Abstract
Immunosenescence is characterized by phenotypic and functional changes of effector memory T cells. In spite of the well-described senescent defects of these experienced T cells, immune responses to new pathogens are also deeply affected in elderly humans, suggesting that naive T cells could also show age-related defects. It has been reported in both, animal models and humans, alterations of the naive T cell turnover associated to advanced age or low thymic function. However, as far as we know, homeostatic mechanisms involved in the deregulation of naive T cell peripheral dynamics and their consequences are still not well understood. Thus, the aim of our study was to analyze homeostatic parameters of peripheral naive T cells and their relationship with thymic function in young and elderly humans. Our results show that lower naive T cell numbers were associated with a lower thymic function and higher activation and proliferating naive T cell levels. We then analyzed sjTREC numbers and relative telomere length from sorted naive T cells. Our results show that the aberrant activation and proliferation status was related to lower sjTREC numbers (a peripheral proliferation marker) and both, higher CD57 expression levels and shortened telomeres (replicative senescence-related markers). Elderly individuals show a greater contraction of the CD8 naive T cell numbers and all homeostatic alterations were more severe in this compartment. In addition, we found that low functional thymus show a CD4-biased thymocyte production. Taken together, our results suggest a homeostatic deregulation, affecting mostly the naive CD8 T cell subset, leading to the accumulation of age-associated defects in, otherwise, phenotypically naive T cells.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-010-9170-8) contains supplementary material, which is available to authorized users.
Keywords: Aging, Naive T cells, Homeostatic proliferation, Human thymus, Immunosenescence
Introduction
Immunosenescence includes all age-related changes in the immune system (Aw et al. 2007; Gruver et al. 2007). Thymic involution causes a continuous drop in the recent thymic emigrants (RTEs) output (Chiodi 1940; Haynes et al. 2000), while homeostatic mechanisms attempt to maintain constant peripheral T cell numbers (Almeida et al. 2005). T cell aging alterations involve phenotypic changes (Junge et al. 2007; Czesnikiewicz-Guzik et al. 2008) and diminished function (Aspinal 2003; Linton and Dorshkind 2004). Effector memory T cells show proliferation defects, replicative senescence-related markers, as CD57, and shorter telomere length (Pawelec et al. 2004). Aberrant immune activation, due to cytomegalovirus (CMV) infection, seems to drive these alterations (Pawelec et al. 2006).
In spite of the widely described effector T cell defects, elderly immune responses to new pathogens are more affected than responses to previously encountered ones (Miller 1996; Akbar and Fletcher 2005) suggesting naive T cells age-related defects. Some studies have described alterations of the naive T cell turnover but whether aging influences the naive pool is only partially understood.
Along this line, the correlation between the age-related contraction of naive T cells and the rise of the naive CD8 T cell proliferation in primates has been reported, while mouse models have shown naive T cell activation in absence of thymic output (Cicin-Saint et al. 2007; Bourgeois et al. 2008). In humans, CD4-increased naive T cells proliferation has been reported in people over 65 and thymectomized children (Naylor et al. 2005; Prelog et al. 2009). The partial lymphopenia due to the loss of thymic function is believed to increase these proliferation rates (Kohler and Thiel, 2008; Nickolich-Zugich 2008). Moreover, percentage of CD4+CD31+ RTEs diminishes with age, while the percentage CD31− non-RTEs remains constant; suggesting that peripheral mechanisms could be influencing naive T cells dynamics (Kilpatrick et al. 2008). On the other hand, naive T cells transferred into T cell-deficient hosts proliferate until the replenishment of the peripheral T cell pool (Ernst et al. 1999). This proliferation, driven by the sense of an immune space due to high survival signals and loss of MHC contacts (Takada and Jameson, 2009), can be avoided when adding a higher concentration of competitor T cells (Jameson 2002; Li et al. 2007).
All these data suggest a relationship between the loss of thymic output and peripheral dynamics of naive T cells. However, homeostatic mechanisms involved in this deregulation are still only partially understood. Thus, the aim of this study was to analyze homeostatic parameters of peripheral naive T cells and their relationship with the remaining thymic function in elderly humans.
Materials and methods
Study subjects Twenty-eight consecutive individuals who underwent cardiac surgery (valvular repair or ischemic cardiopathy) at Virgen del Rocio University Hospital in Seville, Spain, between May 2007 and March 2008 were included. Thymic tissue and peripheral blood samples were simultaneously collected from each individual. The median age of this group was 69.1 years (interquartile range (IQR; 61.0–74.6) and all of them were over 50 years. Peripheral blood samples were collected in the same period of time from 16 young healthy volunteers (median age = 32.8 years, IQR (28.1–42.1)). Since 50 years old has been considered a critical period in thymic functionality (Nobile et al. 2004), the young control group was selected under 50 years. None of the study subjects had received any treatment that could influence their immune status and there were no clinical data of active infections, including an HIV-negative test. All of them signed an informed consent and the study was approved by the Ethical Committee of the Hospital.
Thymocytes and peripheral blood mononuclear cells isolation Thymic tissue samples were extracted during cardiac surgery procedures and immediately processed. As previously confirmed, the aged thymus in these subjects remained clearly distinguishable despite the adipose tissue of the mediastinum. The existence of Hassall's Corpuscles even in tissues showing 0% DP guarantee the accuracy of thymus identification and representative sample taking (Ferrando-Martinez et al. 2009). Thymic tissue was disaggregated with a wire mesh. Thymocytes were isolated by Ficoll–Hypaque density gradient, washed twice with PBS and immediately stained for flow cytometry analysis. Peripheral blood mononuclear cells (PBMCs) were isolated in vacutainer CPT™ tubes with sodium heparin as anticoagulant and stained for flow cytometry or cryopreserved in liquid nitrogen (fetal bovine serum/10% DMSO) until further use. Blood samples and tissues were only obtained for clinical reasons, and never for research purposes.
CMV serostatus determination Presence of CMV IgG antibodies were determined in duplicate from criopreserved serum samples using the CMV IgG Enzyme Immunoassay Test Kit (GenWay, San Diego, CA) according to manufacturers’ instructions.
IL-7 quantification Interleukin (IL)-7 plasmatic levels were performed in duplicate using a highly sensitive colorimetric enzyme-linked immunoabsorbent assay (IL-7 Quantikine ELISA kit, R&D systems, MN), according to manufacturers' instructions. Cytokine concentration of each duplicate sample was extrapolated from the standard curve and calculated using the mean value.
Flow cytometry CD8+ and CD4+ absolute numbers were determined in fresh whole blood using the Epics XL-MCL (Beckman Coulter Inc., CA) flow cytometer, according to the manufacturers’ instructions. Frequency of double positive (DP, CD4+CD8+) cells was determined in fresh thymocytes by flow cytometry using anti-CD4 FITC and anti-CD8 PE monoclonal antibodies (Becton Dickinson, San Jose, CA). CD4+ and CD8+ peripheral T cell subsets were positively selected from fresh PBMCs using magnetic microbeads and MACS® Cell Separation Reagents (Miltenyi Biotec, Germany) according to the manufacturers' instructions. CD4+ and CD8+ isolated T cells were then separately stained using anti-CD45RA FITC, anti-CD45RO PE-Cy7 (Becton Dickinson, San Jose, CA) and anti-CD57 FITC, anti-CD38 PE, anti-CD45RA ECD, anti-HLADR ECD, anti-CD27 PE-Cy5 (Beckman Coulter, FL) monoclonal antibodies. T cell subsets were defined as follows: naive T cells CD45RA+CD27+; memory T cells CD45RO+CD27+; effector T cells CD45RA+/-CD27-. The accuracy of these phenotypes was recently reported (Ferrando-Martinez et al. 2010a). The percentage of proliferating naive T cells (CD45RA+CD27+Ki67+) was determined by surface staining (CD45RA+CD27+) and then by intracellular immunostaining with anti-Ki67 PE (Becton Dickinson, San Jose CA) monoclonal antibody. Cells were permeabilized using the Cytofix/Citoperm, Perm/Wash and Cytoperm plus buffers (BD biosciences).
Cell sorting Cryopreserved PBMCs were stained using anti-CD45RA FITC, anti-CD4 PE, anti-CD27 PE-Cy5 and anti-CD8 PE-Cy7 monoclonal antibodies (Becton Dickinson, San Jose, CA). Naive CD8 (CD8+CD45RA+CD27+) and CD4 (CD4+CD45RA+CD27+) T cells were isolated using a MoFlo™ Cell sorter (Beckman Coulter Inc., CA). Purity over 97% was routinely tested by flow cytometry. DNA from each sample was extracted using the QIAmp DNA Micro Kit (QIAGEN, Chatsworth, CA) according to manufacturers' instructions.
Signal-joint T cell Receptor Excision Circle levels Signal-joint T cell Receptor Excision Circle (sj-TREC) numbers were analyzed in sorted naive T cells. Delta-deletion TRECs formed by δRec-ψJα rearrangement were amplified and quantified by real-time polymerase chain reaction (PCR) using fluorescently labeled oligonucleotides as reporter probes in a 20 μL PCR reaction using the Light-Cycler® 2.0 (Roche Diagnostics, IN) as previously described (Douek et al. 1998). TREC abundance was normalized to cell number by amplification of β-globin with the GH20 and PC04 primers (Bauer et al. 1991). Serial dilutions of plasmid clones containing TRECs or β-globin were run to generate standard curves (Franco et al. 2002). Data were expressed as TRECs per naive T cell using mean values from triplicate assays for TRECs and duplicate assays for β-globin.
Relative telomere length quantification Naive T cell telomeric sequences were amplified by real-time PCR using the Light-Cycler® 480 as described elsewhere (Cawthon 2002; Kilpatrick et al. 2008). Isolated cord blood naive T cells and a no-template control were included in each PCR. β-globin quantification was used to normalize DNA amounts. Samples lengths are expressed as percentage of cord blood naive T cells length.
Statistical analysis Continuous variables are expressed as median (IQR) and categorical ones as percentage. Pearson's test was used to analyze correlations between Gaussian variables and Spearman's test was used to analyze correlations between non-Gaussian variables. Differences among categorical variables were analyzed using the Chi-square test. The Mann–Whitney U test was used to analyze differences between continuous variables. Statistical analysis was performed using the Statistical Package for the Social Sciences software (SPSS 17.0, Chicago, IL).
Results
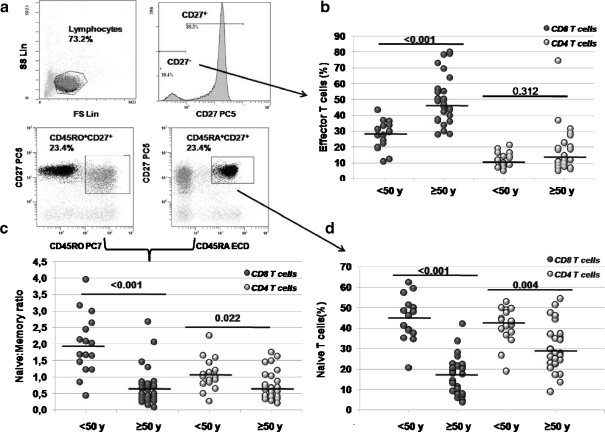
Age-related changes of the T-lymphocytes subsets Forty-four individuals were analyzed and divided in two groups (<50 and ≥ 50 years) according to their ages. Characteristics of the cohort are summarized in Table 1. In the elder group, CD8 absolute counts showed a significant reduction, whereas CD4 T cells numbers were similar. CD4 and CD8 T cells were then isolated and T-lymphocyte subsets were analyzed, by flow cytometry, as shown in Fig. 1a. Percentage of effector T cells (as defined by CD4+CD27- or CD8+CD27−) was higher in the elder group, showing statistical significance in CD8 T cells (Fig. 1b). The peripheral naive to memory T cell ratio from elder individuals was decreased in both, CD4 and CD8 subsets but, once again, the contraction was greater in CD8 T cells (Fig. 1c). Decrease in the naive/memory ratio could be explained by increased percentages of memory T cells but also by a naive T cell drop. In our cohort, memory T cells were not increased in elder individuals (data not shown). However, naive T cells showed a steep drop with age. As expected after the naive/memory results, the decrease was higher in the naive CD8 T cell compartment (Fig. 1d).
Table 1.
Characteristics of the cohort
| Total | < 50 years | ≥ 50 years | p | |
|---|---|---|---|---|
| Number of patients | 44 | 16 | 28 | |
| Age (years)a | 44.0 (31.7–64.0) | 32.8 (28.1–42.1) | 69.1 (61.0–74.6) | <0.001 |
| Sex (% males) | 26/44 (59.1) | 9/16 (56.3) | 17/28 (60.7) | 0.509 |
| CMV (% positive) | 39/44 (86.6) | 12/16 (75) | 27/28 (96.4) | 0.051b |
| Lymphocytes (cells/mm3)a | 1,456 (1,152–1,805) | 1,891 (1,344–2,109) | 1,391 (1,016–1,630) | 0.013 |
| CD4 (cells/mm3)a | 698 (571–844) | 699 (642–941) | 694 (504–833) | 0.197 |
| CD8 (cells/mm3)a | 345 (240–504) | 507 (342–588) | 288 (220–430) | 0.001 |
aMedian (IQR)
bChi-square test
Fig. 1.
a Representative flow cytometry strategy used to discriminate T cell subsets. CD8+ or CD4+ isolated cells were selected by their forward and side scatter profile. Phenotypes were determined as follows: effector T cells as CD45RA+/−CD27−; memory T cells as CD45RO+CD27+ and naive T cells as CD45RA+CD27+. Differences among the age groups in the b effector subsets, c naive to memory peripheral T cell ratio, and d naive subsets were then analyzed in both, CD8+ and CD4+ T cells
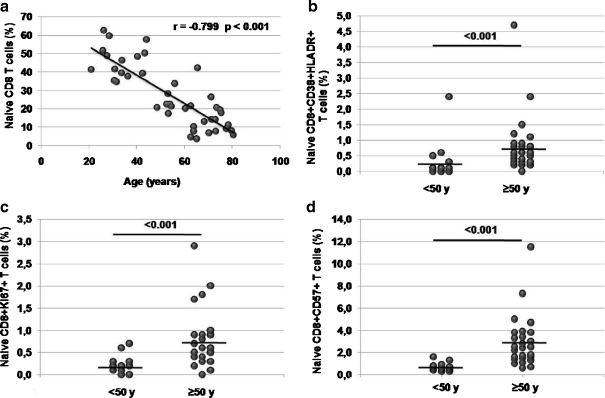
Analysis of naive T cells homeostatic parameters Results showed a strong age-related naive T cell drop in both, CD8 (Fig. 2a) and CD4 (Electronic supplementary Figure S1a) T cell subsets. Despite the T cell number decrease, IL-7 plasmatic concentrations were similar in both groups (15.7 pg/mL IQR[11.7–19.3] vs. 14.0 pg/mL IQR[10.8 –18.1] p = 0.434, Mann–Whitney U test). IL-7 provides survival signals on naive T cells but animal models showed that, when IL-7-derived signal is stronger, this could lead to activation and proliferation of naive T cells (Takada and Jameson, 2009). Thus, we analyzed whether the drop in naive T cell numbers without IL-7 levels reduction could alter their homeostatic parameters. In order to check the homeostatic parameters of the naive T cell subset we analyzed activation (CD38+HLADR+), proliferating (Ki67+) and replicative senescent (CD57+) naive T cell levels in both age groups. We found higher levels of activation, CD57 expression and proliferating naive CD8 T cells in the elder group (Fig. 2b–d). Percentage of peripheral naive CD8 T cells were directly correlated with the percentage of DP thymocytes (Fig. 4b) but showed inverse correlations with percentages of activation, Ki67 and CD57 expression. In addition, statistical significant relationships were found between all the homeostatic parameters (Table 2). A similar, but weaker, age-related rise was found at the CD4 compartment. Both, levels of activation and CD57 expression, were significantly higher at the elder group while proliferating naive CD4 T cell levels remained unaltered (Electronic supplementary Figure S1b). Correlations between the percentage of naive T cells and both, percentage of DP or activation levels, were lost. However, a decrease of the naive T cell numbers was significantly associated with an increase in proliferation and replicative senescence levels. In addition, higher naive CD4 T cell proliferating levels were associated with higher CD57 expression (Electronic supplementary Table S1). These results suggest a failure of the naive T cell homeostasis, with a different regulation of the naive CD4 and CD8 T cell compartments, with a greater deregulation of the naive CD8 T cells.
Fig. 2.
a Age-related drop of naive CD8 T cells. Differences in percentages of b activation (CD38+HLADR+ expressing T cells); c proliferation (Ki67+ expressing T cells) and d replicative senescence (CD57+ expressing T cells) naive CD8 T cells among the age groups
Fig. 4.
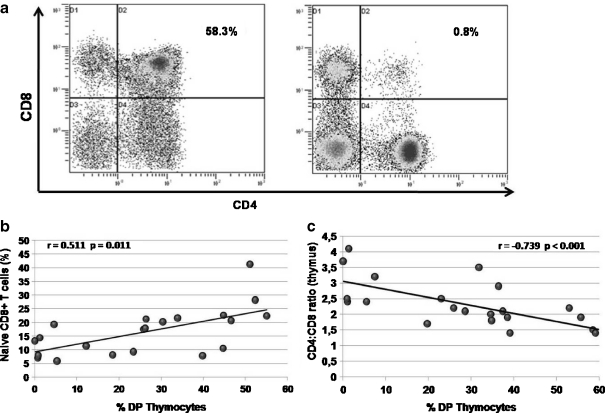
a Representative flow cytometry plots showing CD4+CD8+ double positive thymocyte staining for high (left) and low (right) thymic function. b Relationship between percentage of peripheral naive CD8+ T cells and percentages of double positive (DP) thymocytes. c Relationship between thymic functionality (as measured by percentage of DP thymocytes) and the intrathymic CD4/CD8 single positive thymocytes ratio
Table 2.
Relationships between homeostatic parameters and the naive CD8+ T cell subset
| % DP | % CD38+HLADR+ expression | % Ki67+ expression | % CD57+ expression | |
|---|---|---|---|---|
| % CD45RA+CD27+ | r = 0.511 | r = −0.485 | r = −0.720 | r = −0.827 |
| Naive T cells | p = 0.001 | p = 0.001 | p < 0.001 | p < 0.001 |
| % CD38+HLADR+ expression | NS | – | r = 0.548 | r = 0.476 |
| p = 0.001 | p = 0.002 | |||
| % Ki67+ expression | NS | – | – | r = 0.635 |
| p < 0.001 |
p = Spearman's test
NS non-significant association
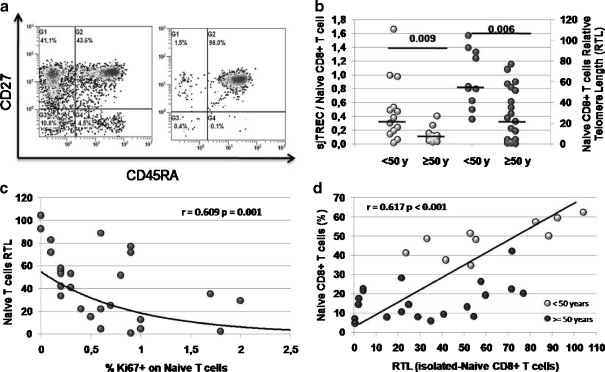
Homeostatic defects on naive T cells Aberrant and sustained activation and proliferation rates lead to immunosenescence defects on highly differentiated effector T cells. Analyzing the naive CD8 T cell compartment we have found that abnormal activation and proliferation rates are associated with higher CD57 expression, a replicative senescence marker (Fig. 2d and Table 2). To determine their replicative history, DNA extracted from cell-sorter isolated CD8+CD45RA+CD27+ naive T cells (Fig. 3a) was analyzed to determine sjTREC counts and telomere length. As shown in Fig. 3b, both sjTREC numbers per naive T cell and telomere length were significantly reduced in the elder group. Diminished sjTREC numbers are an indirect measure of proliferation, since telomere length also indicates replicative senescence. In addition, higher proliferation rates were associated with shorter telomere length (Fig. 3c); all results suggesting that defects are due to proliferation in phenotipically naive T cells. Moreover, analyzing the relationship between shortened telomere lengths (a replicative defect) and the percentage of naive CD8 T cells we found that both parameters were closed related (Fig. 3d). When analyzing the naive CD4 T cell subset, telomere length did not show a significant correlation with proliferation levels (data not shown). However, we also found low sjTREC numbers and shortened telomere length in CD4 naive T cells from elder individuals (Electronic supplementary Figure S1c). In addition, percentage of peripheral naive CD4 T cells showed an association with the telomere length, but it was weaker than the association found in the CD8 subset (Electronic supplementary Figure S1d). Taken together, these results suggest that higher activation and proliferation rates shown in elderly naive T cells could affect both, phenotype (by increasing CD57 expression) and replicative history (as showed by decreased sjTREC numbers and smaller telomere length) thus, probably affecting their functionality.
Fig. 3.
a Representative flow cytometry plot before (left) and after (right) cell sorting. Cells were selected from the CD4+ or CD8+ gate and then analyzed for their CD45RA and CD27 expression. High purity cell sorting (right) was routinely obtained. b sj-TREC levels per naive CD8+ T cell (grey circles) and Relative naive CD8+ T cell Telomere Length differences (black circles) among the age groups. c Relationship between proliferation rates and telomere lengths in naive CD8+ T cells. d Relationship between peripheral percentages of naive CD8+ T cells and their Relative Telomere Length
Age-related thymic alterations on CD8 T cell production Naive CD8 T cell subset showed a greater age-related reduction than CD4 T cells (Fig. 1d). As previously showed (Ferrando-Martinez et al. 2009) elderly individuals show a wide range of thymic functionality that directly affects the naive T cell pool. Percentages of DP thymocytes were analyzed as thymic function gold standard and a representative flow cytometry example is shown as Fig. 4a. Consistent with our results, Fig. 4b shows the relationship between the percentages of naive CD8 T cells and DP thymocytes. Interestingly, naive CD4 T cell levels, which were better preserved, did not show a relationship with the percentage of DP thymocytes (Electronic supplementary Table S1).
A quantitative reduction of RTEs, without any other change in the thymocyte distribution, would lead to a similar reduction in both, CD8 and CD4 naive T cells. To analyze whether differences on thymic production could affect peripheral dynamics we evaluated thymocyte distribution in elder individuals. Percentage of DP thymocytes from our old group showed an age-related reduction (r = −0.601 p = 0.002), in agreement with the physiologic thymic failure. A strong relationship was found between the CD4/CD8 ratio from thymic tissue and the percentage of DP thymocytes (Fig. 4c). Interestingly, all subjects with a very low thymic function, as defined by percentage of DP less than 10%, showed CD4/CD8 ratios higher than two. However, individuals with intermediate thymic function (percentage of DP between 10% and 40%) also had intermediate CD4/CD8 ratios, while higher thymic function (as defined by percentage of DP higher than 40%) showed lower ratios, with similar CD4 and CD8 T cell production. These results suggest that elderly thymic output could be biased to CD4 production.
Discussion
Results of this study show an age-related homeostatic deregulation of the naive T cell compartment. The age-related loss of thymic function leads to decreased naive T cell numbers that are related to higher activation and proliferation rates (mainly in the CD8 T cell compartment) in elderly subjects. Consistent with greater proliferative history, naive T cells from elderly subjects have less sjTREC numbers and shorter telomere lengths. Notably, downsized telomere length (also considered a replicative senescence-related marker) correlates with both, higher proliferation rates and lower percentages of naive CD8+ T cells. Naive CD4+ T cells, with better preserved numbers in elderly individuals showed, to a lesser extent, a similar deregulated phenotype.
The great rise of CD8+ effector cells could be explained by the well known accumulation of clonal populations in elderly people (mostly CMV-specific effector cells), which are senescent and apoptosis-resistant (Pawelec et al. 2004). Focusing on naive T cells, alterations of the naive/memory T cell ratio has been described in elderly individuals and some immune diseases (Hara et al. 2007; Gress and Deeks, 2009). In our cohort, memory T cell numbers did not show an age-related drop, so this ratio was diminished mainly because of the naive T cell loss. In spite of elderly impaired responses to new pathogens, involving naive rather than effector T cells have been described, as far as we know, aged naive T cells have still not been reported.
Naylor et al. (2005) firstly reported an age-related naive homeostatic alteration by showing the CD4 T cell repertoire contraction in very old subjects. This interesting study reports an abrupt drop of naive CD4 T cell receptor diversity in people over 75 but, surprisingly, minimal thymic function in 55-year-old people while we show higher heterogeneity of thymic function and earlier naive T cell age-related defects. These differences are probably due to discrepant thymic function measurements and naive T cell definitions, since Naylor et al. defined the naive T cell subset as CD45RA+, while an age-related accumulation of terminally differentiated effector T cells that express CD45RA has been described (Czesnikiewicz-Guzik et al. 2008).
In non-human primate models Cicin-Saint et al. (2007)) showed an age-related homeostatic increase in both, CD4 and CD8 naive T cell pools. The increase in proliferation rates were strongly related to the naive T cell pool contraction in the CD8 subset. Moreover, Prelog et al. (2009) recently reported that young adults thymectomized in their childhood (an informative group of premature immunosenescence) showed higher rates of naive CD4 T cell proliferation that, eventually, leads to diminished sjTREC numbers at the CD4 naive T cell pool. This study indirectly showed, for the first time in humans, the potential relationship between the loss of thymic function and increased naive turnover rates at the CD4 T cell subset. However, sjTRECs is now preferentially used as peripheral proliferation rather than thymic function measurement (Harris et al. 2005 and Ferrando-Martinez et al. 2010b). Finally, despite the principal age-related impairment is showed at the CD8 T cell subset (Pawelec et al. 2004), human studies are focused on naive CD4 T cells.
Despite the methodological differences, in agreement with these elegant previous studies, we found alterations at the naive T cell compartment that are consistent with antigen-independent homeostatic proliferation, leading to senescence defects. The slight lymphopenia shown in elderly people reduces self-MHC contacts, needed for naive homeostasis (Takada and Jameson, 2009). Besides, IL-7 availability is maintained, or even increased (Ferrando-Martinez et al. 2009). Since naive T cell compartment is independently regulated, less MHC contacts, and stronger IL-7-derived survival signals (described as sense of a naive immune space) allow naive T cells to proliferate in a similar way as naive T cells transferred to lymphopenic hosts do (Ernst et al. 1999).
As a consequence of higher proliferation rates, naive T cells show higher percentages of replicative senescence markers (CD57), shorter telomeres and low sj-TREC numbers. Despite CD57 expression having long been assumed a replicative senescence marker (Brenchley et al. 2003; Pawelec et al. 2006; Czesnikiewicz-Guzik et al. 2008), some studies focusing on chronic infections have reported that CD57 expression correlates high effector capacities (Chattopadhyay et al. 2009; Barbour et al. 2009). Independently of their effector capacities, naive T cells need high proliferation rates to favor a correct immune response. Thus, replicative senescent naive T cells could be considered as non-functional T cells.
Besides, both, low sj-TREC numbers and short telomere lengths are associated with high proliferation rates. A recent murine model shows that short telomeres are enough to induce age-associated defects, resembling those seen in dyskeratosis congenital (Armanios et al. 2009). Thus, results suggest that replicative senescent naive T cells could be functionally defective. In this work we used a relative telomere length (RTL) measurement technique previously reported (Cawthon 2002). Even if this method is not as precise as the quantitative ones, we only wanted to point out differences between two age groups, and our results are independent of the absolute length of the telomeric sequences. Therefore we think that the RTL method is accurate enough for this purpose.
We proposed a dysfunctional aged thymus that could be biased to naive CD4 T cell production. If thymic output is simply reduced, both CD4 and CD8 naive T cells should be identically reduced. However, the naive CD8 T cell subset is more affected. When analyzing thymic production, thymi with higher percentage of DP thymocytes (fully or intermediate functional thymi) showed a CD4/CD8 single positive thymocyte ratio between one and two. Low function-thymi showed intrathymic ratio values twofold higher. Higher death rates shown by peripheral senescent CD4 T cells, but apoptotic-resistant clonal peripheral CD8 T cells, could be affecting thymic production. In this way, accumulation of elderly senescent T cells, which reverse the CD4/CD8 peripheral T cell ratio (Pawelec et al. 2004), could be forcing a higher CD4 thymic production. However, additional mechanisms could also be contributing. Since naive CD8 T cells show a faster turnover rates, a higher naive to memory conversion could be expected at the CD8 subset. In spite of not observing a higher increase of CD8 memory T cells among the age groups (data not shown) a faster homeostatic memory conversion could not be discarded. Another possible explanation is a higher CD8 naive T cells apoptotic death. Unfortunately, as a limitation of the study, we do not dispose of apoptotic rates or PD-1 expression (Petrovas et al. 2009) from CD4 and/or CD8 T cells to clarify this hypothesis and further research is necessary to really clarify this point.
Some authors point out that using CD45RA+CCR7+CD27+ complete naive T cell phenotype is necessary to fully describe this subset. However, due to technical issues, a two-marker simplified phenotype is usually analyzed. Recently, we have reported that the three-marker phenotype is necessary to discriminate intermediate or central memory subsets, but naive T cells are accurately defined by the phenotype used in this study (CD45RA+CD27+) (Ferrando-Martinez et al. 2010a).
In conclusion, we show an age-related homeostasis deregulation, leading to accumulation of age-associated defects on phenotipically naive T cells. Functionally defective naive T cells, added to greatly diminished numbers, could be an important cause of elderly impaired responses to newly encountered pathogens. However, further in vitro functional studies are necessary to guarantee that phenotipically senescent naive T cells show loss of functionality.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Age-related drop of naive CD4 T cells. B) Differences in percentages of activation (CD38+HLADR+ expressing T cells, dark grey circles), replicative senescence (CD57+ expressing T cells, black circles) and proliferating (Ki67+ expressing T cells, light grey circles) naive CD4 T cells among the age groups. C) sj-TREC levels per naive CD4+ T cell between the age groups (grey circles) and Relative naive CD4+ T cell Telomere Length differences between the age groups (black circles). D) Relationship between peripheral percentage of Naive CD4+ T cells and their Relative Telomere Length. (GIF 0 kb)
(DOC 30 kb)
Acknowledgements
Authors want to thank Dr. Ricardo Pardal and the Laboratorio of Investigaciones Biológicas (LIB) for their priceless help in cell sorting. We also want to thank all the Cardiac surgery staff for their kindness and patience. SFM and ERM have grants from the Fondo de Investigaciones Sanitarias (FIS06/00176 and CP08/00172, respectively). This study is supported by Redes Temáticas de Investigación en SIDA (ISCIII RETIC RD06/0006/0021), Redes Temáticas de Cardiovascular (ISCIII RECAVA RD06/0014), Proyecto de Excelencia, Consejería de Innovación, Ciencia y Empresa (P06-CTS-01579) and Consejería de Salud, Servicio Andaluz de Salud (156/2006 and PI0366/07).
References
- Akbar AN, Fletcher JM. Memory T cell homeostasis and senescence during aging. Curr Opin Immunol. 2005;17:480–485. doi: 10.1016/j.coi.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Almeida AR, Rocha B, Freitas AA, Tarichot C. Homeostasis of T cell numbers: from thymus production to peripheral compartimentalization and the indexation of regulatory T cells. Semin Immunol. 2005;17:239–249. doi: 10.1016/j.smim.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Armanios M, Alder JK, Parry EM, Karim B, Strong MA, Greider CW. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am J Hum Gen. 2009;85:823–832. doi: 10.1016/j.ajhg.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspinal R. Age-related changes in the function of T cells. Microsc Res Tech. 2003;62:508–513. doi: 10.1002/jemt.10412. [DOI] [PubMed] [Google Scholar]
- Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120:435–446. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour JD, Ndhlovu LC, Xuan Tan Q, Ho T, Epling L, Bredt BM, Levy JA, Hecht FM, Sinclair E. High CD8+ T cell activation marks a less differentiated HIV-1 specific CD8+ T cell response that is not altered by suppression of viral replication. PLoS One. 2009;4:e4408. doi: 10.1371/journal.pone.0004408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer HM, Ting Y, Greer C, Chambers JC, Tashiro CJ, Chimera J, Reingold A, Manos MM. Genital human papillomavirus infection in female university student as determined by PCR-based method. JAMA. 1991;265:472–477. doi: 10.1001/jama.265.4.472. [DOI] [PubMed] [Google Scholar]
- Bourgeois C, Hao Z, Rajewsky K, Potocnik AJ, Stockinger B. Ablation of thymic export causes accelerated decay of naive CD4 T cells in the periphery because of activation by environmental antigen. Proc Natl Acad Sci USA. 2008;105:8691–8696. doi: 10.1073/pnas.0803732105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, Roederer M, Douek DC, Kroup RA. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8 T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay PK, Betts MR, Price DA, Gostick E, Horton H, Roederer M, Rosa SC. The cytolytic enzymes granzyme A, granzyme B, and perforina: expression patterns, cell distribution, and their relationship to cell maturity and bright CD57 expression. J Leukoc Biol. 2009;85:88–97. doi: 10.1189/jlb.0208107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodi H. The relationship between the thymus and the sexual organs. Endocrinology. 1940;26:107–116. doi: 10.1210/endo-26-1-107. [DOI] [Google Scholar]
- Cicin-Saint L, Messaoudi I, Park B, Currier N, Planer S, Fischer M, Tackitt S, Nikolich-Zugich D, Legasse A, Axthelm MK, Picker LJ, Mori M, Nikolich-Zugich J. Dramatic increase in naive T cell turnover is linked to loss of naive T cells from old primates. Proc Natl Acad Sci USA. 2007;104:19960–19965. doi: 10.1073/pnas.0705905104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, Ouslander JG, Weyand CM, Goronzy JJ. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, Polis MA, Haase AT, Feinberg MB, Sullivan JL, Jamieson BD, Zack JA, Picker LJ, Koup RA. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/S1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- Ferrando-Martinez S, Franco JM, Hernandez A, Ordoñez A, Gutierrez E, Abad A, Leal M. Thymopoiesis in elderly human is associated with systemic inflammatory status. AGE. 2009;31:87–97. doi: 10.1007/s11357-008-9084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrando-Martinez S, Ruiz-Mateos E, Leal M. CD27 and CCR7 expression on naive T cells, are both necessary? Immunol Lett. 2010;127:157–158. doi: 10.1016/j.imlet.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Ferrando-Martinez S, Franco JM, Ruiz-Mateos E, Hernández A, Ordoñez A, Gutierrez E, Leal M. A reliable and simplified sj/beta-TREC ratio quantification method for human thymic output measurement. J Immunol Methods. 2010;352:111–117. doi: 10.1016/j.jim.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Franco JM, Rubio A, Martinez-Moya M, Leal M, Merchante E, Sánchez-Quijano A, Lissen E. T-cell repopulation and thymic volumen in HIV-1 infected adult patients afther highly active antiretroviral therapy. Blood. 2002;99:3702–3706. doi: 10.1182/blood.V99.10.3702. [DOI] [PubMed] [Google Scholar]
- Gress RE, Deeks SG. Reduced thymus activity and infection prematurely age the immune system. J Clin Invest. 2009;119:2884–2887. doi: 10.1172/JCI40855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of aging. J Pathol. 2007;211:144–156. doi: 10.1002/path.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M, Matsuzaki Y, Shimizu T, Tomita M, Ayabe T, Enomoto Y, Onitsuka T. Preoperative peripheral naive/memory ration and prognosis of nonsmall-cell lung cancer patients. Ann Thorac Cardiovasc Surg. 2007;13:384–390. [PubMed] [Google Scholar]
- Haynes BF, Market ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation and HIV-1 infection. Annu Rev Immunol. 2000;18:529–560. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- Harris JM, Hazenberg MD, Poulin JF, Higuera-Alhino D, Schmidt D, Gotway M, McCune JM. Multiparameter evaluation of human thymic function: interpretations and caveats. Clin Immunol. 2005;115:138–146. doi: 10.1016/j.clim.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- Junge S, Cloeckener-Gruissem B, Zufferey R, Keisker A, Salgo B, Fauchere JC, Scherer F, Shalaby T, Grotzer M, Siler U, Seger R, Güngör T. Correlation between recent thymic emigrants and CD31+ (PECAM-1) CD4+ T cells in normal individuals during aging and in lymphopenic children. Eur J Immunol. 2007;37:3270–3280. doi: 10.1002/eji.200636976. [DOI] [PubMed] [Google Scholar]
- Kilpatrick RD, Rickabaugh T, Hultin LE, Hultin P, Hausner MA, Detels R, Phair J, Jamieson BD. Homeostasis of the naive CD4+ T cell compartment during aging. J Immunol. 2008;180:1499–1507. doi: 10.4049/jimmunol.180.3.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S, Thiel A. Life after the thymus, CD31+ and CD31- human naive CD4+ T-cell subsets. Blood. 2008;113:769–774. doi: 10.1182/blood-2008-02-139154. [DOI] [PubMed] [Google Scholar]
- Li CR, Santoso S, Lo DD. Quantitative analysis of T cell homeostatic proliferation. Cell Immunol. 2007;250:40–54. doi: 10.1016/j.cellimm.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton PJ, Dorshkind L. Age related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- Nickolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile M, Correa R, Borghans JA, D’Agostino C, Schneider P, Boer RJ, Pantaleo G, Swiss HIV Cohort Study De novo T cell generation in patients at different ages and stages of HIV-1 disease. Blood. 2004;104:470–477. doi: 10.1182/blood-2003-12-4265. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Akbar A, Caruso C, Effros R, Grucbeck-Loebenstein B, Wikby A. Is immunosenescence infectious? Trends Immunol. 2004;25:406–410. doi: 10.1016/j.it.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Koch S, Gouttefangeas C, Wikby A. Immunorejuvenation in the elderly. Rejuvenation Res. 2006;9:111–116. doi: 10.1089/rej.2006.9.111. [DOI] [PubMed] [Google Scholar]
- Petrovas C, Chaon B, Ambrozak DR, Price DA, Melenhorst JJ, Hill BJ, Geldmacher C, et al. Differential association of programmed death-1 and CD57 with ex vivo survival of CD8 T cells in HIV infection. J Immunol. 2009;183:1120–1132. doi: 10.4049/jimmunol.0900182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelog M, Keller M, Geiger R, Brandstätter A, Würzner R, Schweigmann U, Zlamy M, Zimmerhackl LB, Grubeck-Loebenstein B. Thymectomy in early childhood: significant alterations of the CD4(+)CD45RA(+)CD62L(+) T cell compartment in later life. Clin Immunol. 2009;130:123–132. doi: 10.1016/j.clim.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nat Rev Immunol. 2009;9:823–832. doi: 10.1038/nri2657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
Age-related drop of naive CD4 T cells. B) Differences in percentages of activation (CD38+HLADR+ expressing T cells, dark grey circles), replicative senescence (CD57+ expressing T cells, black circles) and proliferating (Ki67+ expressing T cells, light grey circles) naive CD4 T cells among the age groups. C) sj-TREC levels per naive CD4+ T cell between the age groups (grey circles) and Relative naive CD4+ T cell Telomere Length differences between the age groups (black circles). D) Relationship between peripheral percentage of Naive CD4+ T cells and their Relative Telomere Length. (GIF 0 kb)
(DOC 30 kb)