Isolated tubercular lesions of the cervical spine have grave sequalae; clinicians need to be vigilant when reviewing patients with neck pain.
Case report
A 22-year-old woman presented to our emergency department with a 9-week history of worsening neck stiffness, inability to grip objects, lift arms up, self-care and lower limb weakness. The patient was from Pakistan and had been living in the UK for over 4 years. She reported no recent travel or history of trauma and denied previous TB exposure. Systemically, there was no loss of appetite, weight loss or sweats. On examination, she was apyrexial, normotensive, normoglycaemic with no clubbing. Her neck was held in right lateral flexion with marked kyphosis. Neurological examination revealed upper limb hypertonia and power of 3/5 with lower limb hypotonia and 4/5 weakness. Reflexes were present and symmetrical and sensation to light touch was reduced in dermatomes C3–C5 with axillary sparing. Paraesthesia was noted in both arms and Lhermitte's sign was positive. No sphincteric dysfunction was noted. Base-line blood investigations were normal despite a C-reactive protein of 74 IU.
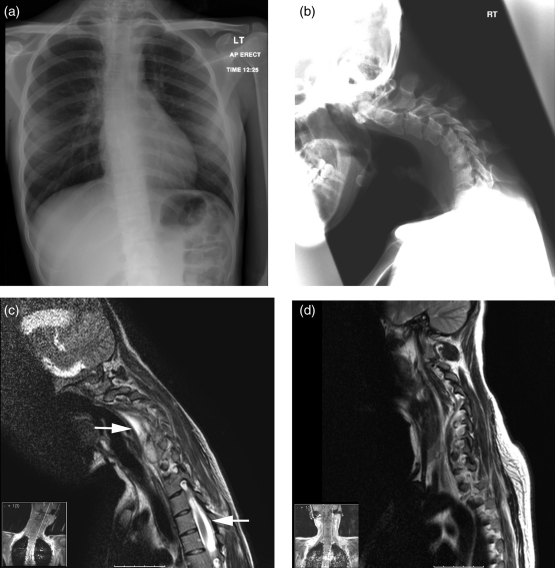
A chest radiograph demonstrated a left upper/middle zone nodular lesion and thoracic scoliosis with concavity to the left, centred around the 6 thoracic vertebral body (Figure 1a). A lateral cervical spine radiograph demonstrated angulated kyphosis centred on the C4/C5 intervertebral disc with soft tissue density in the pre-vertebral and retropharyngeal soft tissues (Figure 1b). Sputum was sent for acid-fast bacilli (later noted to be negative).
Figure 1.
Radiographic imaging of cervical tuberculosis. a. Chest radiograph demonstrating that the lungs appear clear with the exception of some possible granuloma within the left upper/mid zone. There is a slight thoracic scoliosis with concavity to the left, centred around the T6 vertebral body. b. Lateral cervical spine radiograph demonstrating angulated kyphosis centred around the C4–C5 intervertebral disc with anterolisthesis of C5–C4 and a pars defect at C5 is seen. Additionally there is increased soft tissue density in the pre-vertebral and retropharyngeal soft tissues. c. A sagittal T2 post-contrast MRI scan demonstrating a pre-vertebral and epidural collection extending from the C3–C4 disc space to the bottom of T1. There is no enhancement within the cord but there is high signal intensity within it on the T2 weighted scans indicative of oedema. d. A postoperative follow-up MRI scan demonstrating resolution of the pre-vertebral and epidural collections
An MRI of the cervical spine demonstrated altered signal intensity within the C4–C6 vertebral bodies with an epidural collection extending from the C3/4 disc space to the bottom of T1 (Figure 1c). There was no evidence of enhancement within the cord, but there was high signal intensity within it on T2 weighted scans; indicative of cord oedema. Collectively, the appearances were consistent with an epidural abscess lying anterior to the cord in the cervical spine with large vertebral abscesses (Figure 1c). A diagnosis of tuberculosis was made and the patient was commenced on quadruple anti-tubercular therapy (Rifampicin 450 mg o.d., Isoniazid 300 mg o.d., pyrazinamide 1.5 g o.d. and ethambutol 600 mg o.d.).
Four days later the patient underwent anterior decompression of C6–C7 with insertion of a carbon fibre disc and drainage of the pustular collection at C3–C7. Tissue from the abscess was sent for pathological analysis, which grew Mycobacterium tuberulosis. The patient made an uneventful recovery and tolerated the application of a Miami-J collar. Two weeks later, a MRI was performed and demonstrated improvement in cervical kyphosis with reduction in the volume of inflammatory tissue within and anterior to the cervical neural canal (Figure 1d). Following neuro-rehabilitation the patients' neurological symptoms improved.
Discussion
Tuberculosis of the vertebral column has been around for over 5000 years; evidence of the disease has been observed in mummies from Ancient Egypt.1 The London surgeon Sir Perceival Pott was the first to report this extra-pulmonary manifestation of tuberculosis (TB) in association with paraplegia and kyphotic deformity of the spine.2 The commonest extra-pulmonary skeletal manifestation of TB is within the spine, with a predilection to the thoracic and lumbar regions of the vertebral column. In 2–3% of cases, the cervical spine may be affected with resultant lesions giving rise to instability and neurological deficits.3,4 With the number of cases of TB within London alone up by 50% from 1999 to 2009,5 clinicians need to have a heightened awareness of its many presentations so that an early diagnosis can prevent long-lasting sequelae.
The incidence of TB has increased globally, with the ease of travel and socioeconomic migration fuelling the rise. Cases are often not new migrants but are individuals that have been born abroad and have been resident in Europe for over 2 years.6 The majority will have involvement of the thoracic or lumbar spine.
There exist few reports in the literature describing TB of the cervical spine.7,8 The duration between onset of symptoms and presentation is 11–15 months.7,8 The patients are typically young with a mean age of 38 years (range 29–52).7,8 The delay in presentation is secondary to the low intensity of the initial symptoms and incorrect attribution to musculoskeletal pain. In the largest case series (n = 61), constitutional symptoms such as fever, malaise and weight loss did not contribute to the diagnosis in a single patient.7
Neurological deficits are the most serious complication of spinal TB with patients presenting with para- or tetraplegia, hemiplegia or monoplegia.6 When the cervical spine is involved the commonest presenting symptom is neck pain and can precede the diagnosis by 24 months.7 Over 50% of patients will have muscular weakness.7 The development of kyphosis, secondary to spinal TB, is the rule rather than the exception.6 Our patient presented with severe cervical kyphosis (Figure 1b). In severe cases kyphosis can be as great as 60 degrees.6
TB of the cervical spine complicated by worsening neurological deficit and or progressive deformity should be treated early.9 The gold standard treatment, following decompression, is anterior spinal instrumentation to support the collapsed anterior weight-bearing column of the cervical spine.6–8 Our patient underwent decompression and stabilization within 4 days of presentation, with significant improvement in kyphotic deformity (Figure 1d). In the absence of gross deformity or neurological deficit TB of the spine is a medical disease and should be treated with antituberculous medication, rest and mobilization with suitable orthosis.6
The best treatment for TB of the cervical spine with paraplegia is to prevent the development of paraplegia.6 This can only be achieved by approaching patients with worsening neck pain with caution and spotting TB early before it represents with concomitant neurological deficits.
DECLARATIONS
Competing interests
None declared
Funding
None
Ethical approval
Written consent to publication was obtained from the patient or next of kin
Guarantor
ARS
Contributorship
ARS wrote the first draft of the paper and coordinated the review of all drafts; WW reviewed all drafts of the paper; TJ reviewed and commented on all drafts of the paper and all radiographic images
Acknowledgements
ARS is in receipt of the Jason Brice Fellowship in neurosurgical research and the Walport Academic Clinical Fellowship in Neurosurgery
Reviewer
Imtiaz Wani
References
- 1.Derry DC Pott's disease in ancient Egypt. Med Pres Circ 1938;197:196–9 [Google Scholar]
- 2.Pott P The chirurgical works of Percivall Pott, F.R.S., surgeon to St. Bartholomew's Hospital, a new edition, with his last corrections. 1808. Clin Orthop Relat Res 2002;(398):4–10 [DOI] [PubMed] [Google Scholar]
- 3.Hsu LC, Leong JC Tuberculosis of the lower cervical spine (C2 to C7). A report on 40 cases. J Bone Joint Surg Br 1984;66:1–5 [DOI] [PubMed] [Google Scholar]
- 4.Behari S, Nayak SR, Bhargava V, Banerji D, Chhabra DK, Jain VK Craniocervical tuberculosis: protocol of surgical management. Neurosurgery 2003;52:72–80; discussion 80–1 [PubMed] [Google Scholar]
- 5.Zumla A The white plague returns to London with a vengeance. Lancet 2011;377:10–11 [DOI] [PubMed] [Google Scholar]
- 6.Jain AK Tuberculosis of the spine: a fresh look at an old disease. J Bone Joint Surg Br 2010;92:905–13 [DOI] [PubMed] [Google Scholar]
- 7.Ramani PS, Sharma A, Jituri S, Muzumdar DP Anterior instrumentation for cervical spine tuberculosis: an analysis of surgical experience with 61 cases. Neurol India 2005;53:83–9; discussion 89 [DOI] [PubMed] [Google Scholar]
- 8.Abdeen K Surgery for tuberculosis of the cervical spine. The Internet Journal of Neurosurgery 2006;3:2 [Google Scholar]
- 9.Yilmaz C, Selek HY, Gurkan I, Erdemli B, Korkusuz Z Anterior instrumentation for the treatment of spinal tuberculosis. J Bone Joint Surg Am 1999;81:1261–7 [DOI] [PubMed] [Google Scholar]