Abstract
Background: Studies in mice indicate that the gut microbiome influences both sides of the energy-balance equation by contributing to nutrient absorption and regulating host genes that affect adiposity. However, it remains uncertain as to what extent gut microbiota are an important regulator of nutrient absorption in humans.
Objective: With the use of a carefully monitored inpatient study cohort, we tested how gut bacterial community structure is affected by altering the nutrient load in lean and obese individuals and whether their microbiota are correlated with the efficiency of dietary energy harvest.
Design: We investigated dynamic changes of gut microbiota during diets that varied in caloric content (2400 compared with 3400 kcal/d) by pyrosequencing bacterial 16S ribosomal RNA (rRNA) genes present in the feces of 12 lean and 9 obese individuals and by measuring ingested and stool calories with the use of bomb calorimetry.
Results: The alteration of the nutrient load induced rapid changes in the gut microbiota. These changes were directly correlated with stool energy loss in lean individuals such that a 20% increase in Firmicutes and a corresponding decrease in Bacteroidetes were associated with an increased energy harvest of ≈150 kcal. A high degree of overfeeding in lean individuals was accompanied by a greater fractional decrease in stool energy loss.
Conclusions: These results show that the nutrient load is a key variable that can influence the gut (fecal) bacterial community structure over short time scales. Furthermore, the observed associations between gut microbes and nutrient absorption indicate a possible role of the human gut microbiota in the regulation of the nutrient harvest. This trial was registered at clinicaltrials.gov as NCT00414063.
See corresponding article on page 1.
INTRODUCTION
The alteration of the energy-balance equation, which is defined by the equilibrium of energy intake and energy expenditure (1–5), leads to weight gain. One less-extensively-studied component of the energy-balance equation is energy loss in stools and urine. Previous studies of healthy adults showed that ≈5% of ingested calories were lost in stools and urine (6). Individuals who consume high-fiber diets exhibit a higher fecal energy loss than individuals who consume low-fiber diets with an equivalent energy content (7, 8). Webb and Annis (9) studied stool energy loss in 4 lean and 4 obese individuals and showed a tendency to lower the fecal energy excretion in obese compared with lean study participants.
Recent studies in mice have indicated an interrelation between energy balance, diet, and the composition of the gut microbial community and its pool of genes (10–14). Gut-microbiota transplants into germ-free mouse recipients indicated that associations between the gut microbial ecology and obesity may be causal rather than casual (13, 14), which was underscored by the finding that the transplantation of a gut microbiome from obese donors into germ-free recipients resulted in a greater increase in recipient adiposity than did transplants from lean donors (13). Accordingly, the microbiota influences nutrient partitioning by modulating the expression of host genes (eg, studies in genetically engineered mice indicated that the microbiota-directed suppression of the intestinal expression of a circulating lipoprotein lipase inhibitor produced in the gut epithelium was one mechanism by which the gut community could increase adiposity) (10). The mouse gut microbiota is also responsive to reduced caloric intake, with an increased representation of Bacteroidetes and reduced representation of Firmicutes, which are the 2 dominant bacterial phyla (15). Recent results of humanized gnotobiotic mice have provided direct evidence of the rapid adaptations that gut microbiota make to changes in the nutrient load in rodents (16). For translation into human physiology, one of the major challenges is the difficulty in adequately controlling and/or monitoring energy intake and expenditure.
Recent data in humans have shown a correlation between obesity and the gut microbial community structure (17, 18) and the abundance of genes in the microbiome involved in processing components of the diet (19). Comparable results have been obtained from studies of a small cohort of individuals studied before and after a gastric bypass (20), whereas reciprocal changes (increases in Bacteroidetes and reductions in Firmicutes) have been documented with weight loss (17, 21, 22). The interrelation between the microbial community structure, dietary nutrient load, and host adiposity has to be further investigated in humans. Therefore, we performed an inpatient study to carefully measure the energy intake and loss in lean and obese individuals as they consumed 2 calorically distinct diets for brief periods of time while simultaneously monitoring the microbial community structure by using culture-independent metagenomic methods.
SUBJECTS AND METHODS
Research volunteers
Twelve lean [body mass index (in kg/m2) >18.5 and <25] and 9 obese (body mass index ≥ 30) adult white men participated in this study. Volunteers were nonsmokers and healthy according to medical histories (including acute and chronic diseases that involve the gastrointestinal tract), physical examinations, and laboratory tests. Antacids and laxatives were discontinued for ≥3 wk before admission. The use of antibiotics or probiotics in ≤3 mo of the study entry was an exclusion criterion. Study participants were also screened for occult blood in their feces (by using stool guaiac testing on 2 occasions), ova, and parasites (on 2 occasions) and celiac disease (by using total immunoglobulin A and antitissue transglutaminase antibodies) to rule out subclinical gastrointestinal conditions that might have affected nutrient absorption. All volunteers provided written and informed consent before participation in this study. The protocol and consent form were approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Disease.
Study design
Volunteers were admitted to our clinical research unit and consumed a weight-maintaining diet for 3 consecutive days [calories for the weight-maintaining diet were calculated on the basis of specific formulas derived in our study unit as body weight (kg) × 0.5 + 1973] (23).
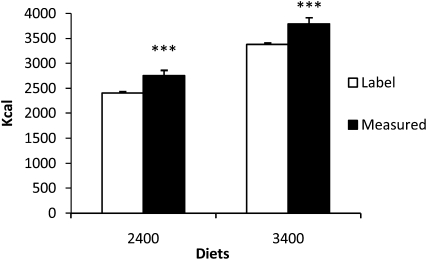
On day 3 after an overnight fast, a 75-g oral-glucose-tolerance test was administered. To minimize effects of fluid consumption on colonic transit time, each volunteer was allowed to drink ≤2000 mL carbonated, noncaffeinated, noncaloric beverages or water plus the fluids brought with each meal. On day 3, the liquid drink for the oral-glucose-tolerance test was not counted toward the 2000 mL of daily fluid. The following day, in a random crossover fashion, volunteers consumed either a 2400- or 3400-kcal/d diet for the next 3 d, each diet followed by a 3-d washout period with a weight-maintaining diet. These diets were chosen on the basis of the intention to directly compare absolute stool calories, rather than stool calories as a percentage of energy intake, between lean and obese individuals when they were fed identical diets. We chose 2 different caloric diets to investigate the response to altering the amount of total calories. However, direct measurements of ingested calories by bomb calorimetry of duplicated meals revealed that the calorie content of each diet was higher and more varied than indicated by the food labels (Figure 1), which meant that we had to express the calorie loss as a percentage of ingested calories. All diets had a similar macronutrient profile (24% protein, 16% fat, and 60% carbohydrates ) and fiber content (see supplemental Tables S1–S6 under “Supplemental data” in the online issue for a complete list of daily food items and macronutrient profiles of the experimental diets). A nonabsorbable dye marker (FD&C blue) was used to determine the beginning and end of the period of the 2400- or 3400-kcal/d diet (see Sample collection, storage, and preparation). Thereafter, the energy content of stool and urine samples was analyzed by using bomb calorimetry. Stools for culture-independent metagenomic studies of the microbiota were collected and stored at −70°C; samples were obtained on day 2 of admission and as close to the midpoint of each diet as feasible.
FIGURE 1.
Mean (±SD) food energy content: product label compared with bomb calorimetry. Open columns represent food calories as shown on the product label. Closed columns represent food calories of duplicated meals measured by bomb calorimetry. ***P < 0.0001 (Student's t test).
Sample collection, storage, and preparation
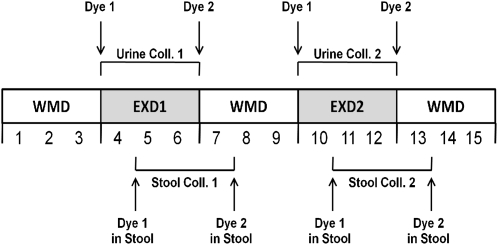
The blue-dye marker was administered at breakfast at the start of the 2400- or 3400-kcal/d diets and again at breakfast on the first day of return to the weight-maintaining diet. Stools were collected from the appearance of the first marker until the appearance of the second marker. Urine was collected after the start of the 2400- or 3400-kcal/d diet until the beginning of breakfast with the weight-maintaining diet (Figure 2). Stool samples for calorimetry were stored at −20°C, and after the 3-d collection period, the sample was weighed, and distilled water equal to the weight of the stool was added. Samples were subsequently homogenized and followed by lyophilization of the feces-water slurry (24). Likewise, food samples were blended and 200 g distilled water was added before lyophilization of the food-water slurry. Daily urine collections underwent direct lyophilization. Lyophilization was performed at −77°C with a Freezemobile 12XL instrument (Virtis, Gardiner, NY). After completion of the drying process, the sample was weighed, and ≈1-g pellets of dried food, feces, or urine were produced with a pellet press (PARR Instrument Co, Moline, IL).
FIGURE 2.
Study design. Numbers below diet boxes represent study days. WMD, weight-maintaining diet; EXD1, experimental diet 1 representing either 2400 or 3400 kcal/d; EXD2, experimental diet 2 representing either 2400 or 3400 kcal/d; Dye 1, administration of dye 1 (FD&C blue) with food; Dye 2, administration of dye 2 (FD&C blue) with food; Dye 1 in stool, appearance of dye 1 in stool; Dye 2 in stool, appearance of dye 2 in stool; Coll., collection.
Calorie content of food
During the time that they were assigned 2400- or 3400-kcal/d diets, volunteers ate under supervision and were asked to consume all of the food provided with each experimental meal. For each meal, 2 identical trays were prepared; one tray was selected at random and given to the volunteer, and the other tray was used to assess the calorie content of the diet. Unconsumed food was returned, and its caloric content was measured by bomb calorimetry. These calories were subtracted from those measured for the meals for that day [one individual consumed ≈260 kcal (≈11.5%) and ≈472 kcal (≈13.6%) less with the 2400- and 3400-kcal/d diets, respectively]. Because of the need to calculate the relative nutrient load that volunteers consumed with the 2 diets, the percentage of weight-maintenance energy needs was calculated for each volunteer who consumed each diet as follows
 |
Bomb calorimetry
To measure the energy content of each biological sample, a pellet (see Sample collection, storage, and preparation) was bombed with an Isoperibol Calorimeter 6200 instrument with a model 1108 oxygen bomb (Parr Instrument Co, Moline, IL). Details about this method have been described elsewhere (25). Briefly, after preparation, the pellet was weighed and placed in a model 1108 oxygen bomb with contact to a 10-cm fuse wire connected to a 2901EB ignition unit (Parr Instrument Co). The bomb was placed in a bomb cylinder surrounded by 2000 mL distilled water. The heat produced at the combustion of the pellet was sensed as a rise of water temperature. Bombs were calibrated by using benzoic acid before use. To give the energy equivalent (W) per change in water temperature (ΔT), benzoic acid standards were run once every 10 burns. The energy content of the pellet (ES) was calculated as follows:
Each sample was run in duplicate (2 pellets were burned) with a CV of 2.1% with the 2400-kcal/d diet and 2.2% with the 3400-kcal/d diet. The total number of calories in the sample was calculated on the basis of weights of the sample, slurry, and lyophilized material.
Community DNA preparation
A fecal sample was collected on day 3 of admission while the subject was consuming the weight-maintaining diet. During the 2400- and 3400-kcal/d diets, a stool sample was collected as close to the midpoint of each diet as feasible. Fecal samples were stored at −70°C before processing. DNA was extracted by bead beating followed by phenol-chloroform extraction, as previously described (18).
Sequencing of 16S ribosomal RNA gene amplicons
The variable region 2 (V2) of bacterial 16S ribosomal RNA (rRNA) genes present in fecal community DNA was targeted for polymerase chain reaction amplification by using primers 8F and 338R (error-correcting, sample-specific barcodes were incorporated into the reverse primer; see supplemental Table S7 under “Supplemental data” in the online issue). Bar-coded amplicons from the various fecal samples were subjected to multiplex pyrosequencing (454 FLX Standard method) (18). Sequencing data of V2 16S rRNA genes were subsequently preprocessed to remove sequences with low-quality scores, sequences with ambiguous characters, and sequences outside the length bounds (200–300 nucleotides). Reads were binned according to their sample-specific, error-correcting barcode. Similar sequences were identified with the cd-hit program (26) with the following variables: a minimum coverage of 99% and minimum pairwise identity of 97% [see references 18 and 27 for more information about phylotype binning (operational taxonomic unit picking), UniFrac-based clustering, taxonomic assignment, and tree building (http://bmf.colorado.edu/unifrac)].
Statistical analyses
Statistical data analyses were carried out with SAS Enterprise Guide application (version 4.1; Cary, NC). Group comparisons were analyzed by using Student's t test for normally distributed variables. Wilcoxon's rank test was used for skewed variables. Pearson's correlations were used to test associations between variables with normal distributions; otherwise, Spearman's rank test was used. To fit linear regression models, skewed variables were log transformed to approach a normal distribution. The paired t test was used to analyze the change in stool calories while subjects consumed the 2 diets. Mixed models were used to account for multiple measurements in the same individual. Gaussian-distributed variables are shown as means ± SDs. α was set at P < 0.05.
RESULTS
Subject characteristics are shown in Table 1. Subjects did not differ by age, glucose-regulation status, or nutrient transit time when consuming either experimental diet (2400 and 3400 kcal/d, in random order), but by design, subjects differed by adiposity.
TABLE 1.
Study-group characteristics
| Lean1 | Obese | P | |
| n | 12 | 9 | — |
| Age (y) | 32.8 ± 9.22 | 35.8 ± 10.6 | 0.4 |
| BMI (kg/m2) | 23.4 ± 1.7 | 40.4 ± 4.6 | <0.001 |
| Body fat (%) | 17.7 ± 6.6 | 37.6 ± 3.2 | <0.001 |
| Fasting plasma glucose (mg/dL)3 | 91.9 ± 5.4 | 91.9 ± 3.6 | 0.87 |
| 2-h plasma glucose (mg/dL)3 | 109.9 ± 32.4 | 124.3 ± 30.6 | 0.28 |
| Fasting plasma insulin (μIU/mL)4 | 6.6 ± 0.4 | 10.3 ± 4.5 | 0.007 |
| 2-h plasma insulin (μIU/mL)4 | 25.9 ± 24.0 | 70.3 ± 37.5 | 0.009 |
| Weight-maintaining calories (kcal)5 | 2672 ± 44 | 3127 ± 185 | <0.001 |
| Transit time with 2400-kcal/d diet (min)6 | 1139 ± 391 | 951 ± 407 | 0.33 |
| Transit time with 3400-kcal/d diet (min)6 | 1158 ± 601 | 1126 ± 340 | 0.89 |
One overweight subject with a BMI (in kg/m2) of 26.1 was included in the lean group.
Mean ± SD (all such values).
Conversion factor to the International System of Units is 0.0555 (mg/dL to mmol/L).
Conversion factor to the International System of Units is 6.945 (μIU/mL to pmol/L).
The following equation was used to calculate individual weight-maintaining calories while in the inpatient clinical research unit: body weight × 9.5 + 1973 (for derivation of equation, see reference 23).
Time between diet ingestion (including the nonabsorbable dye marker) and excretion of the dye marker in stools.
Human gut microbiota
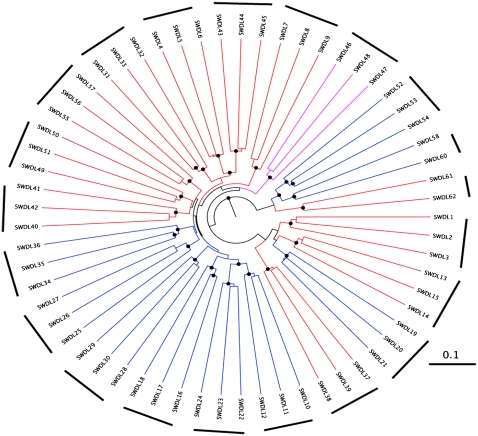
Members of 2 bacterial phyla, Bacteroidetes and Firmicutes, accounted for ≈97% of pyrosequencing reads of 16S rRNA genes (see supplemental Table S7 under “Supplemental data” in the online issue). UniFrac clustering of the microbial community structure showed that fecal samples taken from the same individual, regardless of the current diet, were more similar to each other than were samples obtained from different individuals (Figure 3), which underscored the relatively high level of interpersonal variation noted in other human studies (28, 29). With the initial weight-maintaining diet, we observed no statistically significant difference in the relative abundance of the 3 dominant bacterial phyla between lean and obese individuals as assessed by the sequencing of amplicons derived from V2 of bacterial 16S rRNA genes [22.36 ± 11.58% (lean) compared with 18.77 ± 15.93% (obese) of 16S rRNA gene sequences for Bacteroidetes (P = 0.56), 74.85 ± 12.05% (lean) compared with 78.69 ± 17.05% (obese) of 16S rRNA gene sequences for Firmicutes (P = 0.55), and 0.27% (0.17–0.40%) compared with 0.78% (0.28–0.91%) of 16S rRNA gene sequences for Actinobacteria (P = 0.28)]. Data are shown as median (25th to 75th percentile) due to skewed distribution.
FIGURE 3.
UniFrac analysis of the fecal microbiota of lean and obese individuals fed a 2400- or 3400-kcal/d diet. Unweighted UniFrac clustering was used to measure shared phylogenetic diversity. A radial tree was constructed with FigTree (http://tree.bio.ed.ac.uk/software/figtree/). Black bars indicate samples taken from the same individual; colors indicate samples taken from lean (red) or obese (blue) individuals. Purple represents a sample from an individual with a BMI (in kg/m2) of 26.1 who was included in the lean group in Table 1. Black circles on the nodes indicate a confidence level ≥0.7 (jackknife resampling was used to determine a confidence between 0 and 1). Southwestern diet label (SWDL) numbers represent samples collected during different diets (see supplemental Table S7 under “Supplemental data” in the online issue).
Gut microbiota and nutrient load
We initially made the observation that the nutrient load (ie, the precisely determined calorie content of meals by using bomb calorimetry) with both experimental diets was consistently higher than anticipated from calories listed on the food labels (Figure 1). We calculated the relative nutrient load consumed as
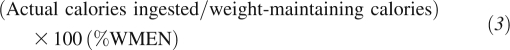 |
for each volunteer while consuming the 2400- and 3400-kcal/d diets and examined its association with the change in the proportional representation of the 2 dominant bacterial phyla.
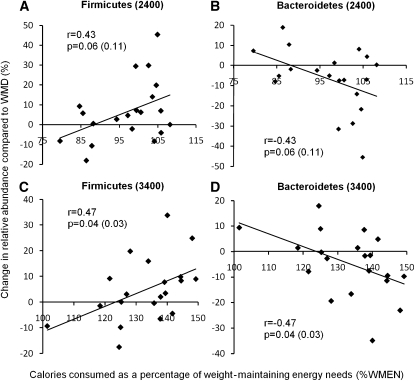
On the 2400-kcal/d diet, %WMEN was associated with changes in gut microbial communities compared with those with the weight-maintaining diet (Firmicutes: r = 0.43, P = 0.06; Bacteroidetes: r = −0.43, P = 0.06; Figure 4, A and B). With the 3400-kcal/d diet, in which the percentage of overfeeding was relatively greater, the change in composition of the microbiota was significantly associated with %WMEN (Firmicutes: r = 0.47, P = 0.04; Bacteroidetes: r = −0.47, P = 0.04; Figure 4, C and D). Whether volunteers received the 2400- or 3400-kcal/d diet first was randomized. However, there appeared to be a possible effect of the diet order in our results such that, when we examined the association between %WMEN and changes in the proportional representation of Firmicutes and Bacteroidetes for the first experimental diet given, the results were consistent with those seen with the 2400- and 3400-kcal/d diets [r = 0.42, P = 0.06 (Firmicutes); r = −0.39, P = 0.09 (Bacteroidetes)], although the same analysis for the second diet was not consistent [r = 0.04, P = 0.87 (Firmicutes); r = −0.03, P = 0.89 (Bacteroidetes)]. One potential confounding effect was the increased time between the initial prediet measurement of the fecal bacterial community structure and the measurement during the first diet relative to during the second diet. However, even after the diet order was controlled for, we observed similar associations between %WMEN and changes in proportional representation of Firmicutes and Bacteroidetes for the 2400-kcal/d diet (P = 0.11 and P = 0.11) and 3400-kcal/d diet (P = 0.03 and P = 0.03).
FIGURE 4.
Associations between the relative abundance of the 2 dominant bacterial phyla in the distal gut and nutrient load. Associations are shown between changes in the relative abundance of Firmicutes and Bacteroidetes from a weight-maintaining diet (WMD) and food energy content as a percentage of individual [n = 20 (diamonds)] weight-maintaining energy needs (%WMEN) with each experimental diet [2400-kcal/d diet (A and B); 3400-kcal/d diet (C and D)]. For 2 individuals, data for only one experimental diet were available. P and r values were derived from Pearson correlations; P values in parentheses were derived from multiple regression models adjusted for diet order.
The dominant class-level representatives of the Bacteroidetes and Firmicutes phyla were Bacteroidetes and Clostridia, irrespective of the experimental diet (2400-kcal diet: 15.4% + 76.4% = 91.8%; 3400-kcal diet: 17.3% + 75.5% = 92.8%). The dominant order-level representatives were Bacteroidales and Clostridiales with either experimental diet (2400-kcal diet: 14.7% + 74.2% = 88.9%; 3400-kcal diet: 16.9% + 73.7% = 90.6%).
Changes in the proportional representation (relative abundance) of the class-level taxa Bacteroidetes and Clostridia with either experimental diet relative to the weight-maintaining diet were variably associated with %WMEN, with decreases in relative abundance noted in the case of Bacteroidetes and increases documented for Clostridia [ie, with the 2400-kcal diet, r = −0.44 and P = 0.05 (0.10 adjusted for diet order) for Bacteroidetes and r = 0.33 and P = 0.15 (0.25) for Clostridia; with the 3400-kcal diet, r = −0.47 and P = 0.04 (0.03) for Bacteroidetes and r = 0.29 and P = 0.22 (0.14) for Clostridia (see supplemental Figure S1 under “Supplemental data” in the online issue)]. Similar observations were made for the order-level taxa Bacteroidales and Clostridiales [with the 2400-kcal diet: r = −0.44, P = 0.05 (0.10) and r = 0.33, P = 0.15 (0.25), respectively; with the 3400-kcal diet: r = −0.47, P = 0.04 (0.03) and r = 0.29, P = 0.21 (0.13), respectively (see supplemental Figure S2 under “Supplemental data” in the online issue)].
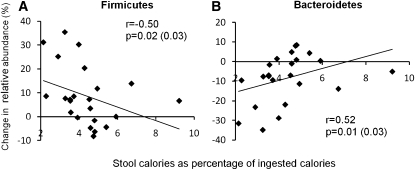
Gut microbiota and stool energy loss
The rapid changes in the gut microbiota observed after only 3 d led us to further explore a possible dynamic link between the microbial community configuration and nutrient absorption. In lean subjects, phylum-level changes in the fecal microbiota from prediet to the 2400- and 3400-kcal diets were significantly associated with calories in stools [Bacteroidetes: r = 0.52, P = 0.01 (0.03), derived from mixed-model accounting for multiple measurements and diet order; Firmicutes: r = −0.50, P = 0.02 (0.03)] (Figure 5). Similar results were seen for class-level taxa (Bacteroidetes and Clostridia) relative to the weight-maintaining diet and percentage of stool calories [r = 0.53, P = 0.009 (0.03) and r = −0.57, P = 0.005 (0.02), respectively]. This was also true for order-level taxa [Bacteriodales and Clostridiales: r = 0.52, P = 0.01 (0.03) and r = −0.53, P = 0.009 (0.03) (see supplemental Figure S3 under “Supplemental data” in the online issue)].
FIGURE 5.
Associations between nutrient absorption (stool calories) and phylum-level changes in the fecal bacterial community structure. Changes in Firmicutes (A) and Bacteroidetes (B) between the first weight-maintaining diet and either the 2400- or 3400-kcal/d diet were associated with nutrient absorption on either experimental diet (2400 or 3400 kcal/d) [n = 12, with 2 data points (diamonds) for each individual]. For one individual, data for only the 2400-kcal/d diet were available. P and r values were derived from Spearman's correlations; P values in parentheses were derived from mixed models to account for repeated measures and were adjusted for diet order.
A 20% increase in the proportional representation of Firmicutes was associated with an increase in nutrient absorption of ≈150 kcal, whereas a 20% increase in Bacteroidetes was associated with a decrease in absorption (≈150 kcal). The change in relative abundance ranged from −8% to 34% in Firmicutes and −35% to 8% in Bacteroidetes. However, under the conditions used for this clinical study, the association between changes in the gut microbial community structure and nutrient absorption was not observed in obese subjects [Bacteroidetes: P = 0.14 (0.59); Firmicutes: P = 0.15 (0.67)]. In the whole group, the interaction term with body weight status was significant for both changes in Bacteroidetes and Firmicutes (P = 0.02 and P = 0.02). There was also no association between stool calories as a percentage of ingested calories and the degree of over- or underfeeding in the whole group (r = −0.21, P = 0.20). These differences might suggest that obese and lean individuals respond differently to differences in nutrition or that the observed effect was dependent on the degree of over- and underfeeding relative to a weight-maintaining diet.
Caloric content of feces and urine
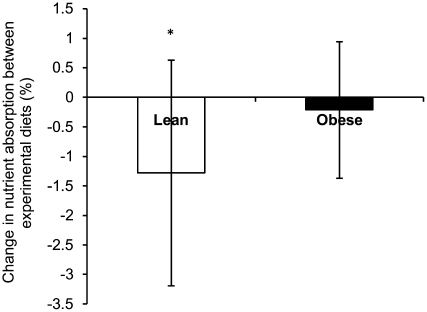
The individual difference in stool energy loss with the 3400-kcal/d diet compared with that with the 2400-kcal/d diet was significant in the case of lean individuals but not for obese individuals [−1.3 ± 1.9% (P = 0.04) and −0.2 ± 1.2% (P = 0.59), respectively; P values derive from paired t tests]. As shown in Figure 6, lean individuals lost relatively less energy in stools with the 3400-kcal/d diet than with the 2400-kcal/d diet. No significant difference was shown in energy excretion in stools between lean and obese subjects who consumed the 2400-kcal/d diet [134.3 ± 48.9 kcal/d (lean) compared with 133.2 ± 44.8 kcal/d (obese) (P = 0.96); 4.9 ± 1.8% compared with 4.8 ± 1.4% (P = 0.87), respectively] or 3400-kcal/d diet [145.1 ± 42.7 kcal/d (lean) compared with 173.7 ± 65.0 kcal/d (obese) (P = 0.21); 3.8 ± 1.1% compared with 4.6 ± 1.8% (P = 0.240), respectively]. However, there was a large interindividual range for the percentage of calories lost in stools (2400-kcal/d diet: 2.1–9.2%; 3400-kcal/d diet: 1.6–7.6%). The gastrointestinal transit time was not significantly different between lean and obese subjects who consumed the 2400- and 3400-kcal/d diets [1139 ± 391 min (lean) compared with 951 ± 407 min (obese) (P = 0.33) and 1158 ± 601 min compared with 1126 ± 340 min (P = 0.89)] and between diets for the whole cohort [1144 ± 489 min (2400 kcal/d) compared with 1055 ± 398 min (3400 kcal/d); P = 0.56]. Moreover, there was no association between the transit time and stool calories with either diet (2400 kcal/d: r = 0.21, P = 0.38; 3400 kcal/d: r = 0.02, P = 0.94).
FIGURE 6.
Mean (±SD) changes in energy content in feces between experimental diets. Changes in energy content in feces are shown between the 3400- and 2400-kcal/d diets in lean (n = 11) and obese individuals (n = 8). *P < 0.05 (paired t test). For one lean individual and one obese individual, data were available for only one of the experimental diets; thus, these individuals were excluded from this analysis.
The change in the percentage of urine calories between the 2400- and 3400-kcal/d diets was not different in either lean or obese subjects [−0.5 ± 0.5% (P = 0.34) and −0.6 ± 0.5% (P = 0.15)]. In addition, calories in urine were not different between lean and obese subjects when expressed as total calories or as the percentage of ingested calories with either the 2400-kcal/d diet [88.4 ± 30.8 kcal/d (lean) compared with 99.3 ± 31.2 kcal/d (obese) (P = 0.48) and 3.2 ± 1.1% (lean) compared with 3.5 ± 1.1% (obese) (P = 0.56)] or the 3400-kcal/d diet [106.0 ± 34.1 kcal/d compared with 112.6 ± 28.4 kcal/d (P = 0.64) and 2.8 ± 0.9% compared with 2.9 ± 0.7% (P = 0.72)]. The total urine mass (freeze-dried mass expressed in grams) was slightly, but not significantly, lower in lean than in obese individuals who consumed the 2400-kcal/d diet [43.1 ± 14.7 g (lean) compared with 53.3 ± 14.7 g (obese); P = 0.13]; similarly, no significant differences were observed with the 3400-kcal/d diet [51.7 ± 18.3 g (lean) compared with 55.1 ± 15.5 g (obese); P = 0.66].
DISCUSSION
In this inpatient study, we showed that an altered nutrient load induced rapid changes in the bacterial composition of the human gut microbiota. Moreover, these changes in the gut microbiota were directly associated with stool energy loss in lean individuals such that a 20% increase in Firmicutes and a corresponding decrease in Bacteroidetes was associated with an increased energy harvest of ≈150 kcal. We also showed that a high degree of overfeeding in lean individuals was associated with a greater fractional decrease in stool energy loss, which indicated that the degree of overnutrition relative to individual weight-maintaining energy needs may have played a role in the determination of the efficiency of nutrient absorption, and may potentially explain the observation of clearer associations in lean compared with obese subjects enrolled in this study.
With the initial weight-maintaining diet, no differences in bacterial abundance between lean and obese individuals were observed. This result was somewhat unexpected because previous reports were able detect a reduced abundance of Bacteroidetes and increased abundance of Firmicutes in obese individuals compared with in their lean counterparts (17, 18). The failure to detect differences in the bacterial abundance at baseline could be due to the relatively small study group or because stools were collected after volunteers were fed a weight-maintaining diet on our unit. There are conflicting data concerning the link between the gut microbiota and adiposity in humans (19, 20, 22, 30, 31), and in mice, for which reports have either shown that the absence of a microbiota does protect or does not protect against diet-induced obesity (11, 32). Several independent studies have documented how switching mice with a mouse microbiota or gnotobiotic mice with a transplanted human gut microbiota from plant polysaccharide-rich feed pellets low in fat to a calorically more-dense high-fat, high-sugar Western diet resulted in an increase in the proportional representation of members of Firmicutes and a decrease in Bacteroidetes (14, 16, 33). These findings were consistent with our observation of an association between the relative abundance of members of these bacterial phyla and relative over- or underfeeding. Although we attributed the relative changes in the proportional representation of the major phyla to a higher caloric load, we could not determine whether it was the increased total fat in the diet (as was the case for the 3400-kcal/d diet; see supplemental Tables S1–S6 under “Supplemental data” in the online issue) that could have led to these observed shifts. Future studies with variations in the macronutrient content at the same caloric level in humans will help to elucidate this question.
Nevertheless, our findings offered a possible explanation for the previously reported results of changes in these phyla with weight loss after an extended diet intervention (17). Diet-induced weight loss is a state of a chronic negative-energy balance in which individuals consume much less than their weight-maintenance needs. Thus, our results were consistent with the hypothesis that the association between changes in the gut bacterial community structure and weight loss is a reflection of the effects of a reduced nutrient load rather than actual weight loss. This raised new questions about the relation between the microbiota and the host. Indeed, such changes may have even altered the baseline bacterial populations in fecal samples collected while subjects were consuming the weight-maintaining diet. If, for example, the obese individuals were in a state of a chronic positive-energy balance before admission (ie, were overeating), the weight-maintaining diet before testing might, itself, have induced changes in the microbiota because the nutrient load would have changed from the free-living condition.
Our findings raise the possibility that the gut senses alterations in nutrient availability and subsequently modulated the nutrient absorption. Specifically, the observed association between the gut microbiota and relative stool calories indicates a possible direct role of gut bacteria in calorie absorption. This is in agreement with a previous study that showed the potential of the gut microbiome to regulate nutrient absorption (13). Whether gut bacteria simply act as a sensor to detect changes in the nutrient load with subsequent feedback to other host components represented in the intestine or located at extraintestinal sites or are more directly involved in the absorption process remains to be determined.
This study is limited by the relatively low number of subjects, and it must be acknowledged that quantification of stool calories may have been an indirect measure of nutrient absorption. However, we believe that bomb calorimetry of duplicate meals and corresponding dyed stool portions represents a unique and accurate technique to assess calorie loss as a percentage of energy consumed, and, thus allows for assumptions to be made on actual nutrient absorption. The failure to detect differences in nutrient absorption between lean and obese individuals at baseline could, in part, be attributed to the similar distribution in the relative bacterial abundances between lean and obese subjects. However, we did not find differences in the mass and calorie content of the urine between these groups who consumed either diet. This could have been due to the sample size, or alternatively it could have represented a bias on the basis of the timing of the urine collections because we were not able to mark the caloric excretion in the urine (as was done in stools). Nonetheless, we believe this effect is minimal, especially because we did not see a difference in the transit time (which indicates a differential rate of calorie absorption) between lean and obese individuals.
The identification of a dynamic interrelation between the nutrient load, absorption, and gut microbiota was facilitated because our study design allowed for interval collections of samples from humans who were living under highly monitored conditions; such associations would likely have been missed in a less well-controlled cohort. These studies provided a template that could be applied to future studies in which a wider range of under- and overfeeding of lean and obese individuals is achieved, controlled manipulations of the nutrient and xenobiotic composition of each diet are performed, deliberate alterations of the microbial community structure are attempted, and analyses, not only of the structure but also direct tests of the functions of the resulting gut microbiomes, are undertaken. Together with animal models, these analyses provided new opportunities, to our knowledge, for translational medicine, and a more mechanistic understanding of the role that the gut microbial community plays in defining and responding to the energetic value of the foods we consume.
Supplementary Material
Acknowledgments
We thank all study volunteers for their contribution to this study and the staff of the Clinical Research Unit on the fifth floor of the Phoenix Indian Medical Center (Phoenix, AZ) (with special thanks to Jennifer Michaels and Shannon Parrington for their excellent contribution by processing caloric-content measurements of biological samples and to Sabrina Wagoner and Jill Manchester for their assistance with metagenomic studies of fecal samples).
The authors’ responsibilities were as follows—JK and DSL: designed the research; DSL, CT, and JK: conducted the research; PJT and JIG: provided essential data by generation and analyses of 16S rRNA data; RJ, JK, PJT, and JIG: analyzed data and conducted statistical analyses; RJ: wrote the manuscript; RJ, JK, PJT, JIG, and CT: had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest.
REFERENCES
- 1.Fontvieille AM, Dwyer J, Ravussin E. Resting metabolic rate and body composition of Pima Indian and Caucasian children. Int J Obes Relat Metab Disord 1992;16:535–42 [PubMed] [Google Scholar]
- 2.Huang KC, Kormas N, Steinbeck K, Loughnan G, Caterson ID. Resting metabolic rate in severely obese diabetic and nondiabetic subjects. Obes Res 2004;12:840–5 [DOI] [PubMed] [Google Scholar]
- 3.Ravussin E. Energy metabolism in obesity. Studies in the Pima Indians. Diabetes Care 1993;16:232–8 [DOI] [PubMed] [Google Scholar]
- 4.Schoeller DA. Balancing energy expenditure and body weight. Am J Clin Nutr 1998;68:956S–61S [DOI] [PubMed] [Google Scholar]
- 5.Tataranni PA, Harper IT, Snitker S, et al. Body weight gain in free-living Pima Indians: effect of energy intake vs expenditure. Int J Obes Relat Metab Disord 2003;27:1578–83 [DOI] [PubMed] [Google Scholar]
- 6.Atwater WO, Rosa EB. A new respiration calorimeter and experiments on the conservation of energy in the human body. I. Phys Rev Ser I 1899;9:129–63 [Google Scholar]
- 7.Beyer PL, Flynn MA. Effects of high- and low-fiber diets on human feces. J Am Diet Assoc 1978;72:271–7 [PubMed] [Google Scholar]
- 8.Wisker E, Maltz A, Feldheim W. Metabolizable energy of diets low or high in dietary fiber from cereals when eaten by humans. J Nutr 1988;118:945–52 [DOI] [PubMed] [Google Scholar]
- 9.Webb P, Annis JF. Adaptation to overeating in lean and overweight men and women. Hum Nutr Clin Nutr 1983;37:117–31 [PubMed] [Google Scholar]
- 10.Backhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 2004;101:15718–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA 2007;104:979–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 2005;102:11070–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–31 [DOI] [PubMed] [Google Scholar]
- 14.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008;3:213–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crawford PA, Crowley JR, Sambandam N, et al. Regulation of myocardial ketone body metabolism by the gut microbiota during nutrient deprivation. Proc Natl Acad Sci USA 2009;106:11276–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 2009;1:6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 2006;444:1022–3 [DOI] [PubMed] [Google Scholar]
- 18.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature 2009;457:480–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duncan SH, Lobley GE, Holtrop G, et al. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes (Lond) 2008;32:1720–4 [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA 2009;106:2365–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadal I, Santacruz A, Marcos A, et al. Shifts in clostridia, bacteroides and immunoglobulin-coating fecal bacteria associated with weight loss in obese adolescents. Int J Obes (Lond) 2009;33:758–67 [DOI] [PubMed] [Google Scholar]
- 22.Santacruz A, Marcos A, Warnberg J, et al. Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity (Silver Spring) 2009;17:1906–15 [DOI] [PubMed] [Google Scholar]
- 23.Ferraro R, Boyce VL, Swinburn B, De Gregorio M, Ravussin E. Energy cost of physical activity on a metabolic ward in relationship to obesity. Am J Clin Nutr 1991;53:1368–71 [DOI] [PubMed] [Google Scholar]
- 24.Zarling EJ, Ruchim MA, Makino D. Improved technique for measuring fecal energy loss in normal and malabsorbing humans. J Lab Clin Med 1986;107:5–9 [PubMed] [Google Scholar]
- 25.Parr Instrument Co Parr manual no 483M, 6200 calorimeter operating instruction manual. Moline, IL: Parr Instrument Co [Google Scholar]
- 26.Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006;22:1658–9 [DOI] [PubMed] [Google Scholar]
- 27.Hamady M, Knight R. Microbial community profiling for human microbiome projects: Tools, techniques, and challenges. Genome Res 2009;19:1141–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science 2009;326:1694–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol 2009;587:4153–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collado MC, Isolauri E, Laitinen K, Salminen S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am J Clin Nutr 2008;88:894–9 [DOI] [PubMed] [Google Scholar]
- 31.Schwiertz A, Taras D, Schafer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010;18:190–5 [DOI] [PubMed] [Google Scholar]
- 32.Fleissner CK, Huebel N, Abd El-Bary MM, Loh G, Klaus S, Blaut M. Absence of intestinal microbiota does not protect mice from diet-induced obesity. Br J Nutr 2010;104:919–29 [DOI] [PubMed] [Google Scholar]
- 33.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009;137:1716–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.