Abstract
The primary purpose of the present set of studies was to provide a direct comparison of the effects of the angiotensin-converting enzyme inhibitor enalapril and the angiotensin receptor blocker losartan on body composition, physical performance, and muscle quality when administered late in life to aged rats. Overall, enalapril treatment consistently attenuated age-related increases in adiposity relative to both placebo and losartan. The maximal effect was achieved after 3 months of treatment (between 24 and 27 months of age), at a dose of 40 mg/kg and was observed in the absence of any changes in physical activity, body temperature, or food intake. In addition, the reduction in fat mass was not due to changes in pathology given that enalapril attenuated age-related increases in tumor development relative to placebo- and losartan-treated animals. Both enalapril and losartan attenuated age-related decreases in grip strength, suggesting that changes in body composition appear dissociated from improvements in physical function and may reflect a differential impact of enalapril and losartan on muscle quality. To link changes in adiposity to improvements in skeletal muscle quality, we performed gene array analyses to generate hypotheses regarding cell signaling pathways altered with enalapril treatment. Based on these results, our primary follow-up pathway was mitochondria-mediated apoptosis of myocytes. Relative to losartan- and placebo-treated rats, only enalapril decreased DNA fragmentation and caspase-dependent apoptotic signaling. These data suggest that attenuation of the severity of skeletal muscle apoptosis promoted by enalapril may represent a distinct mechanism through which this compound improves muscle strength/quality.
Keywords: Age-related adiposity, Body composition, Sarcopenia, Renin–angiotensin system, Physical function, Muscle quality
Introduction
Age-related changes in body composition have important clinical implications, given that loss of muscle and gain of fat mass are independently associated with declining performance as well as increased risk for disability and mortality in older persons (Goodpaster et al. 2001, 2006; Newman et al. 2003; Visser et al. 1998). Behavioral interventions, such as moderate calorie restriction and physical exercise, have proven beneficial against age-associated changes in body composition in experimental settings; however, pharmacological approaches may be particularly relevant late in life, since not all older individuals benefit from or are capable of participating in traditional diet and/or exercise programs. Therefore, the present set of studies was designed to provide a direct comparison of the effects of the angiotensin-converting enzyme inhibitor (ACEi) enalapril and the angiotensin receptor blocker (ARB) losartan on body composition, muscle quality, and physical function, when administered late in life to aged rats.
Developing preclinical models of late-life intervention strategies for combating declining physical function has enormous significance (de Grey 2007; Rae et al. 2010). With the continued “graying” of the worldwide population, the number of individuals at risk of developing physical disability continues to increase and the skyrocketing social, emotional, and economic cost (Olshansky et al. 2009) of caring for such individuals mandates the need for testing the effectiveness of health-promoting interventions within this cohort. To address this need, we have used the Fischer 344 × Brown Norway (F344BN) rat as our model, since several studies have shown that this strain proceeds from 80% to 50% mortality between 24 and 30 months of age. In humans, this same pattern of survival mirrors an exponential increase in disability. In fact, in both rats and humans, assessment of functional limitations in the 50% survival range is highly predictive of future disability and ultimately mortality (Carter et al. 2002; Guralnik et al. 1994). In the context of changing body composition, a similar association exists (Newman et al. 2001, 2003; Goodpaster et al. 2006). In the F344BN, there is a gradual increase in both lean and fat mass through 24 months of age, a decrease in muscle, a further increase in fat through 27 months, and a decrease in both compartments thereafter (Carter et al. 2004; Rice et al. 2005). The similarity between the F344BN rat strain and humans in terms of timing of age-related body composition changes and declining performance makes this strain a reasonable model for studying the relationship between age, adiposity, muscle quality, and physical function.
Observational studies in humans and converging evidence from our history of preclinical studies suggest that the use of ACEis attenuates age-related increases in whole-body adiposity and physical performance decline in the absence of any sizeable muscle hypertrophic effect (Bahi et al. 2004; Foianini et al. 2000; Gayagay et al. 1998; Carter et al. 2004, 2005; Onder et al. 2002). These data lend credence to the hypothesis that it is not necessary to optimize the quantity of muscle that is preserved with an intervention but that it is the quality of muscle that remains which determines functionality. Since then, several other studies have demonstrated similar effects of ACEis, in particular enalapril, on body composition in various strains of rats and mice, across different ages, and under normal and high-fat feeding scenarios (de Cavanagh et al. 2007; Santos et al. 2009; Weisinger et al. 2009b). Taken together, these data suggest that the physiological mechanism by which ACEis may enhance muscle quality and subsequently physical function is through their impact on whole-body adiposity.
However, the point is further complicated given that ACEis have dual physiological actions: (1) they block angiotensin II (ANGII) production by preventing the conversion of angiotensin 1 (ANGI) to ANGII and (2) they block of the proteolytic degradation of bradykinin (BK) (Henriksen and Jacob 2003). Therefore, in the current study, we directly compared the adiposity reducing effectiveness of enalapril with losartan, an ARB which only blocks the action of ANGII by antagonizing the ANGII type 1 (AT1) receptor. This allows for a pharmacological approach in dissociating the contribution of each pathway (ANGII vs BK) in mitigating age-related increases in adiposity. We also measured a variety of physiological endpoints which may themselves cause a reduction in body weight (food intake, temperature, glucose and insulin levels, pathology) and which may be secondary to the effects of enalapril. Furthermore, in order to expand our understanding of how this decrease in adiposity may play a role in preserving skeletal muscle quality, we used gene array technology in order to identify potential cell signaling pathways that are altered with enalapril treatment and which may contribute to declining function. Based on these data, we focused on mitochondria-mediated apoptosis in both enalapril- and losartan-treated animals to further assess whether this pathway may be differentially regulated by these two treatments.
Methods
Animals
For all studies, we used male F344BN rats purchased from the National Institute on Aging (NIA) Colony at Harlan Industries (Indianapolis, IN). The F344BN rat strain has been extensively used for aging studies and was selected for these experiments given that these animals demonstrate age-related body composition changes (i.e., increase in adiposity and decrease in lean mass) resembling those occurring in humans (Schwartz 1998). Animals were received at 22 months of age and housed individually on a 12-h light/dark cycle in a specific pathogen-free facility accredited by the American Association for Accreditation of Laboratory Animal Care. From 22 to 24 months of age, animals were allowed to acclimate to their housing conditions and to establish baseline rates of food intake and body weights. All experimental protocols were approved by the University of Florida’s Animal Care and Use Committee.
Experimental design
The first experiment consisted of a longitudinal study to directly compare the effects of enalapril and losartan and to replicate our previous published findings that enalapril both reduces whole-body adiposity and mitigates declining physical function (Carter et al. 2004). Rats were randomly assigned to receive 40 mg/kg enalapril (n = 16) 30 mg/kg losartan (n = 18) or placebo (n = 19) from 24 to 30 months of age. Drug delivery was accomplished by compounding the various treatments into bacon-flavored food tablets (Bio-Serv, Frenchtown, NJ). Placebo-containing food tablets were identical to those delivering enalapril or losartan, except that the drug was omitted. Determinations of body composition and physical performance were performed at baseline (24 months), 27 and 30 months of age. The results of these experiments demonstrated that only enalapril induces a decrease in adiposity, with the maximal effect occurring between 24 and 27 months of age.
Based on the results of the first experiment, the second experiment was designed to characterize the effects of enalapril (20 or 40 mg/kg; n = 33 in each group for a total of n = 66), losartan (30 mg/kg; n = 39), and placebo (n = 34) on markers of skeletal muscle aging over a narrower age range (24 to 27 months of age). This timeframe represents the critical window determined in the first experiment and reflects timing during the aging process, where males of this strain continue to gain fat mass while simultaneously losing muscle mass (Rice et al. 2005). We performed gene array analyses (enalapril 40 mg/kg and placebo; n = 4 each group) to generate hypotheses regarding cell signaling pathways that might be altered with enalapril treatment. Based on these results, our primary follow-up pathway was mitochondria-mediated apoptosis of myocytes. The rationale to focus on mitochondria-driven apoptosis relies on the major role postulated for this apoptotic pathway in the pathogenesis of sarcopenia of aging which is correlated with declining physical function (Marzetti et al. 2009b). The muscle selected for this study was the gastrocnemius, which undergoes significant age-related atrophy accompanied by elevations in apoptosis markers (Marzetti et al. 2008b, 2009a). To ensure that any observed change in body composition was not secondary to other physiological processes, which by themselves are known to impact body weight and composition, we measured locomotor activity, body temperature, food intake, and glucose and insulin levels. In addition, we assessed overall tissue pathology, in collaboration with the Barshop Longevity Institute, to ensure that the treatment itself did not promote conditions, such as increased tumorigenesis, that would also result in weight loss.
Methods for experiment 1
Determination of body composition via time-domain nuclear magnetic resonance Body composition was determined by time-domain nuclear magnetic resonance (TD-NMR) in restrained but awake and alert rats (TD-NMR Minispec, Bruker Optics, The Woodlands, TX, USA). The MiniSpec identifies three components of body composition (fat, free body fluid, and lean tissue in grams) by acquiring and analyzing TD-NMR signals from all protons in the sample area. Scans were acquired by putting live conscious rats (i.e., without anesthetics) into a sample holder (90-mm diameter and ~250-mm length) with a screw top that tightens to the length of the rat. The sample holder was then inserted into the analyzer. The total scan time for each rat was approximately 2 min. Based on the finding that the greatest change in body weight occurred between approximately 24 and 27 months of age, for each individual animal, we calculated the percent change from baseline, for both lean and fat grams, from 24 to 27 months of age. A two-way repeated analysis of variance (ANOVA) was used for testing the effects of time (24 vs 27 months) and treatment (enalapril 40 mg/kg vs losartan 30 mg/kg vs placebo). This same methodology was applied in experiment 2 at 24 and 27 months of age and analyzed using the same statistical test described above.
Assessment of physical performance Forelimb grip strength was determined using an automated grip strength meter (Columbus Instruments, Columbus, OH). The rat was grasped by the tail and suspended above a grip ring. After about 3 s, the rat was gently lowered toward the grip ring and allowed to grasp the ring with its forepaws. The remainder of the rat’s body was quickly lowered to a horizontal position and the animal's tail pulled until its grasp of the ring was broken. The force in grams was determined with a computerized electronic pull strain gauge that was fitted directly to the grasping ring, for three consecutive pulls, and the maximum was determined. A two-way repeated ANOVA was used for testing the effects of time (24 vs 27 vs 30 months) and treatment (enalapril 40 mg/kg vs losartan 30 mg/kg vs placebo). Data are reported as % change of kilogram of force per kilogram of body weight.
Methods for experiment 2
Measurement of locomotor activity One day prior to the TD-NMR experiments at 24 and 27 months of age, locomotor activity was assessed. At the onset of the dark cycle, rats were brought into the procedure room in their home cages and allowed to acclimate to the environment for 1 h. Rats were then placed into activity monitors (Med Associates, St. Albans VT) and allowed to acclimate for 15 min and then were monitored for 1 h. These devices are cubicles (43.5 × 43.5 × 23 cm), with a series of infrared emitters and receivers arrayed along the sides. These allow the automatic recording of an animal’s position and distance moved (cm) within the chamber. Total distance traveled (cm) across the 1-h session was the final unit of measure. A two-way repeated ANOVA was used for testing the effects of time (24 vs 27 months) and treatment (enalapril 20 mg/kg vs enalapril 40 mg/kg vs losartan 30 mg/kg vs placebo).
Determination of body temperature via implanted microchip A glass-encapsulated IPTT-300 temperature transponder was implanted subcutaneously in each rat (Biomedic Data Systems, Inc., Seaford DE), while under momentary light anesthesia (approximately 2% isoflurane at 2 ml/min O2). The device is designed for harmless nonsurgical subcutaneous implantation and is approximately 14 mm in length by 2 mm in diameter. Temperature data were transmitted to a dedicated handheld reader. Three consecutive temperatures were obtained and averaged, at 24 and 27 months of age. A two-way repeated ANOVA was used for testing the effects of time (24 vs 27 months) and treatment (enalapril 20 mg/kg vs enalapril 40 mg/kg vs losartan 30 mg/kg vs placebo).
Serum measurements Rats were sacrificed by rapid decapitation, using a dedicated guillotine. Whole blood was collected and processed for subsequent determination of serum glucose and insulin. The abdomen was quickly opened, and tissues were removed, placed in 2-ml cryovials, flash-frozen in liquid nitrogen, and stored at −80°C. Measurement of blood glucose and insulin was performed by the Hypertension Core Laboratory at the Wake Forest University School of Medicine, using standardized radioimmunoassay procedures, after solid-phase extraction (Sep-Pak, Waters, Milford, MA). Data are presented as nanogram per deciliter and nanogram per milliliter for glucose and insulin, respectively. A one-way ANOVA was used for testing the effects of treatment (enalapril 20 mg/kg vs enalapril 40 mg/kg vs losartan 30 mg/kg vs placebo).
RNA extraction and Affymetrix gene array Gene array analyses were performed on rats from the 40 mg/kg enalapril and placebo groups (n = 4 per group) in order to develop initial hypotheses regarding enalapril’s effect on skeletal muscle quality. RNA was isolated from the soleus muscle sample using the RNeasy Mini Kit (Qiagen, Germantown, MD). RNA concentration was determined using UV spectrophotometry (NanoDrop ND-1000), and RNA quality was confirmed using Agilent 2100 bioanalyzer (Santa Clara, CA). Following confirmation of RNA quality, 5 μg RNA was synthesized to cRNA using Affymetrix amplification kit (Santa Clara, CA) following the manufacturer’s protocol. Hybridization of cRNA was carried out by the University of Florida Interdisciplinary Center for Biotechnology Research Microarray Core. Hybridization of Affymetrix Rat 1.0 arrays occurred for 17 h at 60°C in accordance with manufacturer’s instructions, and arrays were scanned using an Affymetrix Microarray scanner. Images were analyzed using Affymetrix Gene Chip Operating System software (version 1.1) and scaled to 500. Raw data were normalized by filtering of the probes by “present” and “marginal” flags. A “present,” “absent,” or “marginal” flag was designated to a probe based on signal intensity and background noise. The number of “present” calls was determined across all chips, and the probe was removed if fewer than 75% of the chips exhibited a “present” call for the probe. The remaining probe sets were further filtered to remove expressed sequence tags and probes for hypothetical proteins and pseudo-genes that did not have an indication of biological or molecular function. Following this filtering process, a total of 9,017 network eligible genes remained. Differential expression between groups was determined using two-tailed t tests with an alpha level of 0.025. These differentially expressed transcripts were functionally annotated using the Database for Annotation, Visualization, and Integrative Discovery (david.abcc.ncifcrf.gov) and submitted to Ingenuity Pathway Analysis (IPA, Ingenuity Systems, Redwood City, CA) to determine their roles in networks, cellular functions, and canonical pathways. The IPA program uses a right-tailed Fisher’s exact test to compute the likelihood that the relationship between the list of submitted genes and a set of genes representing a given pathway is due to chance.
Subcellular fractionation of gastrocnemius muscle samples Isolation of cytosolic, mitochondrial, and nuclear fractions was performed as detailed elsewhere (Marzetti et al. 2008b). Protein concentration in the cytosolic and mitochondrial fractions was determined by the method developed by Bradford (1976), whereas the detergent-compatible DC assay (Bio-Rad, Hercules, CA) was employed for nuclear extracts. Subcellular fractions were subsequently aliquoted and stored at −80°C until analysis.
Determination of the extent of skeletal muscle apoptosis Overall levels of apoptosis in the gastrocnemius muscle were quantified by measuring the amount of cytosolic mononucleosomes and oligonucleosomes using an enzyme-linked immunosorbent assay (ELISA) kit (cell death detection ELISA; Roche Diagnostics, Mannheim, Germany), as previously described (Marzetti et al. 2008a). The assay relies on the quantification of histone-complexed fragmented DNA. Although the kit does not allow for the discrimination between apoptotic and necrotic cell death in cytosolic extracts, occurrence of significant necrosis in skeletal muscle during normal aging has not been reported. Therefore, the impact of necrotic cell death in our system may be considered negligible. Absorbance was measured at 405 nm with a Synergy HT multidetection microplate reader (BioTek, Winooski, VT) and reported as arbitrary optical density (OD) units per milligram of protein (apoptotic index). A one-way ANOVA was used for testing the effects of treatment (enalapril 20 mg/kg vs enalapril 40 mg/kg vs losartan 30 mg/kg vs placebo) on all measures.
Western blot analysis for the determination of key mitochondrial apoptotic signaling proteins A vast literature supports a central role for mitochondria-driven apoptosis during the development of sarcopenia (Marzetti et al. 2009b). Therefore, we assessed several proteins integral to the regulation and execution of mitochondrial apoptotic signaling via Western immunoblot analysis of gastrocnemius subcellular fractions. Specifically, we determined expression levels of both caspase-dependent (i.e., cytochrome c, active caspase 9, and cleaved caspase 3) and caspase-independent [apoptosis-inducing factor (AIF) and endonuclease G (EndoG)] mitochondrial apoptogenic mediators in specific subcellular compartments. Furthermore, we measured mitochondrial levels of the anti-apoptotic Bcl-2 and pro-apoptotic Bax, given their central role in controlling cell fate (Marzetti et al. 2009b). Experiments were performed as detailed elsewhere (Marzetti et al. 2009a). The following primary antibodies and relative dilutions were used: rabbit monoclonal anti-cleaved cleaved-caspase-3 (Cell Signaling Technology, Beverly, MA), 1:1,000; rabbit polyclonal anti-Bcl-2 (Santa Cruz Biotechnology, Santa Cruz, CA), 1:200; rabbit polyclonal anti-Bax (Santa Cruz Biotechnology), 1:200; rabbit polyclonal anti-cytochrome c (Santa Cruz Biotechnology), 1:200; rabbit polyclonal anti-active caspase-9 (Santa Cruz Biotechnology), 1:200; rabbit polyclonal anti-EndoG (Abcam, Cambridge, MA), 1:1,000; rabbit polyclonal anti-EndoG (Abcam), 1:200; and rabbit polyclonal anti-AIF (BD Pharmingen, San Diego, CA), 1:500. Generation of the chemiluminescent signal, digital acquisition, and densitometry analysis were performed as previously described (Marzetti et al. 2008b). Spot density of target bands was normalized to the amount of protein loaded in each lane, as determined by densitometric analysis of the corresponding Ponceau S-stained membranes, and expressed as arbitrary OD units (Image Lab 2.0.1, Bio-Rad Laboratories). For each measure, a one-way ANOVA was used for testing the effects of treatment (enalapril 20 mg/kg vs enalapril 40 mg/kg vs losartan 30 mg/kg vs placebo).
Pathology After rats were necropsied for gross pathological lesions, organs and tissues were excised and preserved in 10% buffered formalin. Organs and tissues analyzed included brain, pituitary gland, heart, lung, trachea, thymus, aorta, esophagus, stomach, small intestine, colon, liver, pancreas, spleen, kidneys, urinary bladder, reproductive system (prostate, testes, epididymis, and seminal vesicles), thyroid gland, adrenal glands, parathyroid glands, psoas muscle, knee joint, sternum, and vertebrae. Any other tissue with gross lesions was also excised. Fixed tissues were processed conventionally, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin–eosin. Diagnosis of each histopathological change was established based on histological classifications in aging rats (Maeda et al. 1985; Iwasaki et al. 1988). The prevalence of tumor-bearing rats and overall specific incidence of disease was calculated for each experimental group. The percentage of tumor-bearing rats was calculated as the percentage of animals that had one or more neoplastic lesions. For this assessment, all neoplastic lesions were counted regardless of the severity of tumors, i.e., both incidental (not severe enough to be the cause of death) and fatal (severe enough to be the cause of death) tumors were considered. Chronic nephropathy was graded in the order of increasing severity based on the grading system described by Yu et al. (1982) as follows: grade 0 (no lesions), grade 1, grade 2, grade 3, grade 4, and grade E (very severe). Grading of neoplastic lesions was based on a modification of previously reported criteria (Ikeno et al. 2003): grade 1, primary site only; grade 2, primary site and intra-organ or one other organ metastasis; grade 3, metastasis to two to three organs; and grade 4, metastasis to more than four organs or grade 3 + additional pathology (e.g., pleural effusion, ascites, subcutaneous edema, etc.). Hydrothorax, ascites, and subcutaneous edema were the most common complications associated with advanced neoplastic diseases. Pathological comparisons of tumor-bearing rats and number of neoplastic and non-neoplastic diseases (i.e., chronic nephropathy) among groups were examined by Mantel–Haenszel chi-squared test. The average number of tumors per rat, total number of pathologies, and average severity of nephropathy were compared among groups using the Kruskal–Wallis test.
Statistical analysis
Statistical analyses were performed using SAS 9.1 for Windows (Cary, NC). Multiple pairwise group comparisons were performed using the Bonferroni procedure. The level of significance was set at p < 0.05 for all analyses. All data are presented as means ± standard error.
Results
Experiment 1
Body weight and composition
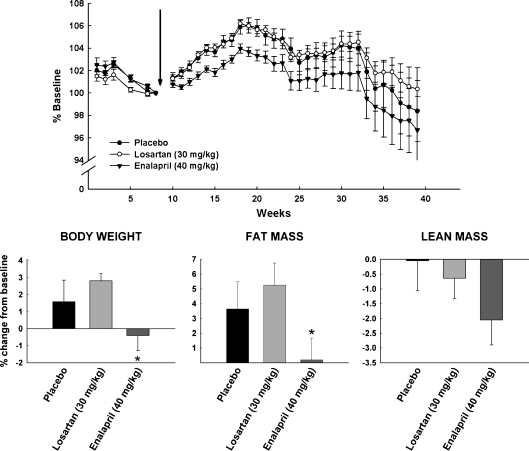
Body weight and composition changes in rats receiving placebo, 40 mg/kg enalapril, and 30 mg/kg losartan from 24 to 30 months of age are depicted in Fig. 1 and expressed as percent change from baseline. During the 6-month intervention, rats in the enalapril group, in general, had lower body weights relative to the other groups, with the peak difference occurring approximately 3 months after the initiation of treatment. The differences in percent change observed, although relatively small (~4%), were statistically significant and reflect the larger gains in body weight observed during the first 3 months of the experiment in the placebo and losartan groups relative to the enalapril-treated rats (p = 0.027 and 0.015, respectively). Analysis of changes in body composition from baseline to 3 months showed an increase in fat mass in the placebo (p = 0.016) group and in rats treated with losartan (p = 0.019), whereas the opposite pattern was detected in the enalapril group (p = 0.017). At 3 months, there was a trend towards a decrease in lean mass loss in the enalapril group, but this change was not statistically significant (p = 0.06). By the end of the study, no statistical differences were observed among groups in any measures of body weight or composition.
Fig. 1.
Body weight (upper panel) changes in rats receiving placebo (closed circles), 40 mg/kg enalapril (closed triangles), and 30 mg/kg losartan (open circles) between 24 and 30 months of age. The lower panel depicts changes in rats receiving placebo, 40 mg/kg enalapril, and 30 mg/kg losartan: body weight (left panel), fat mass (middle panel), and lean mass (right panel) between 24 and 27 months of age. All data expressed as means ± SEM
Physical performance
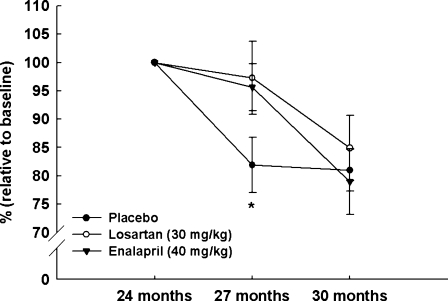
Forelimb grip strength was measured at baseline and at 27 and 30 months of age (Fig. 2). Both enalapril and losartan attenuated the age-related decline in grip strength at 27 months of age (both ps < 0.05). However, this effect was no longer evident for either treatment at the 30-month time point.
Fig. 2.
Forelimb grip strength was measured at baseline and at 27 and 30 months of age in rats receiving placebo (closed circles), 40 mg/kg enalapril (closed triangles), and 30 mg/kg losartan (open circles). All data expressed as means ± SEM
Experiment 2
Body composition, food intake, locomotor activity, glucose, and insulin
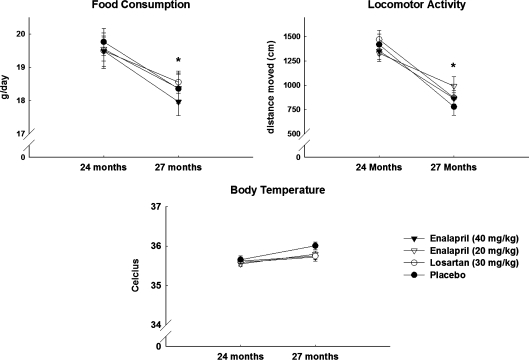
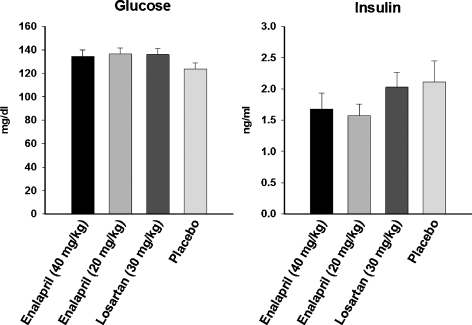
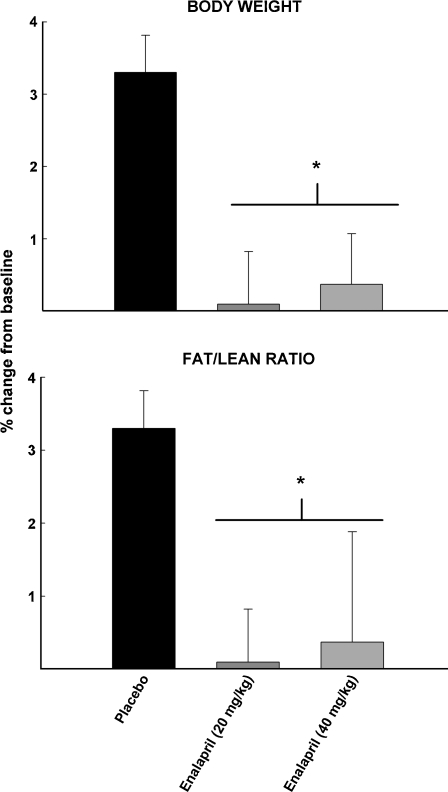
Food intake, body temperature, and locomotor activity were assessed at 24 and 27 months of age in rats treated with 20 or 40 mg/kg enalapril, 30 mg/kg losartan, or placebo (Fig. 3). Blood glucose and insulin levels were determined at 27 months (Fig. 4). Neither enalapril nor losartan affected any of these physiological measures. However, all rats experienced a decrease in food intake (p = 0.026) and locomotor activity (p = 0.021) over time, and most animals demonstrated insulin resistance, which is characteristic of this strain (e.g. levels of glucose > 120 mg/dl; Gabriely and Barzilai 2003). Both doses of enalapril lowered body weight and fat-to-lean ratio (p = 0.002; Fig. 5).
Fig. 3.
Changes in food consumption (upper panel; left), locomotor activity (upper panel; right), and body temperature (lower panel) in rats receiving placebo (closed circles), 30 mg/kg losartan (open circles), 40 mg/kg enalapril (closed triangles), and 20 mg/kg enalapril (open triangles) between 24 and 27 months of age. All data expressed as means ± SEM
Fig. 4.
Glucose and insulin levels in 27-month-old rats receiving placebo, 40 mg/kg enalapril, 20 mg/kg enalapril and 30 mg/kg losartan between 24 and 27 months of age. All data expressed means ± SEM
Fig. 5.
Changes in body weight (upper panel) and fat-to-lean ratio (lower panel) in rats receiving placebo, 40 mg/kg enalapril, and 20 mg/kg enalapril between 24 and 27 months of age. All data expressed as means ± SEM
Transcript profiles and functional annotation
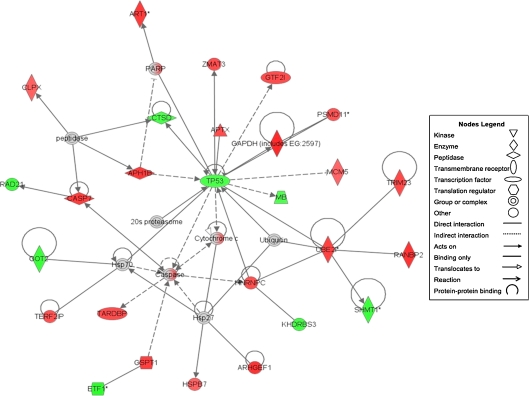
Following 3 months of treatment with 40 mg/kg of enalapril or placebo, 314 unique transcripts were differentially expressed (p < 0.025) between groups in the soleus muscle. Of these transcripts, 237 were significantly upregulated and 77 downregulated by enalapril treatment. The IPA program provides information regarding the top molecular and cellular functions of the experimental treatment based on the number of differentially expressed molecules ascribed a specific cellular function relative to the total number of genomic molecules ascribed that function. Specific molecular and cellular functions are then grouped into categories reflecting a common physiological purpose. Using IPA, we generated and scored 25 functional networks based on transcripts differentially expressed between groups. The IPA network with the highest score of 43 demonstrated differential expression of 27 of the 35 genes in the network (Fig. 6). This network primarily includes genes related to cellular maintenance, including several genes involved in the regulation of apoptosis. The two “hubs” of this network, tumor protein p53 and the caspases, provide a strong indication that regulation of apoptosis lies at the heart of enalapril-mediated effects in aged skeletal muscle. Therefore, we further investigated mitochondrial-driven apoptosis using Western blot analyses.
Fig. 6.
Functional network with the highest score of 43 following IPA analysis. Red indicates upregulation; green indicates downregulation. Note that the two central hubs, TP53 and the caspase group, are associated with myonuclear apoptosis
Skeletal muscle apoptosis and markers of mitochondria-driven apoptotic signaling
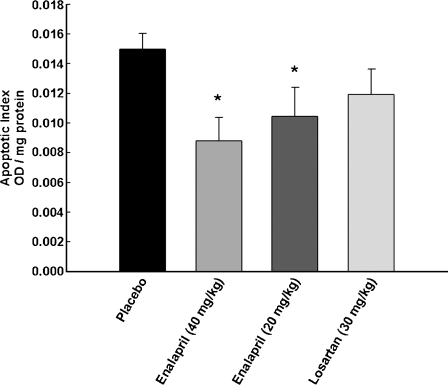
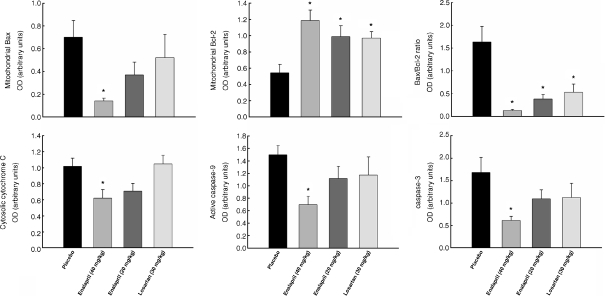
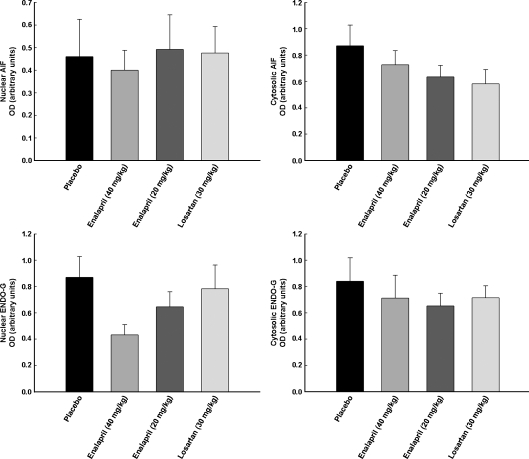
Overall levels of apoptosis and key proteins pertaining to the mitochondrial apoptotic signaling pathways were assessed in the gastrocnemius muscle of rats receiving 20 or 40 mg/kg enalapril, 30 mg/kg losartan, or placebo between 24 and 27 months of age. As shown in Fig. 7, decreased levels of DNA fragmentation were detected in both enalapril-treated groups (both ps = 0.01 vs. placebo). In contrast, the extent of apoptosis in the gastrocnemius muscle was unaffected by losartan. Downregulation of markers of the mitochondrial caspase-dependent apoptotic pathway (i.e., cytosolic cytochrome c, active caspase 9, and cleaved caspase 3) was only detected in rats receiving 40 mg/kg enalapril (ps = 0.038, 0.003, and 0.008, respectively; Fig. 8). On the contrary, neither cytosolic nor nuclear levels of the mitochondrial caspase-independent apoptotic effectors AIF and EndoG were affected by either enalapril or losartan (Fig. 9). Interestingly, in all treatment groups, increased mitochondrial content of Bcl-2 was observed (enalapril 40 mg/kg p = 0.003; enalapril 20 mg/kg p = 0.020, and losartan 30 mg/kg p = 0.012; all relative to placebo as shown in Fig. 8). However, only enalapril given at 40 mg/kg lowered Bax translocation to mitochondria (p = 0.010; Fig. 8). Finally, a lower Bax-to-Bcl-2 ratio, indicative of reduced apoptotic potential, was detected in all treatment arms compared with placebo (enalapril 40 mg/kg p = 0.001; enalapril 20 mg/kg p = 0.025, and losartan 30 mg/kg p = 0.020; all relative to placebo as shown in Fig. 8).
Fig. 7.
Changes in apoptotic index as measured by DNA fragmentation in rats receiving placebo), 40 mg/kg enalapril, 20 mg/kg enalapril, and 30 mg/kg losartan between 24 and 27 months of age. All data expressed means ± SEM
Fig. 8.
Changes in caspase-dependent apoptotic signaling in rats receiving placebo, 40 mg/kg enalapril, 20 mg/kg enalapril, and 30 mg/kg losartan between 24 and 27 months of age. All data expressed means ± SEM
Fig. 9.
Changes in caspase-independent apoptotic signaling in rats receiving placebo, 40 mg/kg enalapril, 20 mg/kg enalapril, and 30 mg/kg losartan between 24 and 27 months of age. All data expressed means ± SEM
Pathology
Pathological analyses were performed on 14 placebo-treated, 20 losartan-treated (30 mg/kg), 22 enalapril-treated (40 mg/kg), and 21 enalapril-treated (20 mg/kg) rats. Tables 1 and 2 show that, overall, the average number of tumor-bearing rats (p = 0.04) and tumors per rat (p = 0.036) was lowest in the enalapril 40 mg/kg group compared with that in the placebo group. In addition, neoplastic events were reduced in both the 40 mg/kg (p = 0.027) and 20 mg/kg (p = 0.012) enalapril groups relative to placebo. Nephropathy was unchanged among groups.
Table 1.
Number of tumor-bearing rats, average number of tumors per rat, total number of pathologies, and average severity of nephropathy of each treatment group
| Placebo | Losartan (30 mg/kg) | Enalapril (40 mg/kg) | Enalapril (20 mg/kg) | |
|---|---|---|---|---|
| Tumor-bearing rats | 11 (78.6%) | 12 (60%) | 9 (39.1%)a | 10 (45.5%) |
| Average number of tumors per rat | 1 | 0.75 | 0.55a | 0.62 |
| Total number of pathologies | 5.93 | 6.25 | 6.55 | 6.38 |
| Average severity of nephropathy | 1.07 | 0.6 | 0.86 | 1 |
Average number of tumors per rat: p = 0.0366 vs group P
aTumor-bearing rats: p = 0.0407 vs group P
Table 2.
Number of tumors in each treatment groups
| Histopathology/group | P (n = 14) | L (n = 20) | EH (n = 22) | EL (n = 21) |
|---|---|---|---|---|
| Neoplastic | 14 | 15 | 12* | 13** |
| Pituitary adenoma | 2 | 4 | 2 | 3 |
| Adenocarcinoma | 4 | 4 | 2 | 2 |
| Pheochromocytoma | 3 | 4 | 3 | 4 |
| Islet cell tumor, pancreas | 4 | 1 | 2 | 1 |
| Others | 1 | 2 | 3 | 3 |
| Non-neoplastic | ||||
| Chronic nephropathy | 9 | 10 | 15 | 14 |
| Others: subcutaneous tumor, skin tumor, and tumor in reproductive system | ||||
*p = 0.027 (EH vs P); ** p = 0.0118 (EL vs P)
Discussion
The primary purpose of the present set of studies was to provide a direct comparison of the effects of the ACEi enalapril and the ARB losartan on body composition and physical performance when administered late in life to aged rats. To ensure that any observed change in body composition was not secondary to other physiological processes, we measured locomotor activity, body temperature, food intake, and glucose and insulin levels. In addition, we assessed overall tissue pathology, to ensure that the treatment itself did not promote conditions, such as increased tumorigenesis, that would also result in weight loss. To link changes in adiposity to improvements in skeletal muscle quality, we performed gene array analyses to generate hypotheses regarding which age-related signaling pathways might be altered with enalapril treatment. Based on these results, our primary follow-up pathway was mitochondria-mediated apoptosis of myocytes.
Our results indicate that enalapril treatment consistently attenuates age-related increases in adiposity relative to both placebo and losartan. The maximal effect was achieved after 3 months of treatment (between 24 and 27 months of age), at a dose of 40 mg/kg, and was observed in the absence of any changes in physical activity, body temperature, or food intake. These data are consistent with our previous findings and a larger developing literature demonstrating selective loss of body fat compartment with ACEi treatment in a variety of species, under various feeding regimens and across various time points in the life span (Weisinger et al. 2009a, b; Santos et al. 2009). However, one exception we note is that most studies, especially those in young animals, report decreased food intake. For example, enalapril administration (10 mg/kg) to young adult rats, fed either regular or a high-fat chow, was accompanied by a decrease in food intake (Santos et al. 2009), although when adjusted for changing body weight, food intake is equivalent or increased. A similar finding has been reported in captopril-treated young mice maintained on a high-fat diet (Weisinger et al. 2009b). One potential difference between our study and those using young rats and mice as subjects is that young rodents are highly leptin sensitive. Leptin is a primary modulator of ingestive behavior, and with age, the F344BN rat becomes leptin resistant (Zhang and Scarpace 2006; Scarpace and Zhang 2009). It is therefore likely that the large losses in fat mass observed in those studies using young animals resulted in decreased leptin levels, initiating an anorectic response. However, it is unlikely that this loss of fat was a direct action of enalapril on leptin signaling given that in the study of Santos et al. (2009), young rats fed regular chow or a high-fat diet had a similar anorectic response to ICV leptin administration regardless of enalapril treatment. Our group has previously demonstrated that enalapril treatment in old rats did indeed result in a reduction in leptin levels commensurate with the loss of fat mass; however, this effect did not translate in any change in food intake (Carter et al. 2004). In fact, in the present study, all animals decrease food intake between 24 and 27 months of age, even though fat mass is increasing. Therefore, enalapril most likely is working through other pathways which modulate fat loss (enhanced fatty acid oxidation, increased adiponectin levels, and improved insulin and peroxisome proliferator-activated receptor (PPAR)-γ signaling; Santos et al. 2009; Weisinger et al. 2009a, b) rather than through decreased food intake. Future studies in older animals are needed to address these mechanisms.
The weight loss observed was also not due to changes in pathology. In fact, quite the opposite occurred in that enalapril, at the highest dose, attenuated the age-related increase in tumor development relative to placebo- and losartan-treated animals. This finding is especially remarkable in light of a recent meta-analysis showing an increased risk for cancer associated with chronic ARB treatment (Sipahi et al. 2010). However, lifespan was not an outcome in our studies given that enalapril administration which occurred was not administered over the entire life of these rats (only between 24 and 30 months of age). However, in a recent study, as part of the NIA Intervention Testing Program, 15 mg/kg enalapril administered to mice from middle age throughout the lifespan did not impact maximal life span (Harrison et al. 2009). In contrast to these findings, others reported increased life span in laboratory rodents treated with enalapril. For example, Santos et al. showed that enalapril, administered for 26 months to Wistar rats, increased mean life span (Santos et al. 2009). The effect on maximum life span was not ascertainable given that the study was censored at 26 months. In addition, Basso et al. found that inhibition of the renin–angiotensin system (RAS) via either enalapril (10 mg/kg) or losartan (30 mg/kg) increased life span in normotensive Wistar rats (Basso et al. 2007). However, in this study, many animals were removed during the experiment for other experimental purposes, thereby changing the composition of the original population and making a conclusive statement regarding life span problematic. Increased longevity has been observed in ACEi-treated mice and rats fed high-fat diets, although these studies were also censored at particular ages (Weisinger et al. 2009b). Hence, additional research is needed to establish the impact of pharmacological RAS inhibition on life span, taking into consideration the dose and duration of the intervention.
In the current study, losartan had no impact on body composition. One interpretation is that blockade of the actions of ANGII at the AT1 receptor has no anti-adiposity action. However, recent reports suggest that ARBs other than losartan, such as telmisartan, prevent weight gain in normally fed and high-fat-fed mice through PPAR/AMPK signaling pathways (Feng et al. 2010; He et al. 2010). Indeed, the same series of studies showed, using an in vitro preparation (3 T3-L1 preadipocytes), that only telmisartan activated PPAR- γ signaling when compared directly with losartan. Furthermore, PPAR- γ and PPAR-delta knockout mice do not respond with weight loss to telmisartan treatment. Most interestingly, mice fed telmisartan had improved exercise endurance, accompanied by a shift to increased slow-twitch muscle fiber profile (Feng et al. 2010). Whether or not this effect of telmisartan is directly regulated at the AT1 receptor or some other direct action of this drug on alternative signaling pathways is yet unknown. However, there is emerging evidence that telmisartan operates as a partial PPAR-γ agonist and that it inhibits the proliferative capacity of some cells that lack ANGII receptors (Benson et al. 2004). These data highlight the need for comparative effectiveness of ARBs in late-life intervention studies, using preclinical models.
Importantly, at 27 months of age, both enalapril and losartan attenuated the age-dependent decline in physical performance. However, in contrast to the enalapril group, in losartan-treated rats, this effect occurred in the absence of changes in body weight or composition. This finding suggests that both compounds might improve physical performance through a direct action on the skeletal muscle, independent of changes in body mass. Therefore, we performed gene array analyses in order to develop working hypotheses regarding potential molecular signaling pathways which might be altered with enalapril treatment, and apoptosis emerged as a leading candidate. This hypothesis is supported by previous studies showing that interventions reducing the severity of skeletal muscle apoptosis improve physical function (Song et al. 2006; Marzetti et al. 2008a). Apoptosis, a process of programmed cell death, is a highly conserved and tightly regulated systematic set of events resulting in cellular self-destruction without the induction of inflammation or damage to the surrounding tissue (Kerr et al. 1972). Cysteine-aspartic proteases (caspases) are the executioner enzymes that carry out the dismantling of the cell and are normally present as inactive zymogens (procaspases) (Danial and Korsmeyer 2004). Upon apoptotic stimuli, initiator caspases (i.e., caspase 8, caspase 9, caspase 12) are activated, subsequently leading to the activation of effector caspases (i.e., caspase 3, caspase 6, caspase 7) that perform the actual cellular degradation (Danial and Korsmeyer 2004). Effector caspases can be activated through extrinsic and intrinsic pathways (Hengartner 2000). The extrinsic apoptotic signaling is initiated by the activation of death receptors present on the cell surface, such as the Fas receptor and tumor necrosis factor receptor (Danial and Korsmeyer 2004). Intrinsic pathways of caspase activation include several internal cellular stimuli mediated by the endoplasmic reticulum or the mitochondria (Danial and Korsmeyer 2004). Mitochondria-mediated intrinsic signaling pathway of apoptosis is probably the more prominent means of programmed cell death and has been the subject of intense scientific scrutiny in the aging community (Jeong and Seol 2008).
In the present study, decreases in DNA fragmentation were only observed in enalapril-treated rats, regardless of the dosage. Interestingly, the Bax-to-Bcl-2 ratio, a critical checkpoint in the apoptotic signaling, was lowered by both enalapril and losartan. Our data do not allow inferring the mechanisms whereby the decreased Bax/Bcl-2 induced by losartan did not translate into a mitigation of muscle apoptotic DNA fragmentation. One explanation for this finding may be that enalapril treatment, besides increasing mitochondrial Bcl-2 expression, was also able to promote Bcl-2 phosphorylation, which is required for its anti-apoptotic activity (Horiuchi et al. 1997). Importantly, phosphorylation of Bcl-2 induced by mitogen-activated protein kinase (MAPK) is inhibited by the activation of ANGII type 2 (AT2) receptor (Horiuchi et al. 1997). It is therefore conceivable that the reduced AT2 receptor signaling elicited by enalapril might have resulted in increased MAPK activation and, hence, enhanced Bcl-2 phosphorylation. On the other hand, the lack of AT2 receptor inhibition by losartan might have induced an incomplete anti-apoptotic response, with increased mitochondrial Bcl-2 levels not supported by adequate Bcl-2 phosphorylation. This possibility warrants further investigation.
In addition, although both enalapril doses were able to reduce the extent of apoptosis in the gastrocnemius muscle, only the highest dose decreased the activation of the mitochondrial caspase-dependent apoptotic pathway. Furthermore, neither enalapril dose affected the caspase-independent pathway. Based on our findings, it is therefore unclear how low-dose enalapril attenuated apoptosis. One possibility is that enalapril given at the lowest dose did indeed result in downregulation of mitochondrial caspase-dependent apoptotic signaling. However, changes in the expression levels of apoptogenic mediators might have been below the detection limit of our Western blot analysis. Alternatively, it may be hypothesized that low-dose enalapril was still able to attenuate the caspase catalytic activity, possibly via upregulation of caspase inhibitors (e.g., cIAPs and cFLIPs). The impact of caspase activity and caspase inhibitors should be addressed in future studies.
In conclusion, enalapril treatment, between 20 and 40 mg/kg, consistently lowers body weight in older animals, even when initiated late in life, with a maximal efficacy achieved after 3 months of treatment. This effect was not observed in losartan-treated animals, suggesting that blocking the AT1 receptor pathway does not mediate these benefits. Changes in body weight and composition appear dissociated from improvements in physical function and may reflect a differential impact of enalapril and losartan on muscle quality. These data suggest that attenuation of the severity of skeletal muscle apoptosis promoted by enalapril may represent a distinct mechanism through which this compound improves muscle strength/quality.
Acknowledgments
This study was supported by the National Institute on Aging Grant AG24526, the University of Florida Institute on Aging, The Claude D. Pepper Older Americans Independence Center Grant NIH P30 AG028740, and the McKnight Foundation.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s11357-011-9243-3
References
- Bahi L, Koulmann N, Sanchez H, Momken I, Veksler V, Bigard AX, et al. Does ACE inhibition enhance endurance performance and muscle energy metabolism in rats? J Appl Physiol. 2004;96:59–64. doi: 10.1152/japplphysiol.00323.2003. [DOI] [PubMed] [Google Scholar]
- Basso N, Cini R, Pietrelli A, Ferder L, Terragno NA, Inserra F. Protective effect of long-term angiotensin II inhibition. Am J Physiol Heart Circ Physiol. 2007;293:H1351–H1358. doi: 10.1152/ajpheart.00393.2007. [DOI] [PubMed] [Google Scholar]
- Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, et al. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPAR gamma-modulating activity. Hypertension. 2004;43:993–1002. doi: 10.1161/01.HYP.0000123072.34629.57. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carter CS, Sonntag WE, Onder G, Pahor M. Physical performance and longevity in aged rats. J Gerontol A Biol Sci Med Sci. 2002;57:B193–B197. doi: 10.1093/gerona/57.5.b193. [DOI] [PubMed] [Google Scholar]
- Carter CS, Cesari M, Ambrosius WT, Hu N, Diz D, Oden S, et al. Angiotensin-converting enzyme inhibition, body composition, and physical performance in aged rats. J Gerontol A Biol Sci Med Sci. 2004;59:416–423. doi: 10.1093/gerona/59.5.b416. [DOI] [PubMed] [Google Scholar]
- Carter CS, Onder G, Kritchevsky SB, Pahor M. Angiotensin-converting enzyme inhibition intervention in elderly persons: effects on body composition and physical performance. J Gerontol A Biol Sci Med Sci. 2005;60:437–1446. doi: 10.1093/gerona/60.11.1437. [DOI] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/S0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Cavanagh EM, Inserra F, Ferder M, Ferder L. From mitochondria to disease: role of the renin–angiotensin system. Am J Nephrol. 2007;27:545–553. doi: 10.1159/000107757. [DOI] [PubMed] [Google Scholar]
- Grey AD. The case for prioritizing research on late-onset life-extension interventions in mammals. Rejuvenation Res. 2007;10:257–259. doi: 10.1089/rej.2007.0547. [DOI] [PubMed] [Google Scholar]
- Feng X, Luo Z, Ma L, Ma S, Yang D, Zhao Z et al (2010) Angiotensin II receptor blocker telmisartan enhances running endurance of skeletal muscle through activation of the PPAR delta/AMPK pathway. J Cell Mol Med (in press) [DOI] [PMC free article] [PubMed]
- Foianini KR, Steen MS, Kinnick TR, Schmit MB, Youngblood EB, Henriksen EJ. Effects of exercise training and ACE inhibition on insulin action in rat skeletal muscle. J Appl Physiol. 2000;89:687–694. doi: 10.1152/jappl.2000.89.2.687. [DOI] [PubMed] [Google Scholar]
- Gabriely I, Barzilai N. Surgical removal of visceral adipose tissue: effects on insulin action. Curr Diab Rep. 2003;3:201–206. doi: 10.1007/s11892-003-0064-3. [DOI] [PubMed] [Google Scholar]
- Gayagay G, Yu B, Hambly B, Boston T, Hahn A, Celermajer DS, et al. Elite endurance athletes and the ACE I allele—the role of genes in athletic performance. Hum Genet. 1998;103:48–50. doi: 10.1007/s004390050781. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: the health ABC Study. J Appl Physiol. 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Yang D, Ma L, Luo Z, Ma S, Feng X, et al. Telmisartan prevents weight gain and obesity through activation of peroxisome proliferator-activated receptor-delta-dependent pathways. Hypertension. 2010;55:869–879. doi: 10.1161/HYPERTENSIONAHA.109.143958. [DOI] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ, Jacob S. Modulation of metabolic control by angiotensin converting enzyme (ACE) inhibition. J Cell Physiol. 2003;196:171–179. doi: 10.1002/jcp.10294. [DOI] [PubMed] [Google Scholar]
- Horiuchi M, Hayashida W, Kambe T, Yamada T, Dzau VJ. Angiotensin type 2 receptor dephosphorylates Bcl-2 by activating mitogen-activated protein kinase phosphatase-1 and induces apoptosis. J Biol Chem. 1997;272:19022–19026. doi: 10.1074/jbc.272.30.19022. [DOI] [PubMed] [Google Scholar]
- Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in Ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci. 2003;58:291–296. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo EJ, Yu BP. The influence of dietary protein source on longevity and age-related disease processes of Fischer rats. J Gerontol. 1988;43:B5–B12. doi: 10.1093/geronj/43.1.b5. [DOI] [PubMed] [Google Scholar]
- Jeong SY, Seol DW. The role of mitochondria in apoptosis. BMB Rep. 2008;41:11–22. doi: 10.5483/BMBRep.2008.41.1.011. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H, Gleiser CA, Masoro EJ, Murata I, McMahan CA, Yu BP. Nutritional influences on aging of Fischer 344 rats: II. Pathology. J Gerontol. 1985;40:671–688. doi: 10.1093/geronj/40.6.671. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Groban L, Wohlgemuth SE, Lees HA, Lin M, Jobe H, et al. Effects of short-term GH supplementation and treadmill exercise training on physical performance and skeletal muscle apoptosis in old rats. Am J Physiol Regul Integr Comp Physiol. 2008;294:R558–R567. doi: 10.1152/ajpregu.00620.2007. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Wohlgemuth SE, Lees HA, Chung HY, Giovannini S, Leeuwenburgh C. Age-related activation of mitochondrial caspase-independent apoptotic signaling in rat gastrocnemius muscle. Mech Ageing Dev. 2008;129:542–549. doi: 10.1016/j.mad.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Carter CS, Wohlgemuth SE, Lees HA, Giovannini S, Anderson B, et al. Changes in IL-15 expression and death-receptor apoptotic signaling in rat gastrocnemius muscle with aging and life-long calorie restriction. Mech Ageing Dev. 2009;130:272–280. doi: 10.1016/j.mad.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Hwang JC, Lees HA, Wohlgemuth SE, Dupont-Versteegden EE, Carter CS, et al. Mitochondrial death effectors: relevance to sarcopenia and disuse muscle atrophy. Biochim Biophys Acta. 2009;1800:235–244. doi: 10.1016/j.bbagen.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AB, Yanez D, Harris T, Duxbury A, Enright PL, Fried LP. Weight change in old age and its association with mortality. J Am Geriatr Soc. 2001;49:1309–1318. doi: 10.1046/j.1532-5415.2001.49258.x. [DOI] [PubMed] [Google Scholar]
- Newman AB, Haggerty CL, Goodpaster B, Harris T, Kritchevsky S, Nevitt M, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51:323–330. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Goldman DP, Zheng Y, Rowe JW. Aging in America in the twenty-first century: demographic forecasts from the MacArthur Foundation Research Network on an Aging Society. Milbank Q. 2009;87:842–862. doi: 10.1111/j.1468-0009.2009.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder G, Penninx BW, Balkrishnan R, Fried LP, Chaves PH, Williamson J, et al. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet. 2002;359:926–930. doi: 10.1016/S0140-6736(02)08024-8. [DOI] [PubMed] [Google Scholar]
- Rae MJ, Butler RN, Campisi J, Grey AD, Finch CE, Gough M, et al. The demographic and biomedical case for late-life interventions in aging. Sci Transl Med. 2010;2:40cm21. doi: 10.1126/scitranslmed.3000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice KM, Linderman JK, Kinnard RS, Blough ER. The Fischer 344/NNiaHSd X Brown Norway/BiNia is a better model of sarcopenia than the Fischer 344/NNiaHSd: a comparative analysis of muscle mass and contractile properties in aging male rat models. Biogerontology. 2005;6:335–343. doi: 10.1007/s10522-005-4808-0. [DOI] [PubMed] [Google Scholar]
- Santos EL, Picoli SK, Silva ED, Batista EC, Martins PJF, D'Almeida V, et al. Long term treatment with ACE inhibitor enalapril decreases body weight gain and increases life span in rats. Biochem Pharmacol. 2009;78:951–958. doi: 10.1016/j.bcp.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Zhang Y. Leptin resistance: a predisposing factor for diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R493–R500. doi: 10.1152/ajpregu.90669.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RS. Obesity in the elderly. In: Bray GA, Bouchard C, James WPT, editors. Handbook of obesity. New York: Marcel Dekker; 1998. pp. 103–114. [Google Scholar]
- Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncol. 2010;11:627–636. doi: 10.1016/S1470-2045(10)70106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Kwak HB, Lawler JM. Exercise training attenuates age-induced changes in apoptotic signaling in rat skeletal muscle. Antioxid Redox Signal. 2006;8:517–528. doi: 10.1089/ars.2006.8.517. [DOI] [PubMed] [Google Scholar]
- Visser M, Langlois J, Guralnik JM, Cauley JA, Kronmal RA, Robbins J, et al. High body fatness, but not low fat-free mass, predicts disability in older men and women: the Cardiovascular Health Study. Am J Clin Nutr. 1998;68:584–590. doi: 10.1093/ajcn/68.3.584. [DOI] [PubMed] [Google Scholar]
- Weisinger RS, Begg DP, Jois M. Antagonists of the renin–angiotensin system and the prevention of obesity. Curr Opin Investig Drugs. 2009;10:1069–1077. [PubMed] [Google Scholar]
- Weisinger RS, Stanley TK, Begg DP, Weisinger HS, Spark KJ, Jois M. Angiotensin converting enzyme inhibition lowers body weight and improves glucose tolerance in C57BL/6 J mice maintained on a high fat diet. Physiol Behav. 2009;98:192–197. doi: 10.1016/j.physbeh.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Yu BP, Masoro EJ, Murata I, Bertrand HA, Lynd FT. Life span study of SPF Fischer 344 male rats fed ad libitum or restricted diets: longevity, growth, lean body mass and disease. J Gerontol. 1982;37:130–141. doi: 10.1093/geronj/37.2.130. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Scarpace PJ. The role of leptin in leptin resistance and obesity. Physiol Behav. 2006;88:249–256. doi: 10.1016/j.physbeh.2006.05.038. [DOI] [PubMed] [Google Scholar]