Abstract
Calorie restriction (CR) is the only proven regimen, which confers lifespan extension benefits across the various phyla right from unicellular organisms like yeast to primates. In a bid to elucidate the mechanism of calorie-restriction-mediated life span extension, the role of mitochondria in the process was investigated. In this study, we found that the mitochondrial content in CR cells remains unaltered as compared to cells grown on nonrestricted media. However, mitochondria isolated from CR cells showed increased respiration and elevated reactive oxygen species levels without augmenting adenosine triphosphate (ATP) generation. The antioxidant defense system was amplified in CR mitochondria, and in CR cells a cross protection to hydrogen-peroxide-induced stress was also observed. Moreover, we also documented that a functional electron transport chain was vital for the life span extension benefits of calorie restriction. Altogether, our results indicate that calorie restriction elicits mitohormetic effect, which ultimately leads to longevity benefit.
Keywords: Calorie restriction, Mitochondria, ROS, Stress resistance, Hormesis, atp2
Introduction
Since the description of longevity benefits of calorie restriction in rats by McCay et al. (1935), it has become an important paradigm in aging research. It is a dietary regimen in which there is a reduction in calorie intake without any nutritional deficiency. Although the life span extension benefits of calorie restriction has been validated in organisms ranging from unicellular yeast to primates, the mechanism of its action still eludes our understanding.
As with other complex biological problem, Saccharomyces cerevisiae has proven to be a useful tool in the study of the phenomenon of aging. Yeast model of calorie restriction has been described wherein it is grown in rich media having low glucose concentration (0.5% glucose as compared to normal 2% glucose), achieving the life span extension as described in higher organisms. Limitation of other nutrients achieving similar result has been described, and it has been established that this intervention is not just a case of glucose derepression (Jiang et al. 2000; Lin et al. 2002).
Mitochondria—the organelle meeting the major energy needs of cell—is an important hub in the cellular metabolic network. They are the major site for energy production in cell. But this activity accounts for the reactive oxygen species (ROS) production too with up to 1–3% of total oxygen consumed by resting cells being converted into superoxide anion (Halliwell and Gutteridge 1999). A progressive accumulation of damaged cellular macromolecules due to the oxidative stress is thought to be the major cause of aging-related disorders ultimately leading to death (Beckman and Ames 1998). Conventional wisdom proposes that calorie restriction slows down cellular metabolism, thereby reducing oxidative stress (Sohal and Weindruch 1996). But, in recent past, several studies have reported longevity benefits despite increased metabolism subsequent to calorie restriction. Overexpression of transcription factor Hap4, which causes a shift in metabolism from fermentation to respiration in yeast, leads to an increased life span (Lin et al. 2002). It has been shown that, upon calorie restriction, the yeast cells show enhanced respiration and higher ROS levels (Agarwal et al. 2005). A longer life span has been described in yeast cells upon MRG19 deletion, wherein the cells show increased levels of ROS with an augmented scavenging enzyme system (Kharade et al. 2005).
Longevity and stress resistance resulting from an adaptive response (termed hormesis) to repeated exposure to mild dosage of an otherwise harmful stress agent have been described in various model organisms (Gems and Partridge 2008). Pretreatment of yeast cells to low doses of oxidant conferred adaptive response to subsequent challenges with higher doses of oxidant (Jamieson 1992). Transient heat stress has been reported to extend life span in yeast cells, and mitochondria were speculated to be vital for this heat-stress-induced life span extension (Shama et al. 1998). Of late, mitohormesis—a new paradigm—has been introduced to explain the benefits of calorie restriction (Tapia 2006). An increase in life span upon glucose restriction has been described in Caenorhabditis elegans, which is mediated by induction of mitochondrial respiration, increased ROS formation, and increased stress (Schulz et al. 2007).
In light of the available information, an important role of mitochondria in the longevity-promoting effects of calorie restriction was anticipated. In this study, the role of mitochondria in calorie-restriction-associated hypermetabolic cellular state was ascertained in yeast. The results of our study provide evidence that increased respiratory activity contributed to the stress response mediated by mitochondria. This mild stress conferred protective benefits to future stress, and it apparently resolves the issue of hypermetabolism in calorie-restricted yeast.
Materials and methods
Yeast strains and culture conditions
The following strains were used in this study: W303-1a (MATa ade2-1 ura3-1 trp1-289 leu2-3, 112 his3-1, 15 can1-100), CCFY100 (W303-1a MATa ade2-1 ura3-1 trp1-289 leu2-3, 112 his3-11, 15 can1-100 HMRΔE::TRP1 rDNA::ADE2, CAN1 VR TEL::URA3), atp2 (W303-1a MATa ade2-1 ura3-1 trp1-289 leu2-3, 112 his3-11, 15 can1-100 atp2::kanMX). Strains were grown at 30°C, with shaking in YPD-NR (non-calorie-restricted; 1% yeast extract, 2% peptone, 2% dextrose media) and YPD-CR (calorie-restricted; 1% yeast extract, 2% peptone, 0.5% dextrose media) conditions. The cells were grown to mid-log phase for all the experiments unless specified.
Mitochondrial content determination using flow cytometry and fluorescence microscopy
To determine if calorie restriction had any effect on the mitochondrial content in yeast cells, W303-1a strain cells were transformed with pVT100U-mtGFP plasmid. It encoded green fluorescent protein (GFP) that targeted specifically to mitochondria (Westermann and Neupert 2000). The transformed cells were grown in yeast complete media lacking uracil (for maintaining the selection pressure on GFP plasmid), i.e., in YC-ura with 2% dextrose (NR) and YC-ura with 0.5% (CR) media. Cells were harvested and sonicated for making single cell suspension before analysis, and the flow cytometry data were acquired using FACScalibur™ flow cytometer (BD Biosciences, San Jose, CA). The GFP was excited by an argon laser, and fluorescence was detected using a 530/30-nm band pass filter in the FL1 channel. The Cell Quest software (BD) was used for data acquisition, and data on scatter parameters and histograms were acquired in log mode. One hundred thousand events were evaluated for each sample, and the median peak channel obtained from the histograms of each standard was used to analyze their fluorescence intensity. For GFP visualization, cells immobilized with 0.5% low melting point agarose were subjected to standard epifluorescence microscopy. For excitation of mtGFP, a 450–490-nm band pass was used. The images were acquired using ACT1 software.
Isolation of mitochondria
The mitochondria were isolated according to Meisinger et al. (2000). In brief, cells were incubated for 20 min at 30°C in 2 ml/g dithiothreitol (DTT) buffer (100 mM Tris H2SO4, pH 9.4, 10 mM DTT) with gentle shaking, washed with zymolyase buffer (1.2 M sorbitol; 20 mM potassium phosphate, pH 7.4), and incubated with 1 mg/g zymolyase-100 T in 7 ml/g zymolyase buffer for 45 min at 30°C for spheroplasting. Homogenization was carried out in a glass Teflon potter in 6.5 ml/g ice-cold homogenization buffer (0.6 M sorbitol; 10 mM Tris–HCl, pH 7.4; 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM PMSF; 0.2% (w/v) bovine serum albumin). Mitochondria were purified using differential centrifugation as described by Meisinger et al. (2000). Mitochondrial pellet was washed with SEM buffer (250 mM sucrose; 1 mM EDTA; 10 mM MOPS, pH 7.2) and resuspended in SEM. Mitochondria prepared in this manner contains intact inner membranes and respiratory complexes. The mitochondria so obtained were used in the experiments described below unless mentioned otherwise.
Mitochondrial oxygen consumption
Oxygen consumption of isolated mitochondria was measured polarographically using a Clark-type oxygen electrode in 1-ml reaction mixture, same as that used for the H2O2 measurement using 5 mM succinate. The rate of oxygen consumption, represented as nanomole per minute per milligram, was calculated using Oxygraph Plus V 1.00 software (Hansatech Instruments Ltd., Norfolk, England).
Mitochondrial H2O2 production
Mitochondrial H2O2 production using succinate (5 mM) as the substrate was measured in terms of fluorescence units due to oxidation of homovanillic acid by H2O2 in the presence of horseradish peroxidase as described elsewhere (Barja 2002). Mitochondrial protein (140 μg), 6 U/ml of horseradish peroxidase, and 0.1 mM homovanillic acid were incubated for 15 min at 37°C in 145 mM KCl, 30 mM Hepes, 5 mM KH2PO4, 3 mM MgCl2, 0.1 mM EGTA, and 0.1% fatty-acid-free albumin at pH 7.4. Antimycin (10 μM) and myxothiazol (30 μM) were used as the inhibitors. Reaction was stopped by adding 0.5 ml of 0.1 M glycine–NaOH (pH 12) and 25 mM EDTA (per each 1.5 ml of reaction volume) and transferring the samples to an ice-cold bath. Emission intensity was recorded at 420 nm with the excitation wavelength of 312 nm. Mitochondrial H2O2 production using NADH (0.4 mM) and pyruvate/malate (2.5 mM) as substrates was measured in terms of fluorescence units due to oxidation of 50 μM Amplex Red in the presence of 1.0 U/ml of horseradish peroxidase at 30°C. The emission intensity at 587 nm was recorded with the excitation wavelength set at 563 nm (Barros et al. 2004).
NADH dehydrogenase activity
The NADH dehydrogenase activity was measured by the reduction of ferricyanide at 410 nm, in a reaction mixture having 1 mM ferricyanide and 115 μg mitochondrial protein. For measuring external and internal dehydrogenase activity, 0.75 mM NADH and pyruvate/malate (2.5 mM each) were used as substrate, respectively. Activity was calculated using an extinction coefficient of ε = 1 mM−1 cm−1 for ferricyanide at 410 nm (Hatefi 1978).
ATP production in isolated mitochondria
Mitochondrial protein (12.5 μg) resuspended in 50 μl of 25 mM Tris–Cl, pH 7.5, 5 mM MgCl2, 10% glycerol, and 10 mM KH2PO4 was used to assay the basal levels of adenosine triphosphate (ATP). After 3 min of incubation at 37°C, the reaction was stopped with 0.1 M TCA. The amount of ATP in the sample was determined by using the ATP bioluminescence assay kit (Sigma Chemicals). ATP generation in isolated mitochondria was monitored with the addition of 5 mM succinate and 5 mM succinate + 10 mM adenosine diphosphate (ADP). The results were expressed as micromolar of ATP per microgram of mitochondrial protein.
Mitochondrial antioxidant defense system
Real-time polymerase chain reaction (PCR) studies were undertaken to study the effect of calorie restriction on mitochondrial antioxidant gene expression. The RNA isolation and cDNA synthesis were done as described by Lin et al. (2002). Quantitative real-time PCR was performed using SYBR Premix Ex Taq (Takara Bio Inc., Japan) kit on Eppendorf Mastercycler® ep realplex Thermal Cycler (Eppendorf, Hamburg, Germany) according to the manufacturer’s protocol. The real-time PCR data were analyzed by the comparative CT method of Schmittgen and Livak (2008). For assaying the superoxide dismutase (SOD) activity in the mitochondria, equal amount of mitochondrial protein was separated on 12% native polyacrylamide gel; activity staining and quantification were done using nitro blue tetrazolium as described previously (Agarwal et al. 2005). Glutathione peroxidase activity (GPx) was determined by using coupled assay (Lawrence and Burk 1976). Oxidized glutathione, produced by the hydroperoxide-dependent oxidation of reduced glutathione, is reduced by glutathione reductase. Decrease in absorbance because of NADPH depletion during this reaction was measured at 340 nm. The assay was carried out in 50 mM sodium phosphate buffer pH 7.0, 1 mM sodium azide, 1 mM EDTA, 1 mM GSH, 0.2 mM NADPH, 1.33 EU glutathione reductase, and mitochondrial protein, and reaction was started by the addition of 1.5 mM cumene hydroperoxide. To eliminate the nonenzymatic NADPH-consuming activity, a blank was taken in which the protein sample was omitted. One unit of activity was defined as the amount of enzyme oxidizing 1 μM of glutathione per minute at 30°C. Millimolar absorption coefficient of 6.22 mM−1 cm−1 for NADPH was used for calculation.
Hydrogen peroxide sensitivity assay
To assess whether calorie restriction provided any protection against other stresses, hydrogen peroxide sensitivity (halo) assay was performed (Flower et al. 2005). The cells were grown in YPD-CR and YPD-NR media to an OD of 1. One hundred microliters of these cells added to 20 ml of YPD agar (Dextrose 2%) was plated and cooled. Four sterile filter paper disks containing 10 μl of hydrogen peroxide at concentrations 1%, 2%, 3%, and 5% were kept on each plate. These plates were incubated at 30°C for 2 days reveal a clear zone (halo) of no growth around the filter disks. The diameters of these halos were measured.
Life span assays
Replicative life span was determined as described previously (Roy and Runge 2000). Cells were taken from logarithmically growing liquid cultures and plated at low density on YPD-NR or YPD-CR medium. The cells were incubated at 30°C for approximately 3 h. At this time, daughter cells that had emerged from mother cells as buds were isolated and moved to uninhabited parts of the plate for analysis of life span. On very rare occasions, a cell was observed to lyse immediately after micromanipulation and was excluded from the data set. Data are average of 60 cells carried out in three independent experiments.
Results
In all experiments, purified mitochondria isolated from cells grown in calorie-restricted and normal media have been used unless otherwise stated. The p values have been calculated using Student paired t test available in Microsoft Excel.
Mitochondrial biogenesis is unaffected by calorie restriction
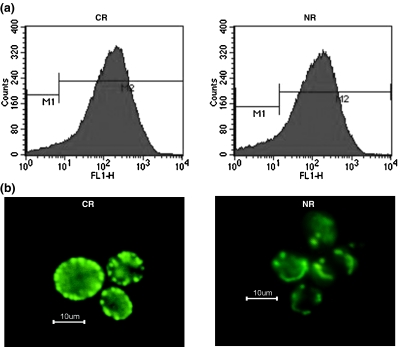
It has been reported that calorie restriction affects mitochondria biogenesis in rats and humans (Civitarese et al. 2007; Nisoli et al. 2005). Therefore, we studied the effect of calorie restriction on mitochondrial content in yeast cells. It was observed that calorie restriction apparently had no effect on the mitochondrial content in yeast cells as is evident from the microscopy images (Fig. 1b) and the FACS data—FL1-H geometric mean fluorescent intensity for CR and NR being 127.58 and 139.11, respectively (Fig. 1a).
Fig. 1.
Similar mitochondrial content in calorie-restricted and normal cells as determined by fluorescence of cells expressing mitochondrial-targeted GFP, analyzed by a flow cytometry and b fluorescence microscopy
Increased respiratory activity and ROS production upon calorie restriction
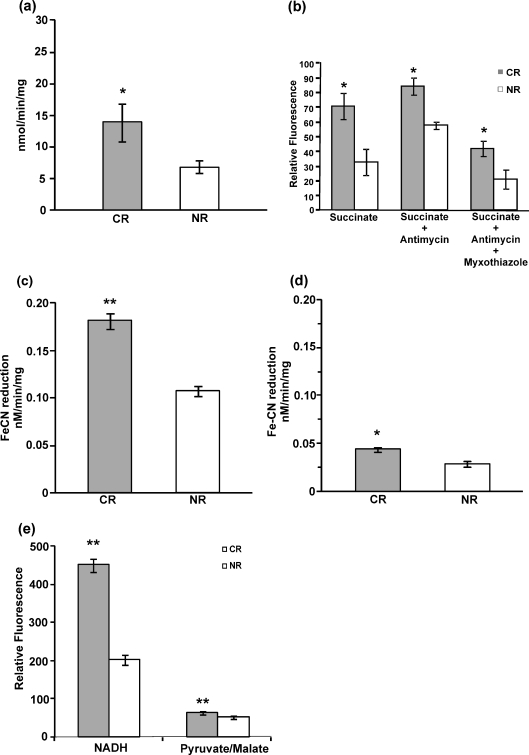
Experiments were performed to gain insight into the effects on mitochondrial physiology in calorie-restricted cells given that mitochondrial content was unaltered. The mitochondria isolated from CR cells showed higher oxygen consumption as compared to those from NR cells as shown in Fig. 2a. CR mitochondria consumed oxygen at a rate of 13.93 nmol/min per milligram whereas mitochondria from NR cells showed an oxygen consumption rate of 6.92 nmol/min per milligram This increased respiratory activity was reflected in reactive oxygen species production. It was seen that H2O2 levels were higher in CR mitochondria as compared to NR mitochondria (Fig. 2b). Further, blockage of the electron transport chain with antimycin produced enhanced levels of H2O2.This enhancement was reduced by 50% in CR and 63% in NR mitochondria when myxothiazol was used concomitantly.
Fig. 2.
Calorie restriction leads to hypermetabolic state. a Oxygen consumption by mitochondria from calorie-restricted cells consume more oxygen. b Mitochondrial H2O2 production using succinate (5 mM) as substrate and antimycin (10 μM) and myxothiazol (30 μM) as inhibitors. c External NADH dehydrogenase activity. d Internal NADH dehydrogenase. e Mitochondrial H2O2 production using NADH and pyruvate/malate as the substrates.*p < 0.05, **p < 0.01
Effect of calorie restriction on mitochondrial NADH dehydrogenase
It has been reported that CR increases NAD/NADH ratio that indirectly activates Sir2 responsible for life span extension. Further, overexpression of external dehydrogenase NDE1 and NDE2 increases yeast life span (Lin et al. 2004). To determine whether CR plays any role in NADH-dehydrogenase-mediated (the complex I equivalents in S. cerevisiae mitochondria) life span extension, its activity was tested. The activity analysis showed that external NADH dehydrogenase activity, i.e., Nde I and Nde II activity (Fig. 2c), was higher (0.073 nM/min per milligram) in CR mitochondria as compared to NR mitochondria whereas internal dehydrogenase activity, i.e., Ndi I activity (Fig. 2d), was only marginally (0.015 nM/min per milligram) increased in CR. To measure the contribution of the NADH dehydrogenases in mitochondrial ROS production, H2O2 production was measured using NADH and pyruvate/malate, the substrates for the external and internal dehydrogenases, respectively. Higher H2O2 production in CR mitochondria was quite evident with NADH as substrate, while the internal dehydrogenase substrates did not result in much difference in the H2O2 levels (Fig. 2e).
ATP levels are unaltered upon calorie restriction
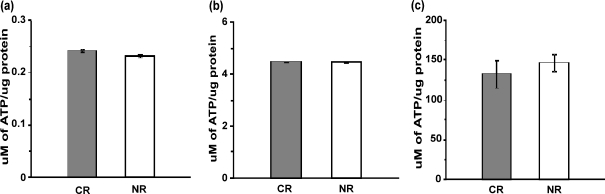
To ascertain the energy status of the cell, the basal levels and the rate of ATP generation in mitochondria isolated from CR and NR cells were evaluated (Fig. 3a–c). The amount of ATP generation was found to be higher when succinate and ADP were included in the incubation medium, but no significant difference was seen in the basal levels and the rate of ATP generation in CR condition.
Fig. 3.
ATP levels are unaltered in calorie-restricted cells. a Basal levels of ATP in isolated mitochondria. b ATP levels in isolated mitochondria in the presence of 5 mM succinate. c ATP levels in isolated mitochondria in the presence of 5 mM succinate and 10 mM ADP
Calorie restriction elicits hormesis-like protective benefits
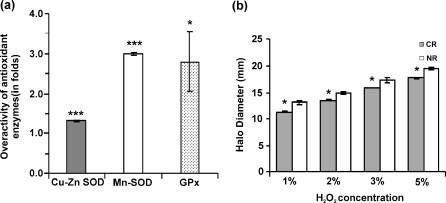
The negative fallout of aerobic metabolism is the generation of reactive oxygen species. In order to detoxify these harmful reactive oxygen species, enzymatic defense mechanism exists in cells. An impaired antioxidant status has been implicated in the phenomenon of aging. We investigated the effect of CR on the antioxidant enzyme status in the mitochondria. The results of the gene expression experiment showed an overexpression antioxidant defense system genes except for GPX2 gene (Table 1). A higher SOD activity was found in the mitochondria of CR cells. The Cu–Zn SOD activity was found to be 1.29 ± 0.02-fold higher whereas the Mn SOD activity was found to be 2.99 ± 0.04-fold higher in the CR mitochondria (Fig. 4a). An enhanced peroxidase activity was observed in CR mitochondria. It was found to be 2.81 ± 0.73-fold higher as compared to NR mitochondria as seen in Fig. 4. These results emphasize that overactivity of the antioxidant enzymes may be one of the mechanism by which CR mediates life extension benefits. We assessed whether calorie restriction in yeast provided any cross protection to future onslaught of stress. The halo diameter as measured in the hydrogen peroxide sensitivity assay (Fig. 4b) shows that halo in case of CR cells were significantly smaller in all the four concentrations of hydrogen peroxide used, which showed that they were more conditioned for the subsequent stress.
Table 1.
The relative expression of the antioxidant enzyme genes in calorie-restricted cells
| Genes | ORF name | Description | Fold change | SEM |
|---|---|---|---|---|
| SOD1 | YJR104C | Cytosolic copper–zinc superoxide dismutase | 2.40 | ±0.28 |
| SOD2 | YHR008C | Mitochondrial superoxide dismutase, protects cells against oxygen toxicity | 3.89 | ±0.43 |
| GPX1 | YKL026C | Phospholipid hydroperoxide glutathione peroxidase induced by glucose starvation that protects cells from phospholipid hydroperoxides and nonphospholipid peroxides during oxidative stress | 3.75 | ±0.63 |
| GPX2 | YBR244W | Phospholipid hydroperoxide glutathione peroxidase induced by glucose starvation that protects cells from phospholipid hydroperoxides and nonphospholipid peroxides during oxidative stress | 0.23 | ±0.03 |
| HYR1 | YIR037W | Thiol peroxidase that functions as a hydroperoxide receptor to sense intracellular hydroperoxide levels and transduce a redox signal to the Yap1p transcription factor | 3.23 | ±0.34 |
Fig. 4.
Calorie restriction elicits a hormetic response in yeast cells. a The ROS defense system is upregulated in calorie-restricted mitochondria. The CR/NR ratio for the antioxidant enzymes (Cu–Zn SOD, Mn SOD, GPx) is shown. b Cross protection bestowed by calorie restriction to future stress as determined by hydrogen peroxide sensitivity (Halo) assay. ***p < 0.001, *p < 0.05
A functional mitochondria is required to exert the longevity benefit of calorie restriction
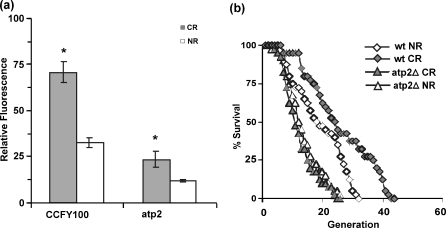
The importance of functional respiratory chain has been cited for life span extension by other stresses, e.g., transient heat stress. Functional respiratory chain requirement for life span extension was testified by measuring H2O2 status along with the life span of the ATP mutant (atp2). H2O2 levels were again found to be elevated in CR; however, the levels were lower when compared to that observed in wild-type (wt) cells as seen in Fig. 5a. The life span analysis yielded some interesting results; CR increased the life span of wild-type cells as reported (Lin et al. 2000)—average (maximum) life span increased to 25.5 (41) generations from 18.27 (30) generations, whereas no significant change in average life span of atp2 was observed upon calorie restriction—life span was 12.77 (25) and 11.45 (23) in calorie-restricted and normal cells, respectively (Fig. 5b). The life span of atp2 cells in CR as well as NR conditions were found to be smaller (average and maximum life span both were decreased).
Fig. 5.
Functional respiratory chain enhances life span of calorie-restricted yeast. a ROS production in mitochondria isolated from CCFY100 and ATP-deleted mutant (atp2). b Life span analysis of wild-type (CCFY100) and ATP-deleted mutant (atp2) in CR and NR conditions. *p < 0.05
Discussion
Most of the studies undertaken to understand the problem of aging have been based on the premise that it is attributable to a decline in cellular functions and impaired stress resistance due to oxidative damage to cellular macromolecules (Rattan 2006). The longevity-promoting effects of CR are hypothesized primarily because of reduced oxidative damage due to decreased oxidative stress (Sohal and Weindruch 1996) in the CR-induced hypometabolic state. Besides the observation of enhanced respiration in yeast cells by Guarente laboratory (Lin et al. 2002), our laboratory has reported enhanced ROS and concomitant stronger antioxidant defense system upon calorie restriction (Agarwal et al. 2005). Though it has been speculated that functional mitochondria are vital for the beneficial effects of CR in yeast (Lin et al. 2004), no experimental evidence is cited, indicating a role of this organelle in CR. Our results give evidence in favor of an interesting role for this organelle in the benefits accrued from calorie restriction in yeast cells.
The mitochondrial content of the cells growing in the normal and calorie-restricted condition was determined to ascertain if the enhanced respiratory activity upon calorie restriction as reported by our and Guarente laboratory was due to increased mitochondrial biogenesis. Apparently, the mitochondrial content remained same in either condition as determined by the fluorescence experiments (Fig. 1).This observation is in contrast to the results obtained in mammalian system where the authors associate calorie restriction with increased mitochondrial content (Civitarese et al. 2007; Nisoli et al. 2005). Our next set of experiments was aimed to see if calorie restriction affected mitochondrial functionality. Oxygen consumption in isolated mitochondria (Fig. 2a) show more than twofold increase upon calorie restriction, which corroborated the previous reported results. H2O2 production was increased in CR mitochondria, ascertaining CR association with hypermetabolic cellular status rather then hypometabolic status. Moreover, this increased ROS generation in CR correlated well with the oxygen consumption (an approximately twofold increase, Fig. 2b). Further, blockage of the electron transport chain with antimycin further increased the H2O2 levels, whereas coincubation with myxothiazol reduced this increase. These findings can be explained on the basis of electron transfer in the complex III and the site of action of these two inhibitors. According to the Q cycle mechanism, two ubiquinone-binding sites are present in the cytochrome bc1 complex. The Qo site or the ubiquinol-oxidizing site is present on the P side of the inner mitochondrial membrane whereas the Qi site, the ubiquinone-reducing site, is located on the N side of the membrane. Antimycin inhibits the reduction of ubiquinone at the Qi site, resulting in the accumulation of unstable semiquinone, which in turn transfers a single electron to molecular oxygen to produce superoxide. Myxothiazol prevents the oxidation of ubiquinol at the Qo site, preventing the formation of ubisemiquinone at this site and having no transfer of electrons to the molecular oxygen (Raha and Robinson 2000). Thus, it can be concluded that complex III is the source of H2O2 production in the CR mitochondria.
Lower ROS production in CR mitochondria has been reported (Barros et al. 2004)—a result contrary to our observation. Closer examination of the data revealed that choice of substrate set (ethanol, malate, and glutamate) for mitochondrial respiration might be the cause of low H2O2 production because of the following reasons. Mitochondrial inner membrane is virtually impermeable to NADH coenzymes. Metabolism of a mixture of ethanol, malate, and glutamate generates intramitochondrial NADH, which can be oxidized by internal NADH dehydrogenase only; it cannot be utilized by external dehydrogenases (Nde1 and Nde2). In this study, we have measured internal dehydrogenase activity and found that the marginal increase in activity is also reflected in slightly increased H2O2 levels, whereas the higher external dehydrogenase activity in CR correlates very well with the H2O2 levels detected with the corresponding substrate for these dehydrogenases (Fig. 2d, e). All these data together indicate that substrates specific to internal dehydrogenase might generate low ROS in mitochondria, which however may not be a true reflection of mitochondrial status in CR.
Indirect evidences have accumulated, conferring a vital role to complex I of respiratory chain in stress and life span regulation of yeast and worms. In contrast to many eukaryotic cells, S. cerevisiae lacks the multi-subunit complex I-type NADH dehydrogenase. Instead, it contains an “internal mitochondrial NADH dehydrogenase,” encoded by NDI1 and an “external NADH dehydrogenase,” encoded by NDE1 and NDE2, which oxidizes mitochondrial and cytosolic NADH, respectively (Horne et al. 2001). Jazwinski’s group has suggested the necessity of mitochondria for life span extension by transient heat stress (Shama et al. 1998). Later, it was established that transient heat stress actually induces oxidative stress, and Nde1 and Nde2 are vital for this induction (Davidson and Schiestl 2001). In CR, NAD levels remain the same, but NADH levels drop down finally, leading to an increase in the NAD/NADH ratio, which mediates Sir2-dependant life span extension. The exact cause of the depletion of NADH levels has however not been documented. As an indirect evidence for overactivity of NADH dehydrogenase, it has been cited that overexpression of Nde1 and Nde2 extends its life span in yeast (Lin et al. 2004) while subsequent mutation in nematode (gas-1) reduces complex I activity and shortens life span (Kayser et al. 2004). Our results suggest that CR specifically upregulate external NADH dehydrogenase activity (Fig. 2c).
ATP levels were determined in isolated mitochondria to see if the increased respiration is reflected in ATP generation. Surprisingly, increased oxygen consumption upon calorie restriction did not entail in higher levels of ATP (Fig. 3a). It could be because of uncoupling of ATP synthase complex with respiratory chain or lack of internal ADP. However, addition of succinate enhanced ATP production, and addition of ADP enhanced it further (Fig. 3b, c), which clearly indicates that isolated mitochondria were well coupled and lack of ATP production is not simply due to lack of ADP or reduced efficiency of ATP synthase complex. Unaltered ATP levels in spite of higher respiratory activity of calorie-restricted mitochondria can be explained on the basis of observed ROS generation (a measure of the electron leak) in the mitochondria. As per the expectation, the increased oxygen consumption should translate into the higher ATP generation. But, instead of that, it is being translated into observed higher H2O2 levels.
Higher levels of H2O2 do not seem to be in accordance with life span extension benefit of calorie restriction, at least in the face value. But this observation seems to make sense when seen in the light of mitohormesis theory (Tapia 2006). According to this theory, ROS from mitochondrial metabolism upon calorie restriction are the key element that initiates a cascade of events that culminates in the life span extension. A higher H2O2 level as detected in calorie-restricted mitochondria provides evidence of stress that the cells are subjected to, when put on that particular diet regimen. If the calorie restriction is a form of hormesis, then a stronger defense mechanism would be expected. This was indeed observed in augmented antioxidant system as evaluated in terms of SOD and GPx activity (Fig. 4a) in CR mitochondria. SOD (Cu–Zn SOD and Mn SOD) activity was found to be elevated in the mitochondria of CR cells. Expression of GPX1 is induced on glucose starvation, expression of GPX2 is induced by oxidative stress in a Yap1p-dependent manner, and expression of GPX3 is constitutive (Inoue et al. 1999). Augmentation of SOD and GPx in response to CR has been reported previously at the cellular level (Agarwal et al. 2005), and probably the same mechanism is applicable to annul the effect of mitochondrial ROS. Life span extension upon calorie restriction has been described in C. elegans, owing to increased stress resistance subsequent to increased respiration and ROS levels (Schulz et al. 2007). Thus, our results suggest that oxidative stress generated from mitochondria activates the scavenging pathway to exert beneficial effects of CR. The result of the Halo assay (Fig. 4b) further provides evidence of calorie restriction eliciting a hormetic response. Such a response should bestow protective effects towards subsequent stress conditions. The calorie-restricted cells were more resistant to the stress in the form of hydrogen peroxide as compared to normal cells. Though the role of an improved defense system in calorie-restriction-mediated life span extension is central, other players cannot be ruled out. It could be that the increased NADH dehydrogenase activity is a part of the hormetic responses and responsible for the lowering of NADH, which in turn augments parallel Sir2-mediated life span extension effects.
In the various reports regarding cellular response to oxidative stress, a role for a functional mitochondrial respiratory chain has been underscored. It has been known that respiratory-deficient yeast strains are sensitive to ROS (Grant et al. 1997) and a fully functional electron transport chain is necessary for maintaining resistance to H2O2 (Thorpe et al. 2004). It is also reported that daughter cells lose age asymmetry in the absence of Atp2 and show clonal-senescence phenotype at higher temperature (Borghouts et al. 2004). The reduction in the mitochondrial ROS generation in respiratory-deficient atp2 cells points out the need of the functional respiratory chain for the generation of ROS. The markedly reduced life span in atp2 in CR and NR as compared to wild type clearly illustrates the role of intact electron transport chain in life span regulation.
To conclude, our study sheds light on the mechanism of life span extension upon calorie restriction in yeast. Evidence is provided supporting a key role for mitochondria in the string of events culminating in the longer life span in CR yeast. Our results buttress the notion that calorie restriction is indeed a form of hormesis—a multipronged response (Rattan 2008) as is evident by higher H2O2 levels, augmented antioxidant defense system, resistance to subsequent stress, and increased NADH dehydrogenase activity. ROS have been traditionally held as damage-causing entities, but they are equally important for normal cellular functioning, and they have been appreciated as mediators of cellular metabolism (Allen and Tresini 2000; Cadenas 2004). It is highly likely that the increased ROS levels activate an array of sensors and mediators, which in turn orchestrate the multipronged response to calorie restriction, ultimately leading to life span extension.
Acknowledgements
The authors thank Westermann B at the Institut für Physiologische Chemie, Germany, for providing pVT100UmtGFP. We acknowledge Dr Girish Varshney and Sukhwinder Kaur at the Institute of Microbial Technology, Sector 39, Chandigarh, India, for their assistance in the flow cytometry experiments. Senior research fellowship from the Indian Council of Medical Research to Praveen Kumar Sharma is also acknowledged. We thank our lab members KD Prajapati and Nitish Mittal for reading the manuscript.
References
- Agarwal S, Sharma S, Agrawal V, Roy N. Caloric restriction augments ROS defense in S. cerevisiae, by a Sir2p independent mechanism. Free Radic Res. 2005;39:55–62. doi: 10.1080/10715760400022343. [DOI] [PubMed] [Google Scholar]
- Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28:463–499. doi: 10.1016/S0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- Barja G. The quantitative measurement of H2O2 generation in isolated mitochondria. J Bioenerg Biomembr. 2002;34:227–233. doi: 10.1023/A:1016039604958. [DOI] [PubMed] [Google Scholar]
- Barros MH, Bandy B, Tahara EB, Kowaltowski AJ. Higher respiratory activity decreases mitochondrial reactive oxygen release and increases life span in Saccharomyces cerevisiae. J Biol Chem. 2004;279:49883–49888. doi: 10.1074/jbc.M408918200. [DOI] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Borghouts C, Benguria A, Wawryn J, Jazwinski SM. Rtg2 protein links metabolism and genome stability in yeast longevity. Genetics. 2004;166:765–777. doi: 10.1534/genetics.166.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas E. Mitochondrial free radical production and cell signaling. Mol Aspects Med. 2004;25:17–26. doi: 10.1016/j.mam.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JF, Schiestl RH. Mitochondrial respiratory electron carriers are involved in oxidative stress during heat stress in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21:8483–8489. doi: 10.1128/MCB.21.24.8483-8489.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower TR, Chesnokova LS, Froelich CA, Dixon C, Witt SN. Heat shock prevents alpha-synuclein-induced apoptosis in a yeast model of Parkinson's disease. J Mol Biol. 2005;351:1081–1100. doi: 10.1016/j.jmb.2005.06.060. [DOI] [PubMed] [Google Scholar]
- Gems D, Partridge L. Stress-response hormesis and aging: “that which does not kill us makes us stronger”. Cell Metab. 2008;7:200–203. doi: 10.1016/j.cmet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Grant CM, MacIver FH, Dawes IW. Mitochondrial function is required for resistance to oxidative stress in the yeast Saccharomyces cerevisiae. FEBS Lett. 1997;410:219–222. doi: 10.1016/S0014-5793(97)00592-9. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 3. Oxford University Press: New York; 1999. pp. 1–35. [Google Scholar]
- Hatefi Y. Preparation and properties of NADH: ubiquinone oxidoreductase (complex I), EC 1.6.5.3. Methods Enzymol. 1978;53:11–14. doi: 10.1016/S0076-6879(78)53006-1. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Matsuda T, Sugiyama K, Izawa S, Kimura A. Genetic analysis of glutathione peroxidase in oxidative stress response of Saccharomyces cerevisiae. J Biol Chem. 1999;274:27002–27009. doi: 10.1074/jbc.274.38.27002. [DOI] [PubMed] [Google Scholar]
- Jamieson DJ. Saccharomyces cerevisiae has distinct adaptive responses to both hydrogen peroxide and menadione. J Bacteriol. 1992;174:6678–6681. doi: 10.1128/jb.174.20.6678-6681.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JC, Jaruga E, Repnevskaya MV, Jazwinski SM. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. FASEB J. 2000;14:2135–2137. doi: 10.1096/fj.00-0242fje. [DOI] [PubMed] [Google Scholar]
- Joseph Horne T, Hollomon DW, Wood PM. Fungal respiration: a fusion of standard and alternative components. Biochim Biophys Acta. 2001;1504:179–195. doi: 10.1016/S0005-2728(00)00251-6. [DOI] [PubMed] [Google Scholar]
- Kayser EB, Sedensky MM, Morgan PG. The effects of complex I function and oxidative damage on lifespan and anesthetic sensitivity in Caenorhabditis elegans. Mech Ageing Dev. 2004;125:455–464. doi: 10.1016/j.mad.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Kharade SV, Mittal N, Das SP, Sinha P, Roy N. Mrg19 depletion increases S. cerevisiae lifespan by augmenting ROS defence. FEBS Lett. 2005;579:6809–6813. doi: 10.1016/j.febslet.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976;71:952–958. doi: 10.1016/0006-291X(76)90747-6. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004;18:12–16. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size: one figure. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- Meisinger C, Sommer T, Pfanner N. Purification of Saccharomyces cerevisiae mitochondria devoid of microsomal and cytosolic contaminations. Anal Biochem. 2000;287:339–342. doi: 10.1006/abio.2000.4868. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- Raha S, Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci. 2000;25:502–508. doi: 10.1016/S0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- Rattan SI. Theories of biological aging: genes, proteins, and free radicals. Free Radic Res. 2006;40:1230–1238. doi: 10.1080/10715760600911303. [DOI] [PubMed] [Google Scholar]
- Rattan SI. Hormesis in aging. Ageing Res Rev. 2008;7:63–78. doi: 10.1016/j.arr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Roy N, Runge KW. Two paralogs involved in transcriptional silencing that antagonistically control yeast life span. Curr Biol. 2000;10:111–114. doi: 10.1016/S0960-9822(00)00298-0. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Shama S, Lai CY, Antoniazzi JM, Jiang JC, Jazwinski SM. Heat stress-induced life span extension in yeast. Exp Cell Res. 1998;245:379–388. doi: 10.1006/excr.1998.4279. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia PC. Sublethal mitochondrial stress with an attendant stoichiometric augmentation of reactive oxygen species may precipitate many of the beneficial alterations in cellular physiology produced by caloric restriction, intermittent fasting, exercise and dietary phytonutrients: “mitohormesis” for health and vitality. Med Hypotheses. 2006;66:832–843. doi: 10.1016/j.mehy.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Thorpe GW, Fong CS, Alic N, Higgins VJ, Dawes IW. Cells have distinct mechanisms to maintain protection against different reactive oxygen species: oxidative-stress-response genes. Proc Natl Acad Sci U S A. 2004;101:6564–6569. doi: 10.1073/pnas.0305888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann B, Neupert W. Mitochondria-targeted green fluorescent proteins: convenient tools for the study of organelle biogenesis in Saccharomyces cerevisiae. Yeast. 2000;16:1421–1427. doi: 10.1002/1097-0061(200011)16:15<1421::AID-YEA624>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]