Abstract
Objectives
To establish whether the provision of numerical data using pictograms and framed as event rates affects subjects' attitudes to colorectal cancer (CRC) screening.
Design
Randomized questionnaire and telephone study comparing a control group given standard NHS CRC information leaflets with an intervention group given the same leaflet but enhanced with additional numerical and pictorial information.
Setting
District General hospital and two general practices in North East England. Study carried out immediately prior to the introduction of CRC screening.
Participants
A total of 478 non-gastroenterological subjects (age range 60–70 years).
Main outcome measures
The difference in the two groups' overall wish to be screened; comparison of the impact of enhanced vs. unenhanced summary points in the NHS information leaflet; the summary point that most influenced their decision on screening; the views of the intervention group on the additional numerical and pictorial information provided.
Results
A total of 256 (54%) responded (124 from the control group and 117 from the intervention group); 22% were interviewed by telephone; 90% of the control group and 85% of the intervention group wished to be screened (P = 0.34). Provision of numerical and pictorial information significantly changed the impact of five of the six summary points on the decision to be screened. Sixty-two percent of the intervention group found the pictograms helpful while 83% of those interviewed by telephone found the numerical data helpful; 73% of the control group when given by telephone the additional numerical information given to the intervention group said this would have been useful in aiding their decision-making.
Conclusion
Providing additional numerical information would enhance the credibility of the screening programme without necessarily reducing the numbers screened.
‘How well we communicate is determined not by how well we say things but how well we are understood.’ Andrew Grove, co-founder of Intel1
Introduction
Four large randomized controlled trials have shown that biennial guaiac-based faecal occult blood testing (GFOBT) reduces colorectal cancer (CRC) mortality by 16% in the population tested and in the UK has the potential to save over 1000 deaths from CRC each year.2–6 The Minnesota group further demonstrated that the incidence of CRC (as opposed to the mortality) was reduced by continued involvement in the screening study but only 18 years after commencement of colonoscopic surveillance, the presumed mechanism being removal of pre-cancerous polyps in the screened group.7 More recently a reduction in CRC incidence and mortality at 12 years has been demonstrated using one-off flexible sigmoidoscopy.8 GFOBT, followed by colonoscopy if positive, is the current method used for CRC screening in the UK initially offered to 60–70-year-olds, with the age range now extended to 75 years.
As with all screening tools the figures are positive in terms of population benefit, but the benefit (and risk) to the individual and the individual's perception of that benefit and risk is less clear-cut, partly because of the lack of sensitivity and specificity of the screening process and partly because the majority screened will not develop CRC whether screened or not. Thus GFOBT has low sensitivity for advanced neoplasia of around 20% and as low as 13% in one study9 and in clinical trials half of cancers are missed by biennial stool testing.10 The chance of an individual benefiting from screening through prevention of death from CRC is estimated at 1 in 862.11 No overall mortality advantage has been shown in those screened, and is unlikely to be, as the screening trials target a disease that contributes a lifetime risk of only 3% to all-cause mortality11 and screening seeks only to reduce this risk in those already with precursor lesions or cancer arising in the screened age group. Assuming no untoward consequences of the screening intervention, this amounts to less than 1/2% mortality reduction (16% of 3%). No study has been powered to detect such small reductions in mortality. Furthermore the risk of a serious adverse effect such as haemorrhage or perforation in those undergoing colonoscopy, of whom only 10% will have cancer, is around 1 in 150.12,13 The current advice leaflet inviting UK subjects to partake in the CRC screening programme focuses on population benefit but does not place CRC mortality in the context of all-cause mortality12 and the absence of this and other patient-focused data lays the CRC programme open to the same adverse publicity surrounding the UK breast cancer screening programme.14 A Cochrane review of trials of decision aids showed that decision aids enhanced risk perceptions and the effect was stronger when probabilities were expressed quantitatively.15 In this study we have compared the attitudes to screening of a control cohort, given the standard NHS information leaflet, to the attitudes of an intervention group given the same information leaflet but enhanced with numerical data expressed in natural frequency format, percentages, information on prolongation of life and pictograms all aimed at the risks and benefits to the individual rather than the population as it is on the individual that the burden of decision-making falls.
Patients and methods
The study was conducted in 2008 shortly before the introduction of CRC screening in our area. Subjects were recruited from patients between the age of 60 and 69 attending non-gastroenterology hospital medical clinics and patients attending two GP surgeries. Subjects were excluded if they presented with or had gastroenterological problems, had previous colon cancer, terminal or advanced disease with limited life expectancy, or previously had a colonoscopy.
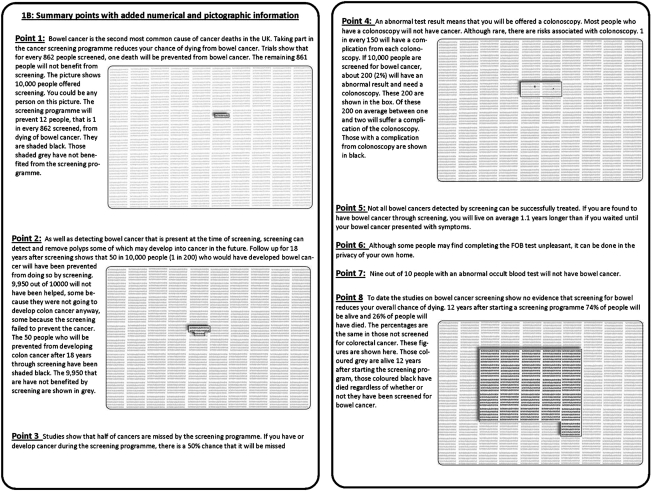
Those consenting to take part were randomized to receive one of two information packs using computer-generated pseudo-random numbers, uniformly distributed between 0 and 1 with allocation determined by whether the number was less than or greater than 0.5. Information packs were concealed in these randomly numbered plain envelopes with researchers and study coordinators blinded to their contents. The control group received the standard NHS information leaflet given to subjects invited to take part in CRC screening including the six summary points contained in the leaflet (Figure 1).12 The intervention group were given the same information but with the summary points augmented with numerical information taken from the four randomized studies of CRC screening and a meta-analysis of all studies (Figure 2).2–7,11 and supplemented with pictorial information to illustrate three of the six summary points. The enhanced information pack was based on Paling's risk charts and was tested and refined on a group of 30 subjects before starting the trial. A format of events per 10,000 subjects was used to illustrate with the same denominator low frequency events such as complications of colonoscopy and high frequency events such as overall mortality at 12 years. The intervention pack contained two additional numerical facts not present in the summary of the standard pack: (1) ‘nine out of ten patients with positive occult blood tests do not have cancer’; and (2) ‘screening does not affect overall mortality’. The overall mortality figures were illustrated with a pictogram (Figure 2).
Figure 1.
Information shown to the control group, as in the standard NHS information pack
Figure 2.
Information shown to the intervention group containing the information shown to the control group embellished with additional numerical information and pictograms
Subjects were asked to read the information leaflets at home and return a questionnaire asking, on the basis of the information received, whether or not they would wish to be screened for CRC and which of the summary points had most influenced their decision. For each summary point subjects were asked whether this made them: ‘definitely want to be screened’; ‘want to be screened’; ‘not want to be screened’; or ‘definitely not want to be screened’. Free-text comments were invited. The questionnaire included questions on demographic data and co-morbidities and subjects were asked their attitude to screening in general. Those failing to return questionnaires within three weeks were sent one reminder.
One-quarter of subjects returning their questionnaire were chosen at random to be interviewed by telephone. The interviewer went through the questionnaire point by point, grading for each point whether that points had been understood fully, partially or not at all. In addition, control subjects were given by telephone the same numerical information that had been given to the intervention group and asked whether having these figures at the onset would have helped their decision-making process or altered their final decision.
We had assumed that, as in national studies, there would be a 50% acceptance of screening and so would need 120 subjects in each group to give an 85% probability of detecting a 20% difference between the two groups, with a two-sided test at the 5% level. Statistical analysis of differences between proportions was carried out using the two-tailed chi-squared test without the Yates correction. The study was approved by the NHS Research Ethics Committee (07/H0905/56, July 2007).
Results
A total of 478 questionnaires were given out of which 256 were returned (54%). Fifteen returns were excluded (age >70 years or <60 years, previous colonoscopy, history of colon cancer or incompletely filled in forms) leaving 124 subjects in the control group and 117 in the intervention group.
There was no significant difference in mean age, smoking prevalence or presence of co-morbidities between the groups. However subjects in the intervention group were more likely to have a relative with bowel cancer than those in the control group (15% v 31%, P = 0.02) (Table 1).
Table 1.
Demographic information and co-morbidities in control and intervention group
| Control group (n = 124) | Intervention group (n = 117) | |
|---|---|---|
| Women (%) | 55.2 | 40.8 |
| Mean age (years) | 64.8 ± 3.1 | 64.7 ± 3.3 |
| Retired (%) | 74.8 | 76.7 |
| Current smokers (%) | 10.0 | 10.4 |
| Ex-smokers (%) | 46.7 | 50.4 |
| Never smoked (%) | 43.3 | 39.1 |
| Co-morbidities (%) | ||
| Hypertension | 48.8 | 41.4 |
| Diabetes mellitus | 17.9 | 20.7 |
| Ischaemic heart disease | 26.0 | 19.0 |
| Bronchitis | 2.4 | 1.7 |
| Indigestion | 22.8 | 13.8 |
| Asthma | 13.8 | 8.6 |
| Kidney disease | 3.3 | 0.9 |
| Others | 35.8 | 31.0 |
| Family history of CRC (%) | 14.6 | 31.0* |
| Friend's history of CRC (%) | 29.4 | 21.9 |
| Attitude to screening (%) | ||
| Positive | 82.8 | 77.0 |
| Neutral | 13.8 | 21.2 |
| Negative | 3.5 | 1.8 |
| Attending GP regularly for screening (%) | 95.2 | 94.9 |
*Significant difference between control and intervention group
P < 0.02
Additional numerical and pictorial information given to the intervention group did not affect their overall decision on whether or not to be screened: 90% in the control group and 85% in the intervention group said they would wish to take part in bowel cancer screening (χ2 = 0.92, P = 0.34). However, there was a decrease in the impact of three of the summary points influencing decision-making when numerical and pictorial information were provided (Table 2). Thus the percentage of subjects who said that that Point 1 made them ‘definitely want to be screened’ fell from 61% of the control group who were told ‘Taking part in the cancer screening programme reduces your chance of dying from bowel cancer’, to 41% (P = 0.002) in the intervention group when informed by text and pictogram that: ‘The screening programme will prevent 12 people [per 10,000], that is 1 in every 862 screened, from dying of bowel cancer’.
Table 2.
Numbers (%) showing the impact of each of the CRC screening summary points in the control group (n= 124) and intervention group (n = 117)
| This makes me … (summary points) | Definitely want to be screened | Want to be screened | Not want to be screened | Definitely not want to be screened | No impact | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Intervention | Control | Intervention | Control | Intervention | Control Group | Intervention | Control | Intervention | |
| 1 CRC mortality is reduced by screening | 76 (61.3)†P = 0.002 | 48 (41.0) | 37 (29.8) | 55 (47) | 3 (2.4) | 5 (4.3) | 3 (2.4) | 1 (0.9) | 5 (4.0) | 8 (6.8) |
| 2 Polyp detection prevents CRC | 70 (56.4)†P = 0.046 | 51 (43.6) | 41 (33.1) | 52 (44.4) | 3 (2.4) | 6 (5.1) | 2 (1.6) | 1 (0.9) | 8 (6.5) | 6 (5.1) |
| 3* CRC may be missed | 45 (36.3)†P = 0.010 | 24 (20.5) | 38 (30.6) | 36 (30.8) | 6 (4.8) †P =0.0017 | 18 (15.3) | 3 (2.4) | 4 (3.4) | 32 (25.8) | 35 (29.9) |
| 4* Colonoscopy is risky | 36 (29.0) | 23 (19.7) | 52 (41.9) | 42 (35.9) | 9 (7.3)†P = 0.004 | 24 (20.5) | 4 (3.2) | 4 (3.4) | 22 (17.7) | 24 (20.5) |
| 5 Not all CRC is treatable | 32 (25.8)†P = 0.035 | 45 (38.5) | 38 (30.6) | 40 (34.2) | 10 (8.1) | 12 (10.3) | 5 (4.0) | 2 (1.7) | 39 (31.5) †P =0.003 | 18 (15.3) |
| 6 Ease of use/privacy | 46 (37.1) | 50 (42.7) | 34 (27.4) | 32 (27.4) | 5 (4.0) | 3 (2.6) | 2 (1.6) | 2 (1.7) | 36 (29.0) | 29 (24.8) |
| 7 9/10 with +ve OB do not have cancer | – | 37 (31.6) | – | 38 (32.5) | – | 16 (13.6) | – | 32.6 | – | 23 (19.6) |
| 8 Screening does not affect all-cause mortality | – | 33 (28.2) | – | 47 (40.2) | – | 12 (10.3) | – | 2 (1.7) | – | 23 (19.7) |
*Summing the numbers in whom this point made subjects ‘not want to be screened’ to those in whom it made them ‘definitely not want to be screened’ showed differences between the control and intervention groups of 7.3% vs. 18.8% (P = 0.008) for summary point 3; and 10.5% vs. 23.9% (P = 0.006) for summary point 4, respectively
†Significant difference between control and intervention groups
P values refer to significance of differences between control and intervention group
When the small risk of colonoscopy complication was illustrated graphically the numbers who said this made them not want to be screened or definitely not want to be screened increased from 11% to 24% (P = 0.006) and a similar increase (7% to 19%, P = 0.008) was seen when subjects were told that half of CRC cancers may be missed by the screening programme (Table 2).
Conversely, in the intervention group the information that ‘Not all bowel cancers detected by screening can be successfully treated. If you are found to have bowel cancer through screening, you will live on average 1.1 years longer than if you waited until your bowel cancer presented with symptoms’ had a positive impact on screening intention compared to the information given to the control group who were only told: ‘not all bowel cancers detected by screening can be successfully treated’: 39% in the intervention group vs. 26% in the control group said this point would definitely make them want to be screened (P = 0.035) and there was a halving of those who said this point had no impact (Table 2).
There was also a difference in the summary point which subjects felt had most influenced their overall decision on whether or not to be screened: 65% of those in the control group said the information contained in summary point 1 (taking part in the CRC screening programme can reduce your chance of dying from bowel cancer) was the most important point in helping them reach their decision compared to 40% in the intervention group (P = 0.0001), while polyp detection was seen as the most important summary point by more subjects in the intervention group compared to the control group (21% v 7%, respectively; P = 0.0006) (Table 3).
Table 3.
Table showing which summary point most influenced subjects' decision on whether or not to be screened. Comparisons between controls and intervention have been made excluding from the intervention group total the nine subjects who put points 7 and 8 as their most important point in decision-making as these choices were not available to the control group
| Summary point | For each summary point numbers (%) saying that this point was the most important point in their decision-making | |
|---|---|---|
| Control group (n = 124) | Intervention group (n = 117) | |
| 1 CRC mortality is reduced by screening | 81 (65.3)* P = 0.0001 | 47 (40.2) |
| 2 Polyp detection prevents CRC | 9 (7.3)* P = 0.0006 | 25 (21.4) |
| 3 CRC may be missed | 5 (4.0) | 6 (5.1) |
| 4 Colonoscopy risky | 7 (5.6) | 6 (5.1) |
| 5 Not all CRC treatable | 5 (4.0) | 7 (6.0) |
| 6 Ease of use/privacy | 9 (7.3) | 9 (7.7) |
| 7 9/10 with +ve OB do not have cancer | – | 5 (4.3) |
| 8 Screening does not affect all-cause mortality | – | 4 (3.4) |
| Not sure/no specific point | 8 (6.4) | 8 (6.8) |
*Significant difference between control and intervention groups
P values refer to significance of differences between control and intervention group
In their questionnaire responses, 62% of the intervention group found the pictograms helpful (23% very helpful and 39% quite helpful) while 34% found them unhelpful and 4% very unhelpful.
Fifty-seven subjects were interviewed by telephone. Of the 33 in the control group 73% said the additional numerical information given to the intervention group would have been useful to them in coming to their decision. One subject said that knowledge of these figures would have reversed his decision on whether to be screened. Of the 24 subjects in the intervention group who were interviewed by telephone, 83% said the numerical data were useful and felt it had influenced their decision-making. In the intervention group one subject was judged to have poor understanding of all of the summary points, the remainder were judged to have full understanding. In the control group one was judged to have poor understanding, one partial understanding, and the remainder full understanding of the summary points under test.
Discussion
Statement of principal findings
Our study suggests that provision of numerical and pictorial data does not influence subjects' overall desire to be screened but does inform their decision-making process. Thus a significant difference in impact of the summary points was seen when numerical information was given; in four of the summary points moving subjects away from screening and in one point towards screening. Seventy-three percent of those interviewed by telephone in the control group felt the numerical information given to the intervention group would have been useful and would have aided their decision-making and over half of the intervention group found the pictograms very or quite helpful.
Strengths and weaknesses of the study
There are possible caveats surrounding this study. First, the increased number of subjects in the intervention group with a family history of bowel cancer could have biased the intervention group towards screening and so lessened the potential negative impact of the numerical information they were given. Excluding all patients with a family history of CRC reduced the percentage wishing to be screened to 90.1% in the control group and 80.1% in the intervention group but the difference between control and intervention groups did not reach statistical significance (χ2 = 3.84, P = 0.05). Second, although not significant, there was a trend to increased co-morbidity in the control group which could have affected their attitude to screening and without a larger sample size we cannot exclude this possibility. Finally, we chose to recruit our subjects from non-gastroenterological hospital outpatient clinics and GP surgeries. These subjects may already be health-seekers and more likely to be in favour of screening and therefore may not be representative of all 60–70-year-olds. Nevertheless the comparison between the two groups stands and many 60–70-year-olds do attend clinics regularly.
The meta-analysis of CRC screening by Moayyedi showed the relative risk of non-CRC death in the screened group to be 1.02 (1.00–1.04, P = 0.015),11 this increase exactly balancing the reduced risk of CRC death in the screened group. ‘Mortality substitution’ in the screened group was also supported by Whynes et al. in their analysis of deaths in the Nottingham Trial.16 Furthermore Moayyedi's meta-analysis found 16% of those in whom cancer was diagnosed by screening died within 2 years of unrelated causes or postoperative complications and so may have been disadvantaged by earlier diagnosis. We were surprised that including data on all cause mortality in our study was not viewed negatively; indeed 68% said this numerical information still made them want to be screened. Fear of mortality from a specific illness or event, particularly cancer, may be a stronger drive to screening than concern about all-cause mortality or mortality from non-specified events.
Strengths and weaknesses in relation to other studies
The recent study by Smith et al. on the use of decision aids in CRC screening has also made use of natural frequencies and diagrams.17 They showed a reduction in screening participation in the intervention group, but their study differs from ours in that its focus was on adults with low education and literacy standards and subjects were not given figures on all cause mortality. Our study suggests that all-cause mortality figures need not be withheld from information leaflets for fear of reducing uptake of CRC in the community.
The number wishing to be screened in our control group (85%) is higher than the figure of 56% presenting for screening in the first round of the UK CRC programme.18 However the figure of 56% for uptake of CRC screening matches our questionnaire return rate of 54%. We speculate that those returning questionnaires on CRC screening may be those who would be more likely to comply with real-life screening programmes. This is supported in our study by the 83% returning the questionnaire who said they were generally positive about screening with only 2.7% overall against screening (Table 1) and 95.4% said they ‘attend their GP surgery for “check-ups” when invited’. An alternative explanation is that subjects faced with a hypothetical questionnaire respond more positively than when faced with the reality of obtaining and testing their own stool and the anxiety of dealing with a possible positive result.
Meaning of the study: possible mechanisms and implications for clinicians or policymakers
The difficult question is how best to frame the numerical information on screening. Natural frequencies, as used in our study, and absolute risk reduction are of relevance to the individual and should be used in preference to relative risk reduction and population-based figures as it is the individual not the population that must decide whether to be screened.19 In their review, Edwards et al. suggest that providing visual aids and graphically representing information increase the effectiveness of risk communication.20 We chose to illustrate numerical facts using pictograms based on Paling's risk charts and have used events per 10,000 so that all events can be viewed against a common denominator.1 Can we be sure even so that the subjects understood the information presented? In the intervention group all but one subject had full understanding of the numerical information and pictograms when interviewed by telephone and Smith's study shows that even in those with low education and literacy standards the use of pictograms and numerical data improved understanding. Sixty-two percent of our group found the pictograms helpful or very helpful. Their wider use in a standardized format may allow easier quantification of risk/benefit for this as well as other health interventions.20,21
Our study shows that the provision of numerical data is welcomed and aids informed decision without any overall impact on attitude to screening. Similar results were seen in a study by Mathieu et al. with visual decision aids to help decision-making for breast cancer screening.22 Even if such data reduce uptake of screening they should be included according to Jørgensen, and rightly so, because concern for uptake should never overrule concern for informed consent. This contrasts with the view of the director of the NHS cancer screening programme who responded to a call to include numerical data in the breast cancer screening leaflet by saying: ‘putting too much numerical information meant women just put the leaflet down’.23
All screening programmes carry a side-effect burden of over-diagnosis and increased levels of anxiety. An invitation for faecal occult blood testing itself causes anxiety in 50% of subjects which is severe in 5% even in those not called for colonoscopy24 and in the CRC programme there are the potential risks and discomfort of bowel preparation and colonoscopy in those with positive faecal occult blood tests. The standard CRC leaflet does give numbers for colonoscopy complications which in terms of total numbers in the screened population are small but not insignificant to those needing colonoscopy. However the leaflet gives little other patient-focused figures and in particular no mention of lack of overall mortality benefit.12 Those responsible for promoting screening programmes are currently those who provide the information to subjects and, as emphasized by Jørgensen, therein lies a conflict of interest.14 High participation rates are essential to ensure the population benefits of screening. Information on the small absolute risk reduction achieved by screening and the lack of evidence of overall mortality reduction could deter subjects from participating. Our study shows that this may not be the case. The numerical and pictorial information was welcomed by most subjects and had no overall effect on their desire to be screened.
In conclusion, our study suggests that providing numerical information, enhanced pictorially, and focusing on the individual's risks and benefits could enhance the credibility of the screening programme without reducing the numbers screened.
DECLARATIONS
Competing interests
None declared
Funding
This study was funded using the County Durham and Darlington Hospitals Foundation Trusts Gastroenterology Research Fund, account number 4037 Eros code CF 9121. This fund is generated from charitable donations made to the hospital by the community it serves
Ethical approval
This study was approved by the County Durham and Darlington Hospitals Foundation NHS Trust Local Research Governance Board and then by the local NHS Research Ethics Committee, Reference 07/H0905/56 (July 2007)
Guarantor
PNT
Contributorship
IMP was the first author and contributed to the study design, ethical approval, recruitment, data collection, data analyses; VB and JI contributed to the recruitment and data collection; PNT came up with the concept and study design, ethical approval, recruitment and data analyses
Acknowledgements
The authors thank Peter Gedling (statistics support), Sampa Chail (data entry support), Chris Wells (initial assistance) and the staff at Clifton Court and Blacketts GP practices in Darlington. The data were presented previously at the British Society of Gastroenterology Meeting in 2009 and published as an abstract in Gut 2009;58 (Suppl. 1):A1–A156)
Reviewer
Karsten Jørgensen
References
- 1.Palin J Helping patients understand risk: 7 Simple Strategies for Successful Communication. 2nd edn [city of publisher]: The Risk Communication Institute, 2006 [Google Scholar]
- 2.Hewitson P, Glasziou PP, Irwig L, Towler B, Watson E Screening for colorectal cancer using the faecal occult blood test, Hemoccult. Cochrane Database Syst Rev 2007;(1):CD001216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. N Engl J Med 1993;328:1365–71 [DOI] [PubMed] [Google Scholar]
- 4.Hardcastle JD, Chamberlain JO, Robinson MHE, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996;348:1472–7 [DOI] [PubMed] [Google Scholar]
- 5.Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996;348:1467–71 [DOI] [PubMed] [Google Scholar]
- 6.Kewenter J, Brevinge H, Engaras B, Haglind E, Ahren C Results of screening, rescreening, and follow-up in a prospective randomized study for detection of colorectal cancer by fecal occult blood testing: results for 68,308 subjects. Scand J Gastroenterol 1994;29:468–73 [DOI] [PubMed] [Google Scholar]
- 7.Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med 2000;343:1603–7 [DOI] [PubMed] [Google Scholar]
- 8.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010;375:1624–33 [DOI] [PubMed] [Google Scholar]
- 9.Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med 2004;351:2704–14 [DOI] [PubMed] [Google Scholar]
- 10.Towler B, Irwig L, Glasziou P, Kewenter J, Weller D, Silagy C A systematic review of the effects of screening for colorectal cancer using the faecal occult blood test, Hemoccult. BMJ 1998;317:559–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moayyedi P, Achkar E Does fecal occult blood testing really reduce mortality? A reanalysis of systematic review data. Am J Gastroenterol 2006;101:380–4 [DOI] [PubMed] [Google Scholar]
- 12.NHS Cancer Screening Programmes Bowel Cancer Screening: The Facts. London: Department of Health, 2008 [Google Scholar]
- 13.Robinson MHE, Hardcastle JD, Moss SM, et al. The risks of screening: data from the Nottingham randomised controlled trial of faecal occult blood screening for colorectal cancer. Gut 1999;45:588–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jorgensen KJ, Gotzsche PC Content of invitations for publicly funded screening mammography. BMJ 2006;332:538–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Connor A, Bennett C, Stacey D, et al. Decision Aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2009;3:CD001431 [DOI] [PubMed] [Google Scholar]
- 16.Whynes DK, Mangham CM, Balfour TW, Scholefield JH Analysis of deaths occuring within the Nottingham trial of faecal occult blood screening for colorectal cancer. Gut 2010;59:1088–93 [DOI] [PubMed] [Google Scholar]
- 17.Smith SK, Trevena LJ, Simpson JM, Barratt A, Nutbeam D, McCaffery KJ A decision aid to support informed choices about bowel cancer screening among adults with low education:randomised controlled trial. BMJ 2010;341:c3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.British Society of Gastroenterology Gastroenterologists call for more investment in cancer screening and services. See http://www.bsg.org.uk/press/press-releases/gastroenterologists-call-for-more-investment-in-cancer-screening-and-services.html [Google Scholar]
- 19.Trevena LJ, Davy HM, Barratt A, Butow P, Caldwell P A systematic review on communicating with patients about evidence. J Evaluation Clin Pract 2006;12:13–23 [DOI] [PubMed] [Google Scholar]
- 20.Edwards A, Elwyn G, Mulley A Explaining risks: turning numerical data into meaningful pictures. BMJ 2002;324:827–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trewby PN, Reddy AV, Trewby CS, Ashton VJ, Brennan G, Inglis J Are preventive drugs preventive enough? A study of patients' expectation of benefit from preventive drugs. Clin Med 2002;2:527–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathieu E, Barratt A, Davey HM, McGeechan K, Howard K, Houssami N Informed choice in mammography screening: a randomized trial of a decision aid for 70-year-old women. Arch Intern Med 2007;167:2039–46 [DOI] [PubMed] [Google Scholar]
- 23.Chris S NHS rips up breast cancer leaflet and starts all over again. The Times 2009. See www.timesonline.co.uk/tol/life_and_style/health/article5776804.ece [Google Scholar]
- 24.Lindholm E, Berglund B, Kewenter J, Haglind E Worry associated with screening for colorectal carcinomas. Scand J Gastroenterol 1997;32:238–45 [DOI] [PubMed] [Google Scholar]