Abstract
Background: The Women's Health Initiative (WHI) randomized clinical trial (RCT) of calcium plus vitamin D (CaD) supplements found a 17% excess in urinary tract stone incidence in the supplemented group. This study evaluated whether this risk is modified by participant characteristics.
Objective: We examined the correlates of urinary tract stone occurrence in the CaD arm of the WHI trial.
Design: We analyzed an RCT involving 36,282 postmenopausal women aged 50–79 y from 40 WHI centers: 18,176 women received 500 mg calcium carbonate plus 200 IU vitamin D3 twice daily (1000 mg and 400 IU daily, respectively), and 18,106 women received a matching placebo for an average of 7.0 y. The incidence of urinary tract stones was determined.
Results: The incidence of self-reported clinically diagnosed urinary tract stones was more common in the active CaD medication group than in the placebo group (hazard ratio: 1.17; 95% CI: 1.02, 1.34): 449 women in the CaD group and 381 women in the placebo group reported a stone during the trial. The rates of self-reported stones did not differ between various demographic, anthropomorphic, dietary, and other hypothesized risk factors according to randomization assignment. Neither the total calcium intake nor the use of calcium supplements at baseline was associated with the risk of stones. In sensitivity analyses that censored participants who were below 80% adherence, the findings were similar.
Conclusions: Daily supplementation with CaD for 7 y was associated with an increase in the number of self-reported urinary tract stones. These findings have implications for CaD supplement use. This trial was registered with the WHI at clinicaltrials.gov as NCT00000611.
See corresponding article on page 5.
INTRODUCTION
Calcium plus vitamin D (CaD) dietary supplements have been recommended for the prevention of osteoporotic fractures in postmenopausal women (1), and millions are using supplements to improve bone health. However, little is known about the long-term adverse effects of such supplements. Because supplemental CaD may alter calcium and vitamin D metabolism and calcium absorption and excretion, the possibility of altered rates of urolithiasis is an important clinical issue, particularly given the preventive nature of such supplement use. In the Women's Health Initiative (WHI) CaD supplementation trial in >36,000 women, urinary tract stones were reported to be 17% more common in those in the active supplementation group (hazard ratio: 1.17; 95% CI: 1.02, 1.34) (2). This, to our knowledge, is the first report of this adverse effect in a large-scale, randomized trial of CaD supplements. In this report, we explored the demographic, dietary, and clinical correlates of and risk factors for urinary tract stone occurrence in this trial to better understand whether specific participant characteristics may have modified the occurrence of this important clinical event.
SUBJECTS AND METHODS
Study population, eligibility, and consent
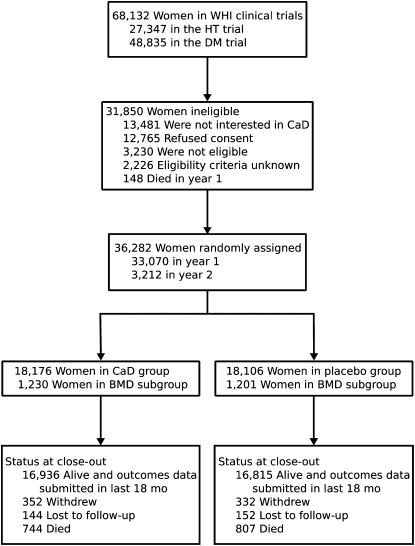
The design, execution, and outcomes of the WHI CaD supplementation trial and the other WHI intervention trials have been described in detail (3–8); subject progress through the trial is shown in Figure 1. Briefly, between 1993 and 1998, postmenopausal women aged 50–79 y were enrolled in the WHI randomized clinical trials designed to assess the risks and benefits of hormone therapy (HT) and dietary modification (DM). One year later, participants enrolled in the HT and/or DM trials were invited to enroll in the CaD supplementation trial. This trial was designed to determine whether supplementation would prevent hip fracture (the primary outcome), other fractures, or colorectal cancer (designated secondary outcomes). Exclusion criteria for enrollment in the HT and DM trials were related to competing risks, safety, adherence, and retention. Additional exclusion criteria for the CaD trial included a predicted survival of <3 y, a history of hypercalcemia or urinary tract stones, current use of corticosteroids, use of calcitriol or ≥600 IU supplemental vitamin D. Although excluded by protocol design, later review of CaD trial intake forms showed that 161 participants in the active CaD arm and 172 in the placebo arm had a self-reported history of urinary tract stones; we conducted intention-to-treat analyses and did not exclude these individuals. The protocol and consent forms were approved by the institutional review board at each participating institution. All women provided written informed consent. Baseline clinical, demographic, anthropomorphic, and dietary information was collected by using self-administered forms supplemented by in-person and telephone interviews, by using common WHI protocols and questionnaires (3).
FIGURE 1.
Progress of subjects through the phases of the Women's Health Initiative (WHI) Calcium plus Vitamin D (CaD) Supplementation Study. HT, hormone therapy; DM, dietary modification; BMD, bone mineral density.
Randomization, blinding, intervention, and monitoring
A permuted-block algorithm was used for randomization, with participants stratified according to clinical center and age. Of the 36,282 participants who enrolled in the CaD trial, 18,176 were randomly assigned to the active arm and received a total daily dose of 1000 mg elemental Ca (calcium carbonate) and 400 IU vitamin D. Each dose contained 500 mg elemental Ca as calcium carbonate combined with 200 IU vitamin D3 and were to be taken twice daily with meals, as either a chewable or (after 1997) a swallowable tablet. The remaining 18,106 participants were randomly assigned to receive an identical-appearing placebo tablet to be taken twice daily. Both active and placebo pills were provided by GlaxoSmithKline (Philadelphia, PA). An independent data and safety monitoring board reviewed the trial data semiannually. Closeout visits occurred as planned between 1 October 2004 and 31 March 2005, with final outcomes assessed before the treatment assignment was revealed. Participants were followed for a mean (±SD) time of 7.0 ± 1.4 y.
Follow-up procedures and ascertainment of outcomes
Four weeks after randomization, participants were telephoned to assess their abdominal symptoms and reinforce adherence. Thereafter, participants were contacted semiannually to obtain self-reported medical history updates. Urinary tract stone occurrence was obtained on the medical history update by asking the following question: “Since (the date given in the front of this form), has your doctor told you for the first time that you have any of the following specific conditions (mark all that apply)” with an item response on that form: “kidney or bladder stones (renal or urinary calculi).” There was no additional follow-up of the clinical evaluation among participants reporting clinical urinary tract stone disease, and information on the type of stone and chemical composition of the stones (ie, oxalate or uric acid) was not obtained. As part of general WHI trial protocols, hospitalization events lasting ≥48 h were solicited from all participants and medical records were obtained. All hospitalizations of CaD trial participants during which urinary tract stone diagnoses or related surgical procedures occurred were tabulated from the WHI database by using the International Classification of Disease, Ninth Revision (ICD-9-CM) (9).
During the study, adherence was assessed by weighing returned pill bottles. Regardless of their level of adherence, participants were followed up until they died, they were lost to follow-up, they requested no further contact, or the study ended. By protocol, study pills were discontinued if urinary tract stones, hypercalcemia, dialysis, calcitriol use, or daily use of supplements providing >1000 IU vitamin D were reported.
Statistical analysis
All analyses were conducted by using SAS version 9.1 (SAS Institute, Cary, NC). Primary analyses used time-to-event methods, according to the intention-to-treat principle. The primary outcome in these analyses was incident self-reported urinary tract stones. We also analyzed hospitalization rates for urinary tract stones or stone-related conditions. Comparison of these outcomes by the 2 groups (active arm compared with placebo) were represented by hazard ratios and 95% CIs from Cox regression models stratified according to baseline age groups and participation in the HT and DM trials (10). Kaplan-Meier estimates were used to present event rates over time. All P values were 2-sided. The effect of CaD on stone occurrence according to potential participant and pathogenetic risk factors was also assessed by Cox regression models with P for interaction derived from likelihood ratio tests. A total of ≈30 subgroup analyses were performed. Thus, ≈2 statistically significant interaction tests would be expected on the basis of chance alone.
To determine the effect of women discontinuing the use of study pills early (active or placebo arm), a sensitivity analysis was conducted, which allowed participants to contribute follow-up time until 6 mo after their first “nonadherent” visit, defined as taking <80% of their study medication. Full adherence hazard ratios were estimated by using inverse adherence censoring weighted estimators with adjustment for 10 covariates associated with adherence.
RESULTS
The randomization scheme worked without difficulty, and no significant differences in the CaD trial participants at screening were found between the active drug and placebo groups with respect to age at screening, other demographic features, family history of study conditions, body mass index, physical activity, prior use of calcium and vitamin D supplements (both as a percentage of users and amounts), total calcium and vitamin D intake, alcohol use and smoking habits, and calculated sun exposure based on geographic residence. Detailed data on these findings were published previously (2). As shown in Table 1, no significant differences were found in the distribution of CaD trial participants enrolled in the other randomized WHI trials. Seventeen percent were enrolled in the HT trials, 69% were enrolled in the DM trials, and 14% participated in both the HT and DM trials.
TABLE 1.
Baseline characteristics of subjects in the Calcium plus Vitamin D (CaD) Supplementation Study according to participation in the other Women's Health Initiative trials
| CaD randomization arm |
||||
| Active drug (n = 18,106) |
Placebo (n = 18,176) |
|||
| n | % | n | % | |
| Estrogen alone | ||||
| No | 15,105 | 83.1 | 15,001 | 82.9 |
| Yes | 881 | 4.8 | 861 | 4.8 |
| Estrogen plus progestin | ||||
| No | 13,193 | 72.6 | 13,176 | 72.8 |
| Yes | 4983 | 27.4 | 4930 | 27.2 |
| Dietary modification | ||||
| No | 5582 | 30.7 | 5490 | 30.3 |
| Yes | 12,594 | 69.3 | 12,616 | 69.7 |
When the trial was concluded on 31 March 2005, 4.3% of participants had died, and 2.7% had withdrawn from the trial or were lost to follow-up. Progress through the trial is shown in Figure 1. Adherence rates, defined as taking ≥80% of the assigned medication, ranged from 60% to 63% for the first 3 trial years; an additional 13–21% took at least half of their study pills. At the trial's conclusion, 76% were taking study medication, and 59% were still taking ≥80% of the doses.
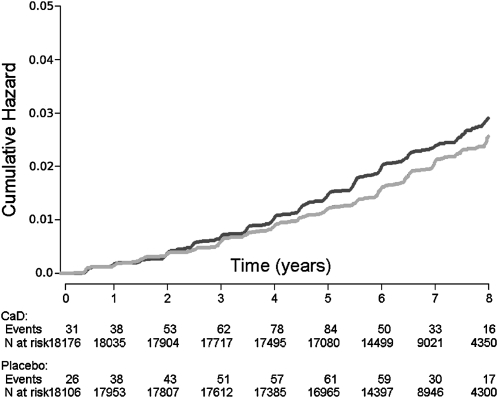
As noted above, and previously reported, self-reported urinary tract stones were 17% more common in the active CaD medication group than in the placebo group (hazard ratio: 1.17; 95% CI: 1.02, 1.34), as shown in the first row of Table 2. Kaplan-Meier analysis of this finding is shown in Figure 2. In the active medication group, 449 participants reported a stone during the trial, as opposed to 381 of those in the placebo group.
TABLE 2.
Number of self-reported urinary tract stones by baseline demographic and dietary characteristics (annualized rate)1
| CaD group(n = 18,106) | Placebo group(n = 18,176) | HR2 (95% CI) | P for interaction3 | |
| n (%) | n (%) | |||
| All self-reported kidney stones | 449 (0.35) | 381 (0.30) | 1.17 (1.02, 1.34) | |
| Age group at screening | 0.194 | |||
| 50–59 y | 151 (0.30) | 140 (0.28) | 1.06 (0.84, 1.33) | |
| 60–69 y | 225 (0.39) | 168 (0.30) | 1.34 (1.10, 1.63) | |
| 70–79 y | 73 (0.34) | 73 (0.34) | 0.99 (0.72, 1.38) | |
| Ethnicity | 0.806 | |||
| White | 367 (0.34) | 302 (0.28) | 1.21 (1.04, 1.41) | |
| Black | 42 (0.36) | 38 (0.33) | 1.10 (0.71, 1.71) | |
| Hispanic | 23 (0.42) | 24 (0.49) | 0.90 (0.50, 1.62) | |
| American Indian | 4 (0.75) | 4 (0.79) | 0.84 (0.20, 3.61) | |
| Asian/Pacific Islander | 10 (0.40) | 8 (0.34) | 1.24 (0.49, 3.17) | |
| Unknown | 3 (0.21) | 5 (0.34) | 0.62 (0.14, 2.73) | |
| Education (collapsed category) | 0.088 | |||
| None–HS diploma/GED | 121 (0.40) | 86 (0.29) | 1.38 (1.05, 1.82) | |
| School after HS | 166 (0.33) | 170 (0.34) | 0.97 (0.78, 1.20) | |
| College degree or higher | 154 (0.33) | 122 (0.26) | 1.25 (0.99, 1.59) | |
| Baseline weight quartile | 126 (0.30) | 111 (0.26) | 1.17 (0.91, 1.52) | 0.866 |
| <67.8 kg | ||||
| 67.8–80.9 kg | 143 (0.33) | 122 (0.29) | 1.12 (0.88, 1.42) | |
| >80.9 kg | 180 (0.42) | 148 (0.35) | 1.22 (0.98, 1.52) | |
| BMI | 0.733 | |||
| <25 kg/m2 | 95 (0.28) | 81 (0.24) | 1.20 (0.89, 1.61) | |
| 25–30 kg/m2 | 153 (0.33) | 141 (0.31) | 1.08 (0.86, 1.35) | |
| >30 kg/m2 | 198 (0.41) | 158 (0.34) | 1.21 (0.99, 1.50) | |
| E alone (n = 1742) | 0.624 | |||
| E alone, active | 48 (0.45) | 37 (0.35) | 1.32 (0.82, 2.02) | |
| E alone, placebo | 42 (0.39) | 38 (0.35) | 1.13 (0.73, 1.75) | |
| E+P (n = 9913) | 0.337 | |||
| E+P, active | 63 (0.36) | 45 (0.26) | 1.41 (0.96, 2.07) | |
| E+P, placebo | 45 (0.26) | 40 (0.24) | 1.07 (0.70, 1.64) | |
| HT intervention (n = 11,655) | ||||
| HT, active | 111 (0.39) | 82 (0.29) | 1.37 (1.03, 1.82) | 0.293 |
| HT, placebo | 87 (0.31) | 78 (0.28) | 1.10 (0.81, 1.49) | |
| DM intervention (n = 25,210) | 0.587 | |||
| DM, intervention | 115 (0.34) | 98 (0.28) | 1.20 (0.91, 1.57) | |
| DM, control | 193 (0.35) | 175 (0.32) | 1.09 (0.88, 1.33) | |
| Thiazides (like) diuretic use | 0.708 | |||
| No | 423 (0.35) | 361 (0.30) | 1.16 (1.01, 1.34) | |
| Yes | 26 (0.43) | 20 (0.34) | 1.30 (0.72, 2.34) | |
| Regional solar irradiance | 0.943 | |||
| 475–500 Langley units | 99 (0.38) | 87 (0.33) | 1.12 (0.84, 1.49) | |
| 400–430 Langley units | 66 (0.30) | 50 (0.23) | 1.28 (0.88, 1.84) | |
| 375–380 Langley units | 53 (0.37) | 45 (0.32) | 1.15 (0.77, 1.71) | |
| 350 Langley units | 102 (0.37) | 82 (0.30) | 1.24 (0.92, 1.66) | |
| 300–325 Langley units | 129 (0.33) | 117 (0.30) | 1.10 (0.85, 1.41) | |
| Smoking | 0.422 | |||
| Never smoker | 234 (0.35) | 187 (0.28) | 1.26 (1.04, 1.53) | |
| Past smoker | 175 (0.34) | 162 (0.32) | 1.06 (0.86, 1.31) | |
| Current smoker | 29 (0.30) | 28 (0.30) | 0.99 (0.59, 1.67) | |
| Alcohol use | 0.945 | |||
| Not a current drinker | 139 (0.40) | 117 (0.33) | 1.19 (0.93, 1.53) | |
| <1 drink/wk | 170 (0.38) | 142 (0.32) | 1.16 (0.93, 1.45) | |
| ≥1 drink/wk | 135 (0.29) | 119 (0.26) | 1.13 (0.89, 1.45) | |
| Physical activity | 0.383 | |||
| >0–3.75 METs/wk | 142 (0.38) | 135 (0.36) | 1.03 (0.81, 1.31) | |
| >3.75–8.75 METs/wk | 150 (0.40) | 112 (0.30) | 1.33 (1.04, 1.70) | |
| >8.75–17.5 METs/wk | 118 (0.31) | 99 (0.26) | 1.18 (0.90, 1.54) | |
| n (%) | n (%) | |||
| HT use at WHI baseline | 0.351 | |||
| Never user | 221 (0.36) | 181 (0.30) | 1.20 (0.99, 1.46) | |
| Past user | 63 (0.28) | 65 (0.30) | 0.92 (0.65, 1.30) | |
| Current user | 163 (0.37) | 135 (0.30) | 1.23 (0.98, 1.55) | |
| HT use (self-report/intervention combination) | 0.207 | |||
| Never user | 142 (0.33) | 126 (0.30) | 1.10 (0.86, 1.40) | |
| Past user | 49 (0.27) | 52 (0.30) | 0.89 (0.60, 1.32) | |
| Current user | 257 (0.39) | 203 (0.30) | 1.29 (1.07, 1.55) | |
| Quartile of caffeine intake | 0.178 | |||
| <77.18 mg | 138 (0.43) | 131 (0.43) | 1.01 (0.79, 1.28) | |
| 77.18 to <177.50 mg | 108 (0.34) | 104 (0.33) | 1.04 (0.79, 1.36) | |
| 177.50 to <265.32 mg | 114 (0.35) | 78 (0.24) | 1.46 (1.10, 1.96) | |
| ≥265.32 mg | 89 (0.28) | 68 (0.21) | 1.34 (0.98, 1.84) | |
| Quartile of protein intake | 128 (0.40) | 96 (0.30) | 1.32 (1.01, 1.72) | 0.678 |
| <14.57% of energy | ||||
| 14.57 to <16.46% of energy | 99 (0.31) | 83 (0.26) | 1.22 (0.91, 1.64) | |
| 16.46 to <18.50% of energy | 107 (0.33) | 101 (0.32) | 1.04 (0.79, 1.37) | |
| ≥18.50% of energy | 115 (0.36) | 101 (0.32) | 1.14 (0.87, 1.49) | |
| Quartile of fat intake | 0.685 | |||
| <32.25% of energy | 101 (0.31) | 80 (0.26) | 1.24 (0.92, 1.66) | |
| 32.25 to <35.78% of energy | 94 (0.30) | 94 (0.29) | 1.01 (0.75, 1.34) | |
| 35.78 to <40.09% of energy | 130 (0.41) | 107 (0.34) | 1.22 (0.94, 1.57) | |
| ≥40.09 % of energy | 124 (0.39) | 100 (0.32) | 1.23 (0.94, 1.60) | |
| Quartile of dietary fat intake | 0.529 | |||
| <45.96 g | 117 (0.37) | 91 (0.29) | 1.27 (0.96, 1.67) | |
| 45.96 to <63.79 g | 96 (0.30) | 93 (0.29) | 1.04 (0.78, 1.38) | |
| 63.79 to <86.95 g | 103 (0.32) | 93 (0.30) | 1.07 (0.81, 1.42) | |
| ≥86.95 g | 133 (0.42) | 104 (0.32) | 1.31 (1.01, 1.69) | |
| Quartile of dietary sodium intake | 117 (0.37) | 97 (0.31) | 1.21 (0.92, 1.58) | 0.899 |
| <2010.04 mg | ||||
| 2010.04 to <2681.65 mg | 104 (0.32) | 94 (0.30) | 1.07 (0.81, 1.41) | |
| 2681.65 to <3514.06 mg | 107 (0.34) | 87 (0.27) | 1.24 (0.93, 1.64) | |
| ≥3514.06 mg | 121 (0.38) | 103 (0.32) | 1.19 (0.91, 1.54) | |
| Quartile of dietary potassium intake | 0.418 | |||
| <1902.56 mg | 125 (0.39) | 114 (0.37) | 1.06 (0.82, 1.37) | |
| 1902.56 to <2486.46 mg | 107 (0.34) | 87 (0.27) | 1.25 (0.94, 1.66) | |
| 2486.46 to <3161.51 mg | 112 (0.34) | 103 (0.33) | 1.06 (0.81, 1.39) | |
| ≥3161.51 mg | 105 (0.33) | 77 (0.24) | 1.41 (1.05, 1.89) | |
| Quartile of dietary iron intake | 0.895 | |||
| <8.77 mg | 114 (0.36) | 103 (0.33) | 1.09 (0.83, 1.42) | |
| 8.77 to <11.86 mg | 115 (0.36) | 100 (0.32) | 1.15 (0.88, 1.50) | |
| 11.86 to <15.53 mg | 104 (0.33) | 83 (0.26) | 1.25 (0.94, 1.67) | |
| ≥15.53 mg | 116 (0.36) | 95 (0.30) | 1.22 (0.93, 1.60) | |
| Quartile of dietary oxalic acid intake | 0.776 | |||
| <201.98 mg | 121 (0.38) | 112 (0.36) | 1.07 (0.83, 1.38) | |
| 201.98 to <276.35 mg | 115 (0.35) | 93 (0.30) | 1.17 (0.89, 1.55) | |
| 276.35 to <371.41 mg | 100 (0.31) | 87 (0.27) | 1.14 (0.86, 1.53) | |
| ≥371.41 mg | 113 (0.36) | 89 (0.28) | 1.30 (0.99, 1.72) | |
| Quartile of dietary vitamin C intake | 0.761 | |||
| <53.28 mg | 122 (0.38) | 97 (0.31) | 1.22 (0.94, 1.60) | |
| 53.28 to <84.68 mg | 128 (0.40) | 103 (0.32) | 1.22 (0.94, 1.59) | |
| 84.68 to <120.79 mg | 105 (0.33) | 103 (0.32) | 1.02 (0.78, 1.34) | |
| ≥120.79 mg | 94 (0.29) | 78 (0.25) | 1.22 (0.90, 1.64) | |
| Quartile of total calcium intake4 | 0.243 | |||
| <674.58 mg | 131 (0.41) | 98 (0.32) | 1.31 (1.01, 1.71) | |
| 674.58 to <1030.36 mg | 102 (0.32) | 102 (0.33) | 0.98 (0.74, 1.29) | |
| 1030.36 to <1490.12 mg | 117 (0.37) | 82 (0.26) | 1.41 (1.06, 1.87) | |
| ≥1490.12 mg | 93 (0.30) | 87 (0.28) | 1.12 (0.83, 1.50) | |
| n (%) | n (%) | |||
| Taking calcium as a single supplement | 0.741 | |||
| No | 374 (0.36) | 315 (0.30) | 1.18 (1.02, 1.37) | |
| Yes | 75 (0.31) | 66 (0.28) | 1.10 (0.79, 1.53) | |
| Quartile of total vitamin C intake5 | 0.982 | |||
| <77.33 mg | 124 (0.38) | 100 (0.31) | 1.21 (0.93, 1.57) | |
| 77.33 to <136.81 mg | 106 (0.33) | 93 (0.29) | 1.11 (0.84, 1.46) | |
| 136.81 to <356.65 mg | 102 (0.32) | 87 (0.27) | 1.19 (0.89, 1.58) | |
| ≥356.65 mg | 117 (0.37) | 101 (0.32) | 1.18 (0.90, 1.54) | |
| Quartile of total vitamin D intake5 | 121 (0.37) | 105 (0.33) | 1.11 (0.85, 1.44) | 0.397 |
| <3.25 μg | ||||
| 3.25 to <6.64 μg | 106 (0.33) | 103 (0.32) | 1.01 (0.77, 1.32) | |
| 6.64 to <13.28 μg | 109 (0.34) | 76 (0.24) | 1.42 (1.06, 1.91) | |
| ≥13.28 μg | 113 (0.37) | 97 (0.30) | 1.21 (0.92, 1.58) | |
| Quartile of total potassium intake5 | 122 (0.38) | 112 (0.36) | 1.06 (0.82, 1.37) | 0.281 |
| <1908.58 mg | ||||
| 1908.58 to <2495.70 mg | 109 (0.34) | 88 (0.27) | 1.25 (0.94, 1.66) | |
| 2495.70 to <3172.10 mg | 112 (0.34) | 106 (0.33) | 1.03 (0.79, 1.35) | |
| ≥3172.10 mg | 106 (0.33) | 75 (0.23) | 1.45 (1.08, 1.95) | |
| Quartile of total iron intake5 | 117 (0.36) | 109 (0.35) | 1.05 (0.81, 1.36) | 0.685 |
| <10.32 mg | ||||
| 10.32 to <15.67 mg | 106 (0.33) | 84 (0.26) | 1.25 (0.94, 1.67) | |
| 15.67 to <27.32 mg | 118 (0.37) | 92 (0.29) | 1.30 (0.99, 1.71) | |
| ≥27.32 mg | 108 (0.34) | 96 (0.30) | 1.15 (0.87, 1.52) | |
| History of kidney stone | 444 (0.35) | 372 (0.30) | 1.18 (1.03, 1.36) | 0.200 |
| No | ||||
| Yes | 5 (0.45) | 9 (0.73) | 0.58 (0.19, 1.75) |
CaD, calcium plus vitamin D; HS, high school; GED, general educational development; E, estrogen; P, progestin; HT, hormone therapy; WHI, Women's Health Initiative; DM, dietary modification; METs, metabolic equivalents; HR, hazard ratio.
From Cox proportional hazards regression models stratified according to age, DM participation, and history of kidney stone.
Test of linear trend for continuous variables. For categorical variables, P values for interaction from likelihood ratio test comparing models with and without interaction terms.
Combination of diet + supplements + medication.
Combination of diet + supplements.
FIGURE 2.
Kaplan-Meier survival curve for risk of self-reported urinary tract stones in the calcium plus vitamin D (CaD; black line) and placebo (gray line) groups.
The remainder of Table 2 shows the rates of self-reported stones for the active medication and placebo groups according to demographic, anthropomorphic, dietary, and other hypothesized risk factors for urinary tract stone disease. Whereas none of these subgroup analyses was statistically significant in a test for interaction with the main outcome effect, some findings were of interest. No obvious effect of age or other sociodemographic characteristics on stone risk was found, and no apparent effect according to participation in any of the other WHI trials was found. Similarly, no significant interactive effect of body weight or body mass index, thiazide diuretic use, regional solar irradiance levels, alcohol use, physical activity, prior menopausal hormone use, or dietary micro- or macronutrient composition on the risk of urinary tract stones was found. Of note, neither the total calcium intake at baseline nor the use of calcium supplements as a single agent at baseline was associated with the risk of stones. Also, as noted in Table 2, reported recurrent stone occurrence during the trial interval was not related to a history of stone disease.
In the sensitivity analysis, including only stone outcomes during active adherence, as defined above, the findings were similar in magnitude to those for the intention-to-treat analysis, yet were statistically insignificant (hazard ratio: 1.21; 95% CI: 0.98, 1.34). Finally, we determined the number of hospital admissions during which any diagnosis used the ICD-9 rubrics for urinary tract stone disease. Only a small proportion of those reporting urinary tract stones had a hospital admission in which urinary tract stone–related diagnoses occurred—35 each in the active medication and placebo groups; these rates were not significantly different.
DISCUSSION
From the literature, reported rates of urinary tract stone occurrence (history) in the developed world vary according to demographic characteristics and have apparently increased in the past 20–30 y, except in Iceland, where there has been a nonsignificant increase in men but no increase in women (11–16). A portion of this increase may be related to more frequent use of diagnostic medical imaging incidentally detecting asymptomatic nephrolithiasis. In a recent review of urinary tract stone occurrence in American adults, the lifetime prevalence increased by 37% between 1976 to 1980 and 1988 to 1994, and was higher among men overall, although the rate of increase was higher in women (46%) than in men (29%) (11). Also, the lifetime prevalence was higher in whites than in Hispanics, Mexican Americans, Asians, and blacks, the latter group having the lowest rates (11, 16). Interestingly, the lifetime cumulative incidence of symptomatic stones in women in one US community increased from 4.3% in 1970 to 9.2% in 1990 and declining to 6.8% in 2000, resulting in a decrease in the male to female ratio from 3:2 to 1:3 over the same period. In this same study, the overall stone incidence rates in the population remained unchanged from 1970 to 2000, but the incidence in males decreased (15). Our finding of a 17% increase in the incidence of clinically diagnosed urinary tract stones suggests that one possible factor, CaD dietary supplement use by women, should be considered as a factor that has influenced the change in the rate of urinary tract stones in the population.
Numerous dietary, anatomic, metabolic, and endocrine risk factors have been variously associated with the risk of stones. With respect to calcium and vitamin D, our basically healthy population of postmenopausal women had a calculated total dietary calcium intake of ≈1150 mg and dietary vitamin D intake of ≈365 IU at baseline before study medication was begun, including dietary and supplement sources, so that the addition of further dietary calcium may have promoted hypercalciuria in the active medication group. However, no clear association of baseline total calcium intake with overall stone risk was observed, and the incident risk of stones was not excessively elevated in those taking calcium as a single supplement at baseline (Table 2). Most urinary tract stones contain calcium oxalate, and many stone formers have elevated concentrations of both urinary calcium and oxalate (17). In the current study, we did not perform urinary biochemical determinations, nor did we have any information regarding the chemical analysis of the incident stones. In women, supplementary calcium intake has been associated with a 17–20% increase in stone risk in observational studies, regardless of the relation to meals (18–20). It is also important to note that this study could not discriminate between the relative effects of calcium and vitamin D with respect to the occurrence of urinary stones.
Calcium intake over 12 y was assessed in 91,731 women (aged 34–59 y at entry) with no history of urinary tract stones in the Nurses’ Health Study (12) to determine the incidence of symptomatic stones. After adjustment for multiple factors, including age, high dietary calcium intake was associated with a lower incidence of stones (35% decrease in the highest quintile, 1098 mg/d), whereas supplement intake, at all amounts, was associated with an increased risk of 20%. Most women took the supplemental calcium either between meals or with meals that may have had a low oxalate content, based on food-frequency questionnaires (20). Magnesium and phosphorus intakes were not associated with a change in stone risk. It was not clear whether the women in that study also took vitamin D, as did the women in the WHI trial, the participants of which were also instructed to take study medications with meals. In addition to enhancing intestinal absorption of calcium, vitamin D may increase the renal tubular absorption of calcium (21).
In one study of men younger than 60 y, high dietary calcium was associated with decreased stone formation, but there was no effect on men older than 60 y, nor was supplemental dietary intake of >500 mg Ca associated with an increase in incident stone risk (22). As in the current study, and in another study (18), past or current hormone use in menopausal women was not associated with an increased rate of stones, regardless of the type of menopause.
Dietary factors variously associated with urinary tract stones, in addition to calcium and vitamin D, have included oxalate, sucrose, sodium, animal protein, and fluids, with some differences observed between men and women (12, 19, 22–24). Because most stones contain calcium oxalate, the role of oxalates is becoming of particular interest to investigators. Oxalate absorption from the gastrointestinal tract is normally low, except in stone formers, in whom it appears to be elevated. The rate of absorption may be related to the amount that is ionized and available at lower oxalate intakes, whereas higher intakes may lead to more complex forms that are not easily absorbed. Dietary calcium may have a protective effect on stone formation by increasing calcium oxalate binding in the gastrointestinal tract, which decreases its absorption (19, 26). A great deal is yet to be learned about the oxalate content of foods, some of which are eaten erratically, and the factors that affect absorption and metabolism. Gene variation and expression may also play a role in the absorption and/or excretion of oxalate (27).
Overall, dietary fluid intake has been associated with a beneficial effect on the occurrence of urinary tract stones. The calcium content of community water does not appear to be associated with an increase in history of urinary tract stones in women (28). Tea and coffee use has been associated with a minimally increased risk, and wine has been associated with a decreased risk (22). Other associations with an increase in stone risk having a greater effect in women than in men are body mass index, obesity, waist circumference and weight gain (29), and histories of either hypertension, especially in larger women (30, 31), or diabetes (32, 33). None of these factors substantially altered the effect of the primary CaD intervention in this study.
With regard to selected nondietary factors, the highest rate of stone occurrence in the United States has been reported in the southeast—associated with an increased sun exposure (10). Similar findings were seen in an Italian study (12). Yet, in the current study using Langley categories, no significant effect of sun exposure on the renal effect of the CaD intervention was seen. A family history of stones has a significant effect on risk, which suggests an important genetic or familial component to the intervention effect; however, little is known about the relative effects of genetics and environment on this finding. Of interest, some participants in the study had a personal history of stone disease, in violation of the WHI protocol; however, this history was not associated with an increase in stone risk. It is possible that calcium citrate as a supplement might be associated with a lesser risk of urinary tract stones, as was suggested in one small study of 18 postmenopausal women (34). This finding warrants further study.
In summary, in this experimental intervention of generally healthy postmenopausal women, a 17% increased risk of self-reported urinary tract stones was observed. This finding was generally not altered by other proven or potential risk factors for such stones. An increased risk of self-reported urinary tract stone was found in women in the intervention group, which could have important population implications because of the large number of women consuming these supplements and the changes in over-the-counter doses now available. The absolute difference in occurrence of urinary tract stones between the groups was small: 0.35% of women in the intervention group and 0.30% of women in the control group reported stones. Furthermore, <10% of women who reported urinary tract stones had related hospitalizations. Note that other adverse effects of calcium and vitamin D supplements have been described, including hypercalcemia, renal function abnormalities, and acute vitamin D toxicity. Furthermore, the possibility exists that an increased calcium intake (without vitamin D supplementation) is associated with a higher risk of myocardial infarction (35).
A major potential limitation of this study was that the reported stone occurrence was not validated by clinical records; recurring stones may not have been reported by some participants because the questionnaire only specifically addressed the first occurrence of urinary tract stones. However, the blinded experimental design greatly enhanced the probability that the reports were not biased. No other intervention study of similar or larger size has reported this effect. It would be very helpful for other studies of similar scope and design to carefully monitor participants for incident urinary tract stone outcomes.
Acknowledgments
The WHI investigators and staff—Program Office: Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller (National Heart, Lung, and Blood Institute, Bethesda, MD). Clinical Coordinating Center: Ross Prentice, Garnet Anderson, Andrea LaCroix, and Charles Kooperberg (Fred Hutchinson Cancer Research Center, Seattle, WA); Evan Stein (Medical Research Laboratories, Highland Heights, KY); and Steven Cummings (University of California at San Francisco, San Francisco, CA). Clinical Centers: Sylvia Wassertheil-Smoller (Albert Einstein College of Medicine, Bronx, NY), Haleh Sangi-Haghpeykar (Baylor College of Medicine, Houston, TX), JoAnn E Manson (Brigham and Women's Hospital, Harvard Medical School, Boston, MA), Charles B Eaton (Brown University, Providence, RI), Lawrence S Phillips (Emory University, Atlanta, GA), Shirley Beresford (Fred Hutchinson Cancer Research Center, Seattle, WA), Lisa Martin (George Washington University Medical Center, Washington, DC), Rowan Chlebowski (Los Angeles Biomedical Research Institute at Harbor–UCLA Medical Center, Torrance, CA), Erin LeBlanc (Kaiser Permanente Center for Health Research, Portland, OR), Bette Caan (Kaiser Permanente Division of Research, Oakland, CA), Jane Morley Kotchen (Medical College of Wisconsin, Milwaukee, WI), Barbara V Howard (MedStar Research Institute/Howard University, Washington, DC), Linda Van Horn (Northwestern University, Chicago/Evanston, IL), Henry Black (Rush Medical Center, Chicago, IL), Marcia L Stefanick (Stanford Prevention Research Center, Stanford, CA), Dorothy Lane (State University of New York at Stony Brook, Stony Brook, NY), Rebecca Jackson (The Ohio State University, Columbus, OH), Cora E Lewis (University of Alabama at Birmingham, Birmingham, AL), Cynthia A Thomson (University of Arizona, Tucson/Phoenix, AZ), Jean Wactawski-Wende (University at Buffalo, Buffalo, NY), John Robbins (University of California at Davis, Sacramento, CA), F Allan Hubbell (University of California at Irvine, CA), Lauren Nathan (University of California at Los Angeles, Los Angeles, CA), Robert D Langer (University of California at San Diego, La Jolla/Chula Vista, CA), Margery Gass (University of Cincinnati, Cincinnati, OH), Marian Limacher (University of Florida, Gainesville/Jacksonville, FL), J David Curb (University of Hawaii, Honolulu, HI), Robert Wallace (University of Iowa, Iowa City/Davenport, IA), Judith Ockene (University of Massachusetts/Fallon Clinic, Worcester, MA), Norman Lasser (University of Medicine and Dentistry of New Jersey, Newark, NJ), Mary Jo O'Sullivan (University of Miami, Miami, FL), Karen Margolis (University of Minnesota, Minneapolis, MN), Robert Brunner (University of Nevada, Reno, NV), Gerardo Heiss (University of North Carolina, Chapel Hill, NC), Lewis Kuller (University of Pittsburgh, Pittsburgh, PA), Karen C Johnson (University of Tennessee Health Science Center, Memphis, TN), Robert Brzyski (University of Texas Health Science Center, San Antonio, TX), Gloria E Sarto (University of Wisconsin, Madison, WI), Mara Vitolins (Wake Forest University School of Medicine, Winston-Salem, NC), and Michael S Simon (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI). WHI Memory Study: Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, NC).
We appreciate the skillful assistance of Michelle Birt-Leeds in the preparation of this manuscript.
The authors’ responsibilities were as follows—RBW, JW-W, MJO, JCL, BC, and MG: designed the research; RBW, JW-W, MJO, BC, MG, and KM: conducted the research; JCL: analyzed the data; RBW, JW-W, MJO, and JCL: wrote the manuscript; and RBW, JW-W, MJO, JCL, BC, MG, and KM: provided significant advice. All authors read and approved the final manuscript. None of the authors declared a conflict of interest.
REFERENCES
- 1.National Osteoporosis Foundation. Available from: http://www.nof.org/prevention (cited 17 June 2008)
- 2.Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 2006;354:669–83 [DOI] [PubMed] [Google Scholar]
- 3.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol 2003;13(suppl):S5–17 [DOI] [PubMed] [Google Scholar]
- 4.Curb JD, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol 2003;13(suppl):S122–8 [DOI] [PubMed] [Google Scholar]
- 5.Hays J, Hunt JR, Hubbell FA, et al. The Women's Health Initiative recruitment methods and results. Ann Epidemiol 2003;13(suppl):S18–77 [DOI] [PubMed] [Google Scholar]
- 6.Jackson RD, LaCroix AZ, Cauley JA, McGowan J. The Women's Health Initiative calcium-vitamin D trial: overview and baseline characteristics of participants. Ann Epidemiol 2003;13(suppl):S98–106 [DOI] [PubMed] [Google Scholar]
- 7.Ritenbaugh C, Patterson RE, Chlebowski RT, et al. The Women's Health Initiative dietary modification trial: overview and baseline characteristics of participants. Ann Epidemiol 2003;13(suppl):S87–97 [DOI] [PubMed] [Google Scholar]
- 8.Stefanick ML, Cochrane BB, Hsia J, Barad DH, Liu JH, Johnson SR. The Women's Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Ann Epidemiol 2003;13(suppl):S78–86 [DOI] [PubMed] [Google Scholar]
- 9.National Center for Health Statistics and the Centers for Medicare and Medicaid Services International classification of diseases, 9th revision, clinical modification. Last update approved 10 October 2010. Available from: http://www.cdc.gov/nchs/icd/icd9cm.htm (cited 4 April 2011)
- 10.Soucie JM, Coates RJ, McClellan W, Austin H, Thun M. Relation between geographic variability in kidney stone prevalence and risk factors for stones. Am J Epidemiol 1996;143:487–95 [DOI] [PubMed] [Google Scholar]
- 11.Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States:1976-1994. Kidney Int 2003;63:1817–23 [DOI] [PubMed] [Google Scholar]
- 12.Serio A, Fraioli A. Epidemiology of nephrolithiasis. Nephron 1999;81(suppl):26–30 [DOI] [PubMed] [Google Scholar]
- 13.Trinchieri A, Coppi F, Montanari E, Del Nero A, Zanetti G, Pisani E. Increase in the prevalence of symptomatic upper urinary tract stones in the last ten years. Eur Urol 2000;37:23–5 [DOI] [PubMed] [Google Scholar]
- 14.Indridason OS, Birgisson S, Edvardsson VO, Sigvaldason H, Sigfusson N, Palsson R. Epidemiology of renal stones in Iceland. Scand J Urol Nephrol 2006;40:215–20 [DOI] [PubMed] [Google Scholar]
- 15.Lieske JC, Pena de la Vega LS, Slezak JM, et al. Renal stone epidemiology in Rochester, Minnesota: an update. Kidney Int 2006;69:760–4 [DOI] [PubMed] [Google Scholar]
- 16.Soucie JM, Thun MJ, Coates RJ, McClellan W, Austin A. Demographic and geographic variability of kidney stones in the United States. Kidney Int 1994;46:893–9 [DOI] [PubMed] [Google Scholar]
- 17.Curhan GC, Taylor EN. 24 hour uric acid excretion and the risk of kidney stones. Kidney Int 2008;73:489–96 [DOI] [PubMed] [Google Scholar]
- 18.Mattix Kramer HJ, Grodstein F, Stampfer MJ, Curhan GC. Menopause and postmenopausal hormone use and risk of incident kidney stones. J Am Soc Nephrol 2003;14:1272–7 [DOI] [PubMed] [Google Scholar]
- 19.Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 1993;328:833–8 [DOI] [PubMed] [Google Scholar]
- 20.Curhan GC, Willett WC, Speizer FE, Spiegleman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk of kidney stones for women. Ann Intern Med 1997;126:497–504 [DOI] [PubMed] [Google Scholar]
- 21.Cochran M, Coates PT, Morris HA. The effect of calcitriol on fasting urinary calcium loss and renal tubular reabsorption of calcium in patients with mild renal failure—actions of a permissive hormone. Clin Nephrol 2005;64:98–102 [DOI] [PubMed] [Google Scholar]
- 22.Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of followup. J Am Soc Nephrol 2004;15:3225–32 [DOI] [PubMed] [Google Scholar]
- 23.Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women. Arch Intern Med 2004;164:885–91 [DOI] [PubMed] [Google Scholar]
- 24.Sowers MR, Jannausch M, Wood C, Pope SK, Lachance L, Peterson B. Prevalence of renal stones in a population based study with dietary calcium, oxalate and medication exposures. Am J Epidemiol 1998;147:914–21 [DOI] [PubMed] [Google Scholar]
- 25.Taylor EN, Curhan GC. Role of nutrition in the formation of calcium-containing kidney stones. Nephron Physiol 2004;98:p55–65 [DOI] [PubMed] [Google Scholar]
- 26.Taylor EN, Curhan GC. Oxalate intake and risk of nephrolithiasis. J Am Soc Nephrol 2007;18:2198–204 [DOI] [PubMed] [Google Scholar]
- 27.Holmes RP, Assimos DG, Goodman HO. Genetic and dietary influences on urinary oxalate excretion. Urol Res 1998;26:195–200 [DOI] [PubMed] [Google Scholar]
- 28.Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Beverage use and risk for kidney stones in women. Ann Intern Med 1998;128:534–40 [DOI] [PubMed] [Google Scholar]
- 29.Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain and the risk of kidney stones. JAMA 2005;293:455–62 [DOI] [PubMed] [Google Scholar]
- 30.Madore F, Stampfer MJ, Willett EC, Speizer FE, Curhan GC. Nephrolithiasis and risk of hypertension in women. Am J Kidney Dis 1998;32:802–7 [DOI] [PubMed] [Google Scholar]
- 31.Gillen DL, Coe FL, Worcester EM. Nephrolithiasis and increased blood pressure among females with high body mass index. Am J Kidney Dis 2005;46:263–9 [DOI] [PubMed] [Google Scholar]
- 32.Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int 2005;68:1230–5 [DOI] [PubMed] [Google Scholar]
- 33.Lieske JC, Pena de la Vega LS, Gettman MT, et al. Diabetes mellitus and the risk of urinary tract stones: a population-based case-control study. Am J Kidney Dis 2006;48:897–904 [DOI] [PubMed] [Google Scholar]
- 34.Sakhaee K, Poindexter JR, Griffith CS, Pak CY. Stone forming risk of calcium citrate supplementation in healthy postmenopausal women. J Urol 2004;172:958–61 [DOI] [PubMed] [Google Scholar]
- 35.Bolland MJ, Avenell A, Baron JA, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ 2010;341:c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]