LLO increased intracellular Ca2+ levels as shown with Ca2+ green-1 (Fig. 3C, i). BAPTA, a membrane permeable Ca2+ chelator, reduced mitochondrial ROS detected with CM-H2Ros (Fig. 3C, ii), and LM-LLO-induced tumor cell death by 50% (Fig. 3C, iii). Moreover, BAPTA combined with apocynin very effectively reduced mitochondrial ROS detected with CM-H2Ros (Fig. 3C, ii) and prevented the LM-LLO-induced cell death by 80% (Fig. 3C, iii). These results imply that NADPH oxidase and excessive intracellular calcium contribute to tumor cell death upon LM-LLO infection causing mitochondrial failure.
As concluded earlier, LM-LLO but not Mage-b311-660 is involved in direct tumor cell kill. Thus, we analyzed the involvement of LM-LLO in tumor cell kill in more detail. Since LLO is required to establish intracellular infections (14, 15), we were wondering whether LLO could be involved in tumor cell death. A sequence rich in proline, glutamic acid, serine and threonine (PEST) at the amino terminus of LLO is thought to control the production of LLO (28). Therefore, we analyzed the effect of LLO protein with and without PEST on 4T1 tumor cells in vitro. Incubation of 4T1 tumor cells with LLO protein killed 80-90% of the 4T1 tumor cells, while LLOΔpest did not induce tumor cell death (Fig. 3D). These results indicate that the PEST sequence is involved in tumor cell death. Moreover, apocynin was able to prevent 50% of the 4T1 tumor cell death induced by LLO protein (Fig. 3D), suggesting that PEST is involved in the activation of NADPH oxidase. LLO protein did not induce an increase in intracellular Ca2+ levels (data not shown).
Expression of Mage-b- and Listerial proteins by tumor cells and immunological consequences
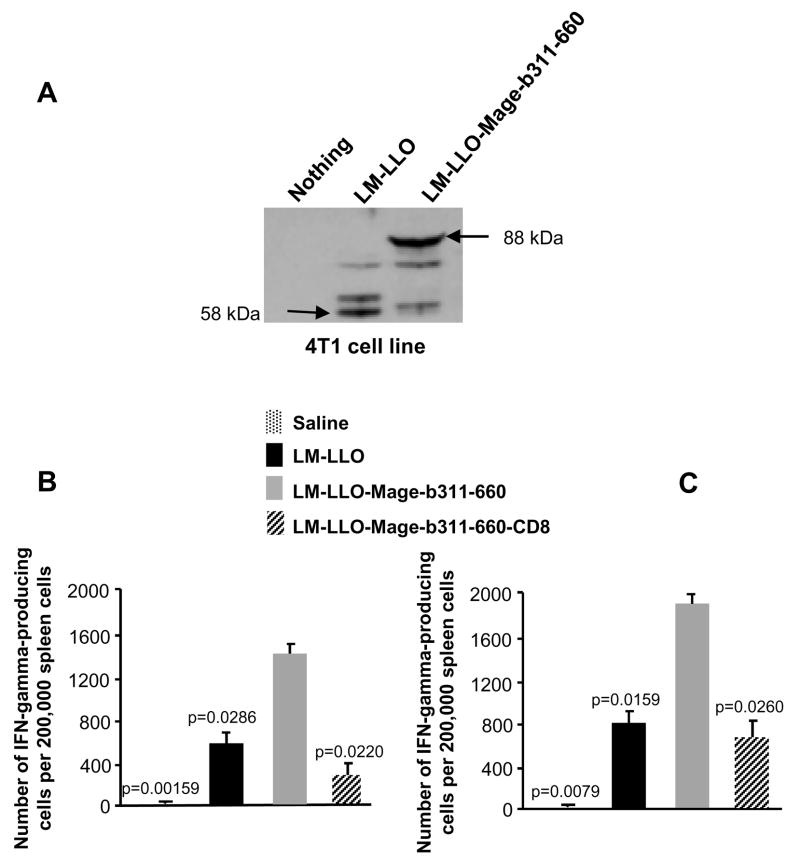
As shown above, LM-LLO and LM-LLO-Mage-b311-660 are able to infect and kill tumor cells in the complete absence of immune cells in vitro. However, infection of the tumor cells with Listeria bacteria does have consequences for the immune system in vivo. We show here that infection of tumor cells in vitro with LM-LLO or LM-LLO-Mage-b311-660 bacteria resulted in overexpression of Listerial proteins and Mage-b311-660 (in addition to relatively low levels of natural Mage-b (16)) (Fig. 4A). In addition, macrophages will be infected as well by LM-LLO or LM-LLO-Mage-b311-660, resulting in strong CTL responses against both Mage-b311-660 (Fig. 4B) and the highly immunogenic Listerial proteins as shown here in vitro (Fig. 4C). Since the combined (preventive/therapeutic) vaccination with LM-LLO and LM-LLO-Mage-b311-660 eradicated metastases and primary tumors almost with the same efficiency, we concluded that most of the in vivo effect here was derived from LM-LLO. Depletion of CD8 T cells in mice that received one preventive and two therapeutic immunizations with LM-LLO showed an increase in tumor growth by 52% compared to LM-LLO alone (Supplementary Fig. S2), suggesting that Listeria-specific CD8 T cells, at least partially, contribute to tumor reduction in vivo. CD8 depletions did not alter the frequency of metastases, and it is possible that direct kill may play a more important role here.
Figure 4.
Expression of Mage-b311-660 and Listerial proteins by 4T1 tumor cells. 4T1 tumor cells were incubated with LM-LLO or LM-LLO-Mage-b311-660 for 2 hours, and subsequently analyzed for the expression of Mage-b311-660 (fused with LLO) (88 kDa) and Listeria (LLO)(58 kDa) proteins by western blotting using anti-PEST antibodies (A). Spleen cells (pooled) of vaccinated (preventive/therapeutic) and control mice bearing 4T1 metastases and primary tumors were analyzed for Mage-b- and Listeria-specific immune responses by ELISPOT using BM cells (transfected with pcDNA3.1-Mage-b and pCMV1-GM-CSF) (B), and BM cells infected with LM-LLO (C), respectively. The involvement of CD8 T cells was determined by negative depletion, using magnetic beads with anti-CD8, antibodies. The results shown here are the average of two independent experiments. n=5 mice per group. The error bars represent the SEM. Significant differences between Mage-b and other groups were determined. Mann-Whitney test: p<0.05 is significant.
DISCUSSION
While LM-based cancer vaccines are supposed to exert their effect through killing of tumors cells by CTL responses to tumor-associate antigens (TAA), our present work shows that this is not necessarily the only and most efficient mechanism. Here we demonstrate a dramatic effect of a Listeria-based vaccine on metastases and primary tumor after one preventive followed by two therapeutic immunizations in a highly aggressive and poorly immunogenic mouse model 4T1. We provide strong evidence that efficient tumor cell kill can be achieved through a dual mode of action by LM involving both direct kill and CTL responses to Listeria antigens. EM studies showed that both the vaccine (LM-LLO-Mage-b311-660) and control strain (LM-LLO) infect tumor cells in vitro and in vivo, and that both kill tumor cells in vitro through the generation of high levels of ROS resulting in oxidative stress. Evidence for tumor cell kill by CTL responses to LM-LLO is based on in vitro and in vivo data. We showed in vitro that infection of the tumor cells with LM-LLO and LM-LLO-Mage-b311-660 resulted in high expression of Listeria and Mage-b proteins, and strong Listeria- and Mage-b-specific CTL responses in the spleen of tumor-bearing vaccinated mice. However, we demonstrated that the combined vaccination with LM-LLO and LM-LLO-Mage-b311-660 eradicated metastases and primary tumors almost with the same efficiency in vivo, indicating that most of the in vivo effect was derived from LM-LLO. Depletion of CD8 T cells in LM-LLO-vaccinated mice resulted in tumor re-growth (about 50%), suggesting that Listeria-specific CTL responses were indeed, at least partially, effective against the primary tumor. CD8 depletions did not alter the frequency of metastases. It is possible that the metastases are directly killed by the Listeria bacteria. Since we only did CD8 depletion studies we cannot rule out that NK cell responses and CD4 T cell responses are involved as well. Although the presence of bacteria in tumors has been recognized earlier (29-31), the direct kill and immunological consequences as shown with the attenuated LM in the current study has not been reported so far.
If Listeria is mediating direct tumor cell kill in vivo, why are normal cells not affected? Indeed, primary cultures of normal mouse or human fibroblasts were infected and killed by LM-LLO as efficiently as the tumor cells in vitro (Supplementary Fig. S3). However, mice that were immunized with LM-LLO or LM-LLO-Mage-b311-660 seemed completely healthy. Analysis of the liver, spleen and gastrointestinal tissues (known targets for LM infection) for pathological damage after three immunizations with LM-LLO or LM-LLO-Mage-b311-660 showed a few inflammatory spots (concentration of lymphocytes) in the liver only (data not shown). Also liver functions such as AST and ALT were unaffected by the Listeria bacteria (Supplementary Table S1). To explain this apparent lack of effect on normal tissues we hypothesize that in vivo the Listeria are cleared very efficiently by the immune system from normal tissues, as we have shown for the spleen in this study (three days after infection Listeria could not be cultured anymore from the spleen). At the same time, vaccine-induced immune responses are at least partially suppressed in the tumor environment, as we found in the draining lymph nodes of the current (data not shown) and a previous study (16), and have also been shown by others (32). Therefore, Listeria bacteria in the tumor microenvironment may be protected from clearance by the immune system, but not in the normal tissues. This is in keeping with the results obtained by others reporting only flu-like symptoms of LM-LLO-based vaccines in cancer patients in Phase I/II clinical trials (33).
In summary, our results demonstrate that different immunization strategies with LM-LLO-Mage-b311-660 provide different effects on metastatic breast cancer in the highly aggressive mouse model 4T1. The combined preventive/therapeutic immunization was superior over three preventive or three therapeutic immunizations. Our results strongly suggest that this is due to the combination of direct kill and Listeria-specific CTL-mediated tumor cell cytolysis. Reduced efficacies after exclusive preventive or therapeutic immunizations may be due to vaccine-induced immune responses or direct kill as separate actions, respectively. This dual mode of action through tumor cell infection has not been described before and opens up new strategies for eradicating breast cancer effectively through the combination of direct kill and immune responses against highly immunogenic antigens rather than against weak TAA.
Supplementary Material
Acknowledgments
This work was supported by NIH grant 1RO1 AG023096-01 and the American Federation for Aging Research (AFAR) A000106. We greatly thank Ivy Hsie and Dr. ZhenHang Meng for their technical assistance with EM and pathology, respectively, as well as Wilber Quispe, Denise Asafu-Adjei and Ilyssa Ramos for finishing the experiments of this manuscript.
References
- 1.Althuis MD, Singh S. Global trends in breast cancer incidence and mortality. 1973–1997. Int J Epidemiol. 2005;34:405–12. doi: 10.1093/ije/dyh414. [DOI] [PubMed] [Google Scholar]
- 2.Berkowitz N, Gupta S, Silberman G. Estimates of the lifetime direct costs of treatment for metastatic breast cancer. Value Health. 2000;3:23–30. doi: 10.1046/j.1524-4733.2000.31003.x. [DOI] [PubMed] [Google Scholar]
- 3.Scart H, Cantin J, Levin M. Clinical practice guidelines for the care and treatment of breast cancer: Mastectomy or lumpectomy? The choice of operation for clinical stages I and II breast cancer (summary of the 2002 update) CMAJ. 2002;167:145–55. [PMC free article] [PubMed] [Google Scholar]
- 4.Kurtz JE, Dufour P. Strategies for improving quality of life in older patients with metastatic breast cancer. Drugs Aging. 2002;19:605–22. doi: 10.2165/00002512-200219080-00006. [DOI] [PubMed] [Google Scholar]
- 5.Alberg AJ, Singh S. Epidemiology of breast cancer in older women: implications for future health care. Drugs Aging. 2001;18:761–62. doi: 10.2165/00002512-200118100-00005. [DOI] [PubMed] [Google Scholar]
- 6.Sypniewska RK, Hoflack L, Tarango M, et al. Prevention of metastases with a Mage-b DNA vaccine in a mouse breast tumor model: potential for breast cancer therapy. BCRT. 2005;91:19–28. doi: 10.1007/s10549-004-6454-7. [DOI] [PubMed] [Google Scholar]
- 7.Gravekamp C, Leal B, Denny A, et al. In vivo responses to vaccination with Mage-b, GM-CSF and thioglycollate in a highly metastatic mouse breast tumor model, 4T1. Cancer Immunol Immunother. 2008;57:1067–77. doi: 10.1007/s00262-007-0438-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JW, Kwon TK, Kim IH, et al. A new strategy for the diagnosis of MAGE-expressing cancers. J Immunol. 2002;79:79–86. doi: 10.1016/s0022-1759(02)00105-9. [DOI] [PubMed] [Google Scholar]
- 9.Paterson Y, Maciag P. Listeria-based vaccines for cancer treatment. Curr Opin Mol Ther. 2005;7:454–60. [PubMed] [Google Scholar]
- 10.Dussurget O, Pizarro-Cerda J, Cossart P. Molecular determinants of Listeria monocytogenes virulence. Ann Rev. 2004;58:587–10. doi: 10.1146/annurev.micro.57.030502.090934. [DOI] [PubMed] [Google Scholar]
- 11.Gedde MM, Higgins DE, Tilney LG, Portnoy DA. Role of Lysteriolysn O in cell-to-cell spread of Listeria monocytogenes. Infect Immun. 2000;68:999–1003. doi: 10.1128/iai.68.2.999-1003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paterson Y, Ikonomidis G. Recombinant Listeria monocytogenes cancer vaccines. Curr Opin Immunol. 1996;8:664–669. doi: 10.1016/s0952-7915(96)80083-5. [DOI] [PubMed] [Google Scholar]
- 13.Singh R, Domineicki ME, Jaffee EM, Paterson Y. Fusion of Listeriolysin O and delivery by Listeria monocytogenes enhances the immunogenicity of HER-2/neu and reveals subdominant epitopes in the FVB/N mouse. J Immunol. 2005;175:3663–73. doi: 10.4049/jimmunol.175.6.3663. [DOI] [PubMed] [Google Scholar]
- 14.Gaillard JL, Berche PP, Sansonetti P. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun. 1986;52:50–55. doi: 10.1128/iai.52.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Portnoy DA, Jacks PS, Hinrichs DJ. Role of hymolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–71. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SH, Castro F, Gonzalez D, Maciag PC, Paterson Y, Gravekamp C. Mage-b vaccine delivered by recombinant Listeria monocytogenes is highly effective against breast cancer metastases. British Journal of Cancer. 2008;99:741–49. doi: 10.1038/sj.bjc.6604526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monoytogenes vaccine vectors that express different molecular forms of human papillomavirus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol. 2001;167:6471–79. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]
- 18.Pan ZK, Okonomidis G, Lazenby A, Pardoll D, Paterson Y. A recombinant Listeria monocytogenes vaccine expressing a model tumor antigen protects mice against lethal tumor challenge and causes regression of established tumors. Nature Med. 1995;1:471–77. doi: 10.1038/nm0595-471. [DOI] [PubMed] [Google Scholar]
- 19.Chambers RS, Johnston SA. High-level generation of polyclonal antibodies by genetic immunization. Nature Biotechnol. 2003;21:1088–92. doi: 10.1038/nbt858. [DOI] [PubMed] [Google Scholar]
- 20.Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;152:99. [PubMed] [Google Scholar]
- 21.Pease DC. Histological techniques for electron microscopy. Academic Press; New York and London: 1964. [Google Scholar]
- 22.Stenberg PE, Shuman MA, Levine SP, Bainton DF. Redistribution of alpha-granules and their contents in thrombin-stimulated platelets. J Bio Chem. 1984;98:748–760. doi: 10.1083/jcb.98.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lancellotti M, Brocchi M, da Silvedeira WD. Bacteria-induced apoptosis: an approach to bacterial pathogenesis. Braz J Morphol Sci. 2006;23:75–86. [Google Scholar]
- 24.North BM. Murine Listeriosis as a model of antimicrobial defense. Immunol Rev. 1997;158:27–36. doi: 10.1111/j.1600-065x.1997.tb00989.x. [DOI] [PubMed] [Google Scholar]
- 25.Rogers HW, Unanue ER. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect Immun. 1993;61:5090–96. doi: 10.1128/iai.61.12.5090-5096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unanue ER. Inter-relationships among macrophages, natural killer cells, and neutrophils in early stages of Listeria resistance. Curr Opin Immunol. 1997;1:25–43. doi: 10.1016/s0952-7915(97)80156-2. [DOI] [PubMed] [Google Scholar]
- 27.Wadsworth S, Goldfine H. Mobilization of proteinase C in macrophages induced by Listeria monocytogenes affects its internalization and escape from the phagosome. Infect Immun. 2002;70:4650–60. doi: 10.1128/IAI.70.8.4650-4660.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schnupf P, Portnoy DA, Decatur AL. Phosphorylation, ubiquitination and degradation of Listeriolysin O in mammalian cells: role of the PEST-like sequence. Cell Microbiol. 2006;8:353–64. doi: 10.1111/j.1462-5822.2005.00631.x. [DOI] [PubMed] [Google Scholar]
- 29.Abelman HW. Cancer as I see it. Philosophical Library; Inc New York: 1951. [Google Scholar]
- 30.Yu YA, Shabahang S, Timiryasova TM, Zhang O, Beltz R, Gentschev I, Goebel W, Szalay AA. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nature Biotechn. 2004;22:313–20. doi: 10.1038/nbt937. [DOI] [PubMed] [Google Scholar]
- 31.Stritzker J, Pilgrim S, Szalay AA, Goebel W. Prodrug converting enzyme gene delivery by L. monocytogenes. BMC Cancer. 2008 doi: 10.1186/1471-2407-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gajewski TF, Meng Y, Harlin H. Immune suppression in tumor microenvironment. J Immunother. 2006;29:233–240. doi: 10.1097/01.cji.0000199193.29048.56. [DOI] [PubMed] [Google Scholar]
- 33.Rothman J. The first use of Live Listeria cancer vaccine in man. AACR Conference; San Diego, CA. April 12–16, 2008; Abstract 225. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.