Abstract
Approximately one-quarter of circulating cobalamin (vitamin B-12) binds to transcobalamin (holoTC) and is thereby available for the cells of the body. For this reason, holoTC is also referred to as active vitamin B-12. HoloTC was suggested as an optimal marker of early vitamin B-12 deficiency >20 y ago. This suggestion led to the development of suitable assays for measurement of the compound and clinical studies that aimed to show the benefit of measurement of holoTC rather than of vitamin B-12. Today holoTC can be analyzed by 3 methods: direct measurement of the complex between transcobalamin and vitamin B-12, measurement of vitamin B-12 attached to transcobalamin, or measurement of the amount of transcobalamin saturated with vitamin B-12. These 3 methods give similar results, but direct measurement of holoTC complex is preferable in the clinical setting from a practical point of view. HoloTC measurement has proven useful for the identification of the few patients who suffer from transcobalamin deficiency. In addition, holoTC is part of the CobaSorb test and therefore useful for assessment of vitamin B-12 absorption. Clinical studies that compare the ability of holoTC and vitamin B-12 to identify individuals with vitamin B-12 deficiency (elevated concentration of methylmalonic acid) suggest that holoTC performs better than total vitamin B-12. To date, holoTC has not been used for population-based assessments of vitamin B-12 status, but we suggest that holoTC is a better marker than total vitamin B-12 for such studies.
INTRODUCTION
Why does one measure cobalamin (vitamin B-12) rather than the fraction of the vitamin that can enter the cells, holotranscobalamin (holoTC)? In the 1980s the late Victor Herbert frequently asked this question (1). At the time, holoTC could only be measured by calculation of the difference between vitamin B-12 (eg, 500 pmol/L) and the fraction of vitamin B-12 that was not attached to transcobalamin (eg, 420 pmol/L). As a result, the uncertainty of the holoTC values was too large to allow proper evaluation of holoTC's clinical usefulness compared with other markers of vitamin B-12 status [plasma cobalamin (vitamin B-12), homocysteine (tHcy), and methylmalonic acid (MMA)]. Today several suitable methods are available to measure holoTC, and data on its analytic and clinical aspects are accumulating rapidly.
The present review summarizes current knowledge about the analytic aspects and concerning determinants of plasma holoTC. This review also addresses the clinical performance of holoTC compared with that of total vitamin B-12. We conclude that measurement of holoTC is also likely to be valuable for population-wide screening of vitamin B-12 status.
HoloTC AND OTHER MARKERS OF VITAMIN B-12 STATUS
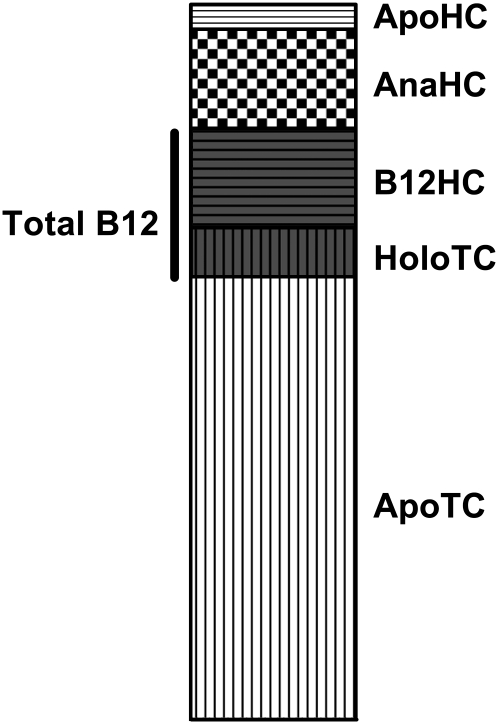
Plasma vitamin B-12 is bound to 2 proteins, transcobalamin and haptocorrin (Figure 1). Transcobalamin carries a minor part of the circulating vitamin B-12, and only ≈10% of the protein is saturated with vitamin B-12 (2). Transcobalamin transports vitamin B-12 into all cells of the body and is responsible for the transport of ≈4 nmol of vitamin B-12 every day (3).
FIGURE 1.
Cobalamin [vitamin B-12 (B12)] and its binding proteins in human plasma. The figure shows the relation between the total concentration of the vitamin B-12 binding proteins in plasma and the distribution of vitamin B-12 and its analogs on the proteins. The average concentrations used are as follows—total transcobalamin: 1000 pmol/L [holotranscobalamin (HoloTC): 100 pmol/L; apotranscobalamin (ApoTC): 900 pmol/L]; total haptocorrin: 450 pmol/L [vitamin B-12 bound to haptocorrin (B12HC): 200 pmol/L; vitamin B-12 analogs bound to haptocorrin (AnaHC): 200 pmol/L; apohaptocorrin (ApoHC): 50 pmol/L]; total vitamin B-12: 300 pmol/L.
Haptocorrin is an almost fully saturated vitamin B-12 binding glycoprotein of unknown function that carries the major part of circulating vitamin B-12 and, in addition, the inactive forms of the vitamin, the so-called analogs. The metabolism of the protein is slow, with a turnover of ≈0.1 nmol vitamin B-12 every day (4–6).
We and others have established methods for research purposes that are suitable for measurement of transcobalamin and haptocorrin, whether saturated or not (7–9). However, clinicians have been interested mainly in measurements of total vitamin B-12 (10) and holoTC.
The fact that only vitamin B-12 that binds to transcobalamin is available for cells has fostered the concept that measurement of holoTC would be more clinically meaningful than measurement of total vitamin B-12 (all of the vitamin B-12 that binds to transcobalamin and haptocorrin). Several years ago, Victor Herbert suggested staging vitamin B-12 status with holoTC as the first marker to decline (11). A few studies on vegetarian populations have confirmed this concept (12, 13).
Whereas plasma vitamin B-12 and holoTC concentrations can be used as a measure of the amount of vitamin B-12 available for the body's cells, the metabolites tHcy and MMA mirror any lack of vitamin B-12 within the cells. Both plasma tHcy and MMA accumulate in vitamin B-12–deficient patients (Figure 2). In general, tHcy has low specificity because it also increases in patients with folate and possibly thiamine and vitamin B-6 deficiency (14). However, in a folate-fortified population, tHcy's specificity as a marker of vitamin B-12 deficiency is considerably better (15). MMA is a sensitive marker of vitamin B-12 deficiency, and an elevated MMA concentration is often used as a gold standard for classification of a patient's status as vitamin B-12 deficient or nondeficient. Major drawbacks are the low specificity of marginally elevated MMA values and the test's limited availability (16).
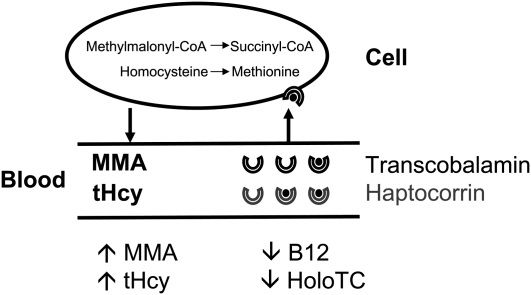
FIGURE 2.
Markers of cobalamin [vitamin B-12 (B12)] status. The figure shows the relation between vitamin B-12 deficiency markers. Holotranscobalamin (holoTC) transports vitamin B-12 into the cells by binding to a specific receptor, CD320. In the cell, vitamin B-12 acts as a coenzyme for 2 enzymes. Methionine synthetase (5-methyltetrahydropteroyl-l-glutamate:l-homocysteine-S-methyltransferase; EC 2.1.1.13), present in the cytoplasm, converts homocysteine (tHcy) to methionine. Methylmalonyl–coenzyme A (-CoA) mutases (methylmalonyl-CoA CoA-carbonylmutase; EC 5.4.99.2), which are present in the mitochondria, are involved in the conversion of methylmalonyl-CoA to succinyl-CoA. A decrease in holoTC results in a decrease in the cellular uptake of vitamin B-12. Once the cells become deficient, methylmalonic acid (MMA) and tHcy accumulate in the bloodstream; total vitamin B-12 concentration decreases.
In otherwise healthy individuals, all 4 tests (vitamin B-12, holoTC, tHcy, and MMA) have a strong relation to vitamin B-12 intake, with steady state concentrations at a daily intake of 4–7 μg vitamin B-12 (17). Indirectly, such studies suggest that all 4 markers may be useful for monitoring a population's vitamin B-12 status over time.
HoloTC MEASUREMENT
Three different types of holoTC measurement methods are available and, as described below, we have shown that these methods give reasonably similar values. The first approach to be published combined ionic precipitation of transcobalamin with measurement of the amount of vitamin B-12 trapped in the precipitate (18–20). Lindemans et al (21) improved this approach with the use of antibodies directed to transcobalamin rather than ionic separation, and Ulleland et al (22) developed a version of this approach that involved measurement of trapped vitamin B-12 by an isotope dilution assay, the holoTC-radioimmunoassay (RIA). In another version, Refsum et al (9) measured vitamin B-12 with the use of a microbiological method. Yet another approach is to remove apo-transcobalamin with vitamin B-12–coated beads followed by enzyme-linked immunosorbent assay measurement of the holoTC that remains in the supernatant fluid (23).
A multicenter study that involved 4 European laboratories evaluated the holoTC-RIA (calibration curve: 10–160 pmol/L) and the holoTC–enzyme-linked immunosorbent assay (calibration curve: 1.6–100 pmol/L) (24). The results from the study showed similar values across laboratories and across the methods tested. The long-term imprecision was 6% for the enzyme-linked immunosorbent assay and >10% for the RIA (24). The RIA assay results were also similar to the results that Refsum et al (9) obtained with the use of the microbiological assay (calibration curve: 1.36–24.5 pmol/L; imprecision: 4–7%).
Today, the holoTC-RIA has been replaced by an assay that uses holoTC-specific monoclonal antibodies (25). On the AxSYM platform (Abbott Labs, North Chicago, IL), the method has a measurement range of 3–100 pmol/L and a total imprecision of 6–9%. The results obtained by this method compare well with those of the holoTC-RIA (26, 27).
HoloTC DETERMINANTS
When a marker is used to monitor a population's vitamin B-12 status, it should be remembered that an elevation in holoTC or vitamin B-12 concentration or a decrease in MMA or tHcy concentration by determinants other than vitamin B-12 deficiency predicts a spurious sufficient status. Obviously, the opposite is true for prediction of a spurious insufficient status.
We and others have documented holoTC determinants not related to vitamin B-12 status. The results are summarized below and, to some extent, in relation to determinants for other markers of vitamin B-12 status.
Blood sampling and storage
HoloTC, like vitamin B-12, is a stable analyte and no special precautions need to be taken to draw blood samples for holoTC measurement. HoloTC is also stable in storage at –20°C to −70°C, probably for years. Loikas et al (28) examined stability systematically for storage at –70°C for up to 16 mo.
Diurnal variation
A major concern has been the possible elevation in holoTC after intake of vitamin B-12–rich food. The few studies that have addressed this issue have shown very limited variations related to intake of a normal diet (9, 29) and because of that, blood can be drawn both from fasting and nonfasting individuals. However, repeated intakes of high physiologic doses of vitamin B-12 (eg, 9 μg 3 times in 1 d) elevate the holoTC concentration (and to a lesser degree the total vitamin B-12 concentration) within 24 h (30). We have used this finding to develop a clinical test for vitamin B-12 absorption, as described below.
Plasma compared with serum
EDTA plasma has shown slightly higher values for holoTC or total transcobalamin in some studies (22, 31) but not in others (9). In general, it is acceptable to use both plasma and serum to analyze holoTC concentrations.
Sex, race, and life cycle
HoloTC, like total vitamin B-12, varies to a limited extent by sex or age. Note, however, that Refsum et al (9) reported slightly lower concentrations in younger women. In contrast, we and others have observed marked alterations in the metabolic markers, especially tHcy, in relation to age and sex (32, 14).
Because haptocorrin decreases, total vitamin B-12 also decreases in pregnant women, to ≈50% at term compared with 2 mo postpartum. In contrast, holoTC concentrations remain unchanged during pregnancy (33, 34). For this reason, holoTC is better than vitamin B-12 for monitoring of vitamin B-12 status in population-based studies that include pregnant women. Information on racial differences in holoTC concentrations and other vitamin B-12 status markers is limited.
Total transcobalamin and holoTC
HoloTC accounts for 5–20% of total transcobalamin (23). Total transcobalamin does not change in patients with vitamin B-12 deficiency (E Nexo, unpublished data, 2011). For this reason, we have gained no additional information from the measurement of transcobalamin saturation as opposed to holoTC measurement alone (32). However, we observed a decrease in total transcobalamin after healthy individuals were given a daily vitamin B-12 dose of 500 μg. The concentration increased toward preloading values within the following 84 d (35). This observation might relate to the fact that the cellular receptor for transcobalamin, CD320, recognizes holoTC rather than apotranscobalamin (36–38). After vitamin B-12 provision, the cells absorb an elevated amount of holoTC. However, once the cells are saturated with vitamin B-12, total transcobalamin reaches a new equilibrium.
Transcobalamin genotypes
Transcobalamin occurs in many genotypes (39), and several authors have clearly documented that the genotype present, notably the P259R (TCN2 776C→G), influences the protein's total concentration. Total transcobalamin concentrations are ≈20% lower in individuals with the 776GG genotype than in individuals with the 776CC genotype; individuals with the 776GC genotype have intermediate values. Genotype distribution varies somewhat between studies but is ≈30% for P259P (776CC), 50% for P259R (776CG), and 20% for R259R (776GG) (9). HoloTC concentrations are influenced to a much lesser degree by the transcobalamin genotypes, although this influence was significant in some studies (9, 40, 41).
Other diseases
Transcobalamin is a relatively small protein with a molecular mass of ≈40 kDa (42), and it is filtered in the kidney. As a result, holoTC concentrations elevate as kidney function decreases (32, 43). The same is true for total vitamin B-12, although to a much lesser degree, because haptocorrin's apparent molecular mass exceeds that of albumin (44). Some studies have shown only a marginal relation between kidney function and holoTC and total vitamin B-12 concentrations (9, 45). The metabolic markers show a marked concentration elevation with an increase in serum creatinine (32, 45). As a result, with the use of the metabolic markers, one might falsely categorize a person with impaired kidney function to have a vitamin B-12 deficiency and (less likely) to not have a deficiency with the use of holoTC.
Experts have expressed concern about a number of other conditions, mainly in relation to holoTC and possibly total vitamin B-12. Originally, we considered transcobalamin to be an acute-phase protein elevated in conditions that involved acute inflammation. However, more recent data indicate that transcobalamin concentrations elevate only in conditions with macrophage activation (31, 46). Other conditions that might elevate holoTC concentrations include liver diseases (43, 47) and the development of autoantibodies against transcobalamin (48, 49). However, we need to do additional studies to examine the influence of autoantibodies in relation to the new generation of holoTC assays.
HoloTC REFERENCE INTERVALS
As we have discussed above, slight differences may occur in the reference interval for holoTC because of transcobalamin genotype, age, and sex (9, 22, 23, 26, 28). The reported variations by these factors to date are relatively small, and additional studies need to address this issue before the need for adjusted reference intervals by, for example, age, sex, and race is determined.
The reference intervals published so far are summarized in Table 1. We have included only data on reasonably large groups of healthy individuals. The current consensus seems to be that a reference interval of 40–200 pmol/L is appropriate. Until results from additional studies are available, clinicians and researchers should confirm this interval locally before introduction of holoTC into daily clinical practice or interpretation of the results in population-based studies.
TABLE 1.
Selected reference intervals for holotranscobalamin (holoTC) with the use of the most common assays for holoTC measurement1
| Method2 | Sample | Reference range | n | Reference |
| μL | pmol/L | |||
| Radioimmunoassay | 400 | 24–160 | 105 | 22 |
| 37–170 | 303 | 28 | ||
| Microbiology | <150 | 42–160 | 500 | 9 |
| ELISA | 100 | 40–150 | 137 | 23 |
| Direct | 200 | 19–130 | 292 | 26 |
| 36–220 | 276 | 50 |
ELISA, enzyme-linked immunosorbent assay.
Radioimmunoassay: precipitation of transcobalamin on antibody-coated beads and measurement of vitamin B-12 with the use of an isotope dilution method. Microbiology: precipitation of transcobalamin by antibody-coated beads and measurement of trapped vitamin B-12 with the use of a microbiological method. ELISA: measurement of holoTC with the use of ELISA after removal of apotranscobalamin with vitamin B-12–coated beads. Direct: measurement of holoTC by ELISA, with the use of an antibody that is specific for holoTC.
USEFULNESS OF holoTC MEASUREMENT
Nissen et al (51) have concluded that measurement of holoTC and/or total transcobalamin is useful as a diagnostic tool in the few patients with transcobalamin deficiency. This condition gives rise to severe symptoms in early childhood and clinicians must diagnose it as early as possible to avoid permanent damage.
Vitamin B-12 status
A number of studies have presented data on the relation between holoTC and total vitamin B-12 concentrations across extreme to insufficient intakes of the vitamin. We studied healthy individuals on a daily oral vitamin B-12 dose of 500 μg and observed a maximal elevation in holoTC of ≈50% by 3 d, with no further elevation by the end of the study (84 d). The pattern for vitamin B-12 bound to haptocorrin was different. The initial elevation was ≈20%, but we observed a continuous increase throughout the study (35). MMA showed no change, and the alteration in tHcy was slower than that of holoTC (52). At the other extreme, studies in a vegan population with an insufficient vitamin B-12 intake suggest that holoTC decreases before alterations in the other vitamin B-12 status markers (12). In between these extremes, holoTC, like the other vitamin B-12 status markers, reflects the intake of vitamin B-12 (17).
Taken together, the above-mentioned studies suggest that holoTC may prove most useful if the aim is to monitor a population with a borderline suboptimal vitamin B-12 supply. In contrast, total vitamin B-12 may be superior if the goal is to monitor a possible surplus load of the vitamin. And finally, any of the markers for vitamin B-12 deficiency may be used if the goal is to distinguish between a normal vitamin B-12 status and an overt deficiency.
Vitamin B-12 uptake
The observation that holoTC is a marker of an acute increase in the intake of vitamin B-12 led us to develop a routine test (CobaSorb) for the study of vitamin B-12 uptake. To perform the test, we remove blood samples before and after 1–2 d intake of an oral dose of 3 times 9 μg vitamin B-12 daily and measure the elevation in holoTC. From an analytic point of view, we are able to detect an elevation in holoTC of ≥10 pmol/L only if baseline holoTC is reasonably low, and we initially recommended the test to be used only if baseline holoTC was <75 pmol/L (53). In an early study on a cohort (n = 17) unable to absorb vitamin B-12 because of inherited lack of either intrinsic factor or its receptor, we observed that none of the patients absorbed vitamin B-12 as judged by the CobaSorb test. In other words, judged by these data, the sensitivity of CobaSorb is 1.00 (54). We calculated the specificity to be 0.98 on the basis of results obtained on 57 healthy individuals with a baseline holoTC <75 pmol/L (53). We later improved the CobaSorb test so that we can also study vitamin B-12 absorption if the baseline holoTC is too high to allow the use of the original test. We benefited from the observation that the oral dose of vitamin B-12 is absorbed unchanged (ie, as cyanocobalamin). On the basis of this observation, we developed a version of the CobaSorb test in which we measure the increase in the amount of cyanocobalamin bound to transcobalamin. We named this version of the test C-Cobasorb (55). Today we recommend the use of CobaSorb to clarify whether a diagnosed vitamin B-12 deficiency is caused by an inability to absorb the vitamin, and recommend the test be judged in the following manner. We judge the patient able to absorb vitamin B-12 if holoTC increases >10 pmol/L and >22% after 2 d intake of an oral dose of 3 times 9 μg vitamin B-12 daily. We recommend C-CobaSorb be performed in patients with a baseline holoTC >65 pmol/L and not judged to absorb vitamin B-12 based on the CobaSorb test (55). We stress that currently the CobaSorb tests have been used only to give a “yes” or “no” answer related to the absorption of vitamin B-12. We do not know whether the tests will be able to classify individuals to have a low, but not absent, capacity for absorption of the vitamin.
Vitamin B-12 deficiency
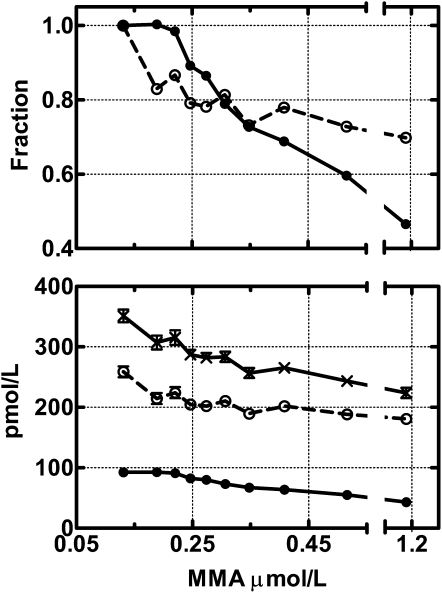
We and others perceive holoTC as an early marker of vitamin B-12 deficiency, but final proof of this concept would require a study in which a cohort of patients is followed as their vitamin B-12 status shifts from normal to overt deficiency. Such a study is not feasible because you would have an obligation to treat the patient as soon as the first sign of deficiency became apparent. However, as shown in Figure 3, we provide indirect evidence that suggests that holoTC is a better marker than is vitamin B-12. We analyzed the combined data from a study on community-dwelling elderly adults (56) and from a study on patients referred to the hospital for evaluation of suspected vitamin B-12 deficiency (32). The figure shows that holoTC accounts for most of the change in vitamin B-12 both expressed in relative (upper panel) or absolute (lower panel) terms in the groups of patients with increasing vitamin B-12 deficiency as judged from an increase in MMA values from 0.25 to >1 μmol. Interestingly, holoTC levels off, whereas total vitamin B-12 continues to increase in the groups of patients without vitamin B-12 deficiency as judged by MMA values <0.25 μmol/L. This result is in accordance with our previous results (35), which showed that prolonged loading with vitamin B-12 results in a change in total vitamin B-12 but not in holoTC.
FIGURE 3.
Holotranscobalamin (holoTC) and cobalamin (vitamin B-12) in relation to methylmalonic acid (MMA). The figure shows combined data from Hvas and Nexo (32) and Clarke et al (56). The figure includes 1842 data sets divided into tertiles in accordance with elevating MMA values. The x axis denotes the mean MMA value in each group. The upper panel shows the fractional change in holoTC (•) and haptocorrin-bound vitamin B-12 (total vitamin B-12 minus holoTC values; ○). To calculate the fractional change, we divided the mean for each group by the mean for the group with the lowest MMA values. The lower panel shows the absolute values for holoTC (•), haptocorrin-bound vitamin B-12 (○), and total vitamin B-12 (×). The figure depicts the mean (±SEM) for each group.
Numerous studies have compared holoTC's performance with that of total vitamin B-12 for identification of patients with vitamin B-12 deficiency. Most of these studies used an elevated MMA and possibly tHcy concentration to identify individuals with and without vitamin B-12 deficiency (Table 2). All the studies shown in Table 2 involved patients with normal kidney function, and all but one of these studies showed that holoTC's performance is better than that of vitamin B-12, independent of the cutoff MMA value they used to classify patients’ vitamin B-12 status (32, 56–59). The one study that showed that vitamin B-12 was superior to holoTC included very few patients for assessment of holoTC (59). The few studies in patients with impaired kidney function showed that holoTC is also superior in these patients (56, 58). Although holoTC seems to identify patients with elevated MMA concentrations more effectively than does vitamin B-12, Miller et al (60) have suggested that the use of both parameters in combination is preferable.
TABLE 2.
Comparison of holotranscobalamin (holoTC) and total vitamin B-12 for diagnosis of vitamin B-12 deficiency1
| Total no. of subjects (total no. of subjects with vitamin B-12 deficiency) | Nationality | Age | Limits for MMA (tHcy) | AUC for holoTC | AUC for vitamin B-12 | Reference |
| y | μmol/L | |||||
| 806 (24) | Danish | >18 | >0.75 (>15) | 0.90 | 0.85 | 32 |
| 1651 (70) | English | >65 | >0.75 | 0.87 | 0.79 | 56 |
| 1651 (129) | English | >65 | >0.45 | 0.80 | 0.73 | 56 |
| 533 (71) | German | 18–98 | >0.40 | — | 0.72 | 59 |
| 125 (16) | German | 18–98 | >0.40 | 0.66 | — | 59 |
| 759 (174) | German | 8–92 | >0.30 | 0.71 | 0.60 | 58 |
| 204 (68) | German, Dutch | 21–73 | >0.27 | 0.88 | 0.84 | 57 |
The limits for MMA and tHcy indicate the cutoffs that were used to classify the patients as vitamin B-12 deficient. The researchers assessed the results with the use of receiver operating curves. An area under the curve (AUC) of 1.0 indicates a perfect test, and an AUC of 0.5 indicates a useless test. MMA, methylmalonic acid; tHcy, homocysteine.
Fedosov (61) recently used mathematic modeling to classify patients’ vitamin B-12 status with the use of all 4 deficiency markers (holoTC, total vitamin B-12, MMA, and tHcy). He then determined how well each marker would have predicted vitamin B-12 deficiency, and showed holoTC [area under the receiver operating curve (AUC) = 0.93] to be similar to MMA (AUC = 0.92) and better than total vitamin B-12 (AUC = 0.88) and tHcy (AUC = 0.87).
CONCLUSIONS
More than 20 y ago, insights into the physiology of vitamin B-12 led to the suggestion that holoTC might be a sensitive marker of early vitamin B-12 deficiency. Since then, holoTC measurement has come of age. Today, we can conclude that holoTC seems more suitable than total vitamin B-12 for diagnosis of vitamin B-12 deficiency. However, to date, holoTC has not acquired wide clinical acceptance, most likely because of the test's cost and limited availability. On the basis of the data we present in this review, we predict that holoTC will also be an excellent marker for monitoring a population's vitamin B-12 status. Currently, a strong need exists for studies that can prove or disprove this prediction.
Acknowledgments
The authors’ responsibilities were as follows—EN: wrote the first draft of the main portion of the article; and EH-L: was responsible for the literature search and wrote the first draft that covered the genetic determinants for transcobalamin. Both authors contributed to the continuous work and approved the final draft. Neither author had any conflicts of interest to declare.
REFERENCES
- 1.Herzlich B, Herbert V. Depletion of serum holotranscobalamin II. An early sign of negative vitamin B12 balance. Lab Invest 1988;58:332–7 [PubMed] [Google Scholar]
- 2.Nexo E, Andersen J. Unsaturated and cobalamin saturated transcobalamin I and II in normal human plasma. Scand J Clin Lab Invest 1977;37:723–8 [DOI] [PubMed] [Google Scholar]
- 3.Hom BL, Olesen HA. Plasma clearance of 57cobalt-labelled vitamin B12 bound in vitro and in vivo to transcobalamin I and II. Scand J Clin Lab Invest 1969;23:201–11 [DOI] [PubMed] [Google Scholar]
- 4.Nexo E, Gimsing P. Turnover in humans of iodine- and cobalamin-labeled transcobalamin I and of iodine-labeled albumin. Scand J Clin Lab Invest 1975;35:391–8 [PubMed] [Google Scholar]
- 5.Kolhouse JF, Kondo H, Allen NC, Podell E, Allen RH. Cobalamin analogues are present in human plasma and can mask cobalamin deficiency because current radioisotope dilution assays are not specific for true cobalamin. N Engl J Med 1978;299:785–92 [DOI] [PubMed] [Google Scholar]
- 6.Hardlei TF, Nexo E. A new principle for measurement of cobalamin and corrinoids, used for studies of cobalamin analogs on serum haptocorrin. Clin Chem 2009;55:1002–10 [DOI] [PubMed] [Google Scholar]
- 7.Carmel R. R-binder deficiency. A clinically benign cause of cobalamin pseudodeficiency. JAMA 1983;250:1886–90 [DOI] [PubMed] [Google Scholar]
- 8.Morkbak AL, Pedersen JF, Nexo E. Glycosylation independent measurement of the cobalamin binding protein haptocorrin. Clin Chim Acta 2005;356:184–90 [DOI] [PubMed] [Google Scholar]
- 9.Refsum H, Johnston C, Guttormsen AB, Nexo E. Holotranscobalamin and total transcobalamin in human plasma: determination, determinants, and reference values in healthy adults. Clin Chem 2006;52:129–37 [DOI] [PubMed] [Google Scholar]
- 10.Carmel R. Biomarkers of cobalamin (vitamin B-12) status in the epidemiologic setting: a critical overview of context, applications, and performance characteristics of cobalamin, methylmalonic acid, and holotranscobalamin II. Am J Clin Nutr 2011;94(suppl):348S–58S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herbert V. Staging vitamin B-12 (cobalamin) status in vegetarians. Am J Clin Nutr 1994;59(suppl):1213S–22S [DOI] [PubMed] [Google Scholar]
- 12.Lloyd-Wright Z, Hvas AM, Møller J, Sanders TA, Nexo E. Holotranscobalamin as an indicator of dietary vitamin B12 deficiency. Clin Chem 2003;49:2076–8 [DOI] [PubMed] [Google Scholar]
- 13.Herrmann W, Schorr H, Obeid R, Geisel J. Vitamin B-12 status, particularly holotranscobalamin II and methylmalonic acid concentrations, and hyperhomocysteinemia in vegetarians. Am J Clin Nutr 2003;78:131–6 [DOI] [PubMed] [Google Scholar]
- 14.Refsum H, Smith AD, Ueland PM, et al. Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem 2004;50:3–32 [DOI] [PubMed] [Google Scholar]
- 15.Green R, Miller JW. Vitamin B12 deficiency is the dominant nutritional cause of hyperhomocysteinemia in a folic acid-fortified population. Clin Chem Lab Med 2005;43:1048–51 [DOI] [PubMed] [Google Scholar]
- 16.Hvas AM, Nexo E. Diagnosis and treatment of vitamin B12 deficiency-an update. Haematologica 2006;91:1506–12 [PubMed] [Google Scholar]
- 17.Bor MV, von Castel-Roberts KM, Kauwell GP, et al. Daily intake of 4 to 7 μg dietary vitamin B-12 is associated with steady concentrations of vitamin B-12–related biomarkers in a healthy young population. Am J Clin Nutr 2010;91:571–7 [DOI] [PubMed] [Google Scholar]
- 18.Begley JA, Hall CA. Measurement of vitamin B12-binding proteins of plasma. I. Technique. Blood 1975;45:281–6 [PubMed] [Google Scholar]
- 19.van Kapel J, Wouters NM, Lindemans J. Application of heparin-conjugated Sepharose for the measurement of cobalamin-saturated and unsaturated transcobalamin II. Clin Chim Acta 1988;172:297–310 [DOI] [PubMed] [Google Scholar]
- 20.Lindgren A, Kilander A, Bagge E, Nexo E. Holotranscobalamin: a sensitive marker of cobalamin malabsorption. Eur J Clin Invest 1999;29:321–9 [DOI] [PubMed] [Google Scholar]
- 21.Lindemans J, Schoester M, van Kapel J. Application of a simple immunoadsorption assay for the measurement of saturated and unsaturated transcobalamin II and R-binders. Clin Chim Acta 1983;132:53–61 [DOI] [PubMed] [Google Scholar]
- 22.Ulleland M, Eilertsen I, Quadros EV, et al. Direct assay for cobalamin bound to transcobalamin (holo-transcobalamin) in serum. Clin Chem 2002;48:526–32 [PubMed] [Google Scholar]
- 23.Nexo E, Christensen AL, Hvas AM, Petersen TE, Fedosov SN. Quantification of holo-transcobalamin, a marker of vitamin B12 deficiency. Clin Chem 2002;48:561–2 [PubMed] [Google Scholar]
- 24.Morkbak AL, Heimdal RM, Emmens K, et al. Evaluation of the technical performance of novel holotranscobalamin (holoTC) assays in a multicenter European demonstration project. Clin Chem Lab Med 2005;43:1058–64 [DOI] [PubMed] [Google Scholar]
- 25.Orning L, Rian A, Campbell A, et al. Characterization of a monoclonal antibody with specificity for holo-transcobalamin. Nutr Metab (Lond) 2006;3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brady J, Wilson L, McGregor L, Valente E, Orning L. Active B12: a rapid, automated assay for holotranscobalamin on the Abbott AxSYM analyzer. Clin Chem 2008;54:567–73 [DOI] [PubMed] [Google Scholar]
- 27.Bamonti F, Moscato GA, Novembrino C, et al. Determination of serum holotranscobalamin concentrations with the AxSYM active B(12) assay: cut-off point evaluation in the clinical laboratory. Clin Chem Lab Med 2010;48:249–53 [DOI] [PubMed] [Google Scholar]
- 28.Loikas S, Löppönen M, Suominen P, et al. RIA for serum holo-transcobalamin: method evaluation in the clinical laboratory and reference interval. Clin Chem 2003;49:455–62 [DOI] [PubMed] [Google Scholar]
- 29.Hvas AM, Gravholt CH, Nexo E. Circadian variation of holo-transcobalamin (holo-TC) and related markers. Clin Chem Lab Med 2005;43:760–4 [DOI] [PubMed] [Google Scholar]
- 30.von Castel-Roberts KM, Morkbak AL, Nexo E, et al. Holo-transcobalamin is an indicator of vitamin B-12 absorption in healthy adults with adequate vitamin B-12 status. Am J Clin Nutr 2007;85:1057–61 [DOI] [PubMed] [Google Scholar]
- 31.Nexo E, Christensen AL, Petersen TE, Fedosov SN. Measurement of transcobalamin by ELISA. Clin Chem 2000;46:1643–9 [PubMed] [Google Scholar]
- 32.Hvas AM, Nexo E. Holotranscobalamin - a first choice assay for diagnosing early vitamin B12 deficiency? J Intern Med 2005;257:289–98 [DOI] [PubMed] [Google Scholar]
- 33.Morkbak AL, Hvas AM, Milman N, Nexo E. Holotranscobalamin remains unchanged during pregnancy. Longitudinal changes of cobalamins and their binding proteins during pregnancy and postpartum. Haematologica 2007;92:1711–2 [DOI] [PubMed] [Google Scholar]
- 34.Murphy MM, Molloy AM, Ueland PM, et al. Longitudinal study of the effect of pregnancy on maternal and fetal cobalamin status in healthy women and their offspring. J Nutr 2007;137:1863–7 [DOI] [PubMed] [Google Scholar]
- 35.Nexo E, Hvas AM, Bleie Ø, et al. Holo-transcobalamin is an early marker of changes in cobalamin homeostasis. A randomized placebo-controlled study. Clin Chem 2002;48:1768–71 [PubMed] [Google Scholar]
- 36.Nexo E, Hollenberg MD. Characterization of the particulate and soluble acceptor for transcobalamin II from human placenta and rabbit liver. Biochim Biophys Acta 1980;628:190–200 [DOI] [PubMed] [Google Scholar]
- 37.Quadros EV, Nakayama Y, Sequeira JM. The binding properties of the human receptor for the cellular uptake of vitamin B12. Biochem Biophys Res Commun 2005;327:1006–10 [DOI] [PubMed] [Google Scholar]
- 38.Quadros EV, Nakayama Y, Sequeira JM. The protein and the gene encoding the receptor for the cellular uptake of transcobalamin-bound cobalamin. Blood 2009;113:186–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li N, Sood GK, Seetharam S, Seetharam B. Polymorphism of human transcobalamin II: substitution of proline and/or glutamine residues by arginine. Biochim Biophys Acta 1994;1219:515–20 [DOI] [PubMed] [Google Scholar]
- 40.Castro R, Barroso M, Rocha M, et al. The TCN2 776CNG polymorphism correlates with vitamin B(12) cellular delivery in healthy adult populations. Clin Biochem 2010;43:645–9 [DOI] [PubMed] [Google Scholar]
- 41.Garrod MG, Allen LH, Haan MN, Green R, Miller JW. Transcobalamin C776G genotype modifies the association between vitamin B12 and homocysteine in older Hispanics. Eur J Clin Nutr 2010;64:503–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Platica O, Janeczko R, Quadros EV, Regec A, Romain R, Rothenberg SP. The cDNA sequence and the deduced amino acid sequence of human transcobalamin II show homology with rat intrinsic factor and human transcobalamin I. J Biol Chem 1991;266:7860–3 [PubMed] [Google Scholar]
- 43.Carmel R, Vasireddy H, Aurangzeb I, George K. High serum cobalamin levels in the clinical setting-clinical associations and holo-transcobalamin changes. Clin Lab Haematol 2001;23:365–71 [DOI] [PubMed] [Google Scholar]
- 44.Nexø E. Transcobalamin I and other human R-binders: purification, structural, spectral and physiological studies. Scand J Haematol 1978;20:221–36 [DOI] [PubMed] [Google Scholar]
- 45.Loikas S, Koskinen P, Irjala K, et al. Renal impairment compromises the use of total homocysteine and methylmalonic acid but not total vitamin B12 and holotranscobalamin in screening for vitamin B12 deficiency in the aged. Clin Chem Lab Med 2007;45:197–201 [DOI] [PubMed] [Google Scholar]
- 46.Moller HJ, Moestrup SK, Weis N, et al. Macrophage serum markers in pneumococcal bacteremia: Prediction of survival by soluble CD163. Crit Care Med 2006;34:2561–6 [DOI] [PubMed] [Google Scholar]
- 47.Baker H, Leevy CB, DeAngelis B, Frank O, Baker ER. Cobalamin (vitamin B12) and holotranscobalamin changes in plasma and liver tissue in alcoholics with liver disease. J Am Coll Nutr 1998;17:235–8 [DOI] [PubMed] [Google Scholar]
- 48.Skouby AP, Hippe E, Olesen H. Antibody to transcobalamin II and B12 binding capacity in patients treated with hydroxocobalamin. Blood 1971;38:769–74 [PubMed] [Google Scholar]
- 49.Carmel R, Tatsis B, Baril L. Circulating antibody to transcobalamin II causing retention of vitamin B12 in the blood. Blood 1977;49:987–1000 [PubMed] [Google Scholar]
- 50.Aarsetøy H, Valente E, Reine A, Mansoor MA, Grundt H, Nilsen DW. Holotranscobalamin and methylmalonic acid as prognostic markers following an acute myocardial infarction. Eur J Clin Nutr 2008;62:411–8 [DOI] [PubMed] [Google Scholar]
- 51.Nissen PH, Nordwall M, Hoffmann-Lücke E, Sorensen BS, Nexo E. Transcobalamin deficiency caused by compound heterozygosity for two novel mutations in the TCN2 gene: a study of two affected siblings, their brother, and their parents. J Inherit Metab Dis (Epub ahead of print 6 July 2010) [DOI] [PubMed] [Google Scholar]
- 52.Bleie Ø, Refsum H, Ueland PM, et al. Changes in basal and postmethionine load concentrations of total homocysteine and cystathionine after B vitamin intervention. Am J Clin Nutr 2004;80:641–8 [DOI] [PubMed] [Google Scholar]
- 53.Hvas AM, Morkbak AL, Nexo E. Plasma holotranscobalamin compared with plasma cobalamins for assessment of vitamin B12 absorption; optimisation of a non-radioactive vitamin B12 absorption test (CobaSorb). Clin Chim Acta 2007;376:150–4 [DOI] [PubMed] [Google Scholar]
- 54.Bor MV, Cetin M, Aytaç S, Altay C, Nexo E. Nonradioactive vitamin B12 absorption test evaluated in controls and in patients with inherited malabsorption of vitamin B12. Clin Chem 2005;51:2151–5 [DOI] [PubMed] [Google Scholar]
- 55.Hardlei TF, Mørkbak AL, Bor MV, Bailey LB, Hvas AM, Nexo E. Assessment of vitamin B(12) absorption based on the accumulation of orally administered cyanocobalamin on transcobalamin. Clin Chem 2010;56:432–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clarke R, Sherliker P, Hin H, et al. Detection of vitamin B12 deficiency in older people by measuring vitamin B12 or the active fraction of vitamin B12, holotranscobalamin. Clin Chem 2007;53:963–70 [DOI] [PubMed] [Google Scholar]
- 57.Herrmann W, Obeid R, Schorr H, Geisel J. Functional vitamin B12 deficiency and determination of holotranscobalamin in populations at risk. Clin Chem Lab Med 2003;41:1478–88 [DOI] [PubMed] [Google Scholar]
- 58.Obeid R, Herrmann W. Holotranscobalamin in laboratory diagnosis of cobalamin deficiency compared to total cobalamin and methylmalonic acid. Clin Chem Lab Med 2007;45:1746–50 [DOI] [PubMed] [Google Scholar]
- 59.Schrempf W, Eulitz M, Neumeister V, Siegert G, Koch R, Reichmann H, Storch A. Utility of measuring vitamin B(12) and its active fraction, holotranscobalamin, in neurological vitamin B(12) deficiency syndromes. J Neurol (Epub ahead of print 2 October 2010) [DOI] [PubMed] [Google Scholar]
- 60.Miller JW, Garrod MG, Rockwood AL, et al. Measurement of total vitamin B12 and holotranscobalamin, singly and in combination, in screening for metabolic vitamin B12 deficiency. Clin Chem 2006;52:278–85 [DOI] [PubMed] [Google Scholar]
- 61.Fedosov SN. Metabolic signs of vitamin B(12) deficiency in humans: computational model and its implications for diagnostics. Metabolism 2010;59:1124–38 [DOI] [PubMed] [Google Scholar]