Abstract
Background: Gestational weight gain (GWG) is associated with a range of health outcomes, but little is known about the factors that influence it.
Objective: The objective was to test the hypothesis that maternal and fetal genetic variants that are reliably associated with adiposity are associated with GWG.
Design: We examined the association of a risk allele score by using 4 adiposity-related single nucleotide polymorphisms (SNPs; rs9939609 in FTO, rs17782313 near MC4R, rs6548238 near TMEM18, and rs10938397 near GNPDA2) with GWG in a pregnancy cohort in which women had detailed repeated assessment of GWG (median number of weight measurements: 10; interquartile range: 8, 11). The numbers included in our analyses varied between 2324 and 7563 for different variant-outcome analyses. A linear spline random-effects model was used to model weight change with gestational age and to relate genetic variants to this. This modeling confirmed 3 distinct periods of GWG: 0–18, 19–28, and ≥29 wk of gestation.
Results: Maternal risk allele score and SNPs in FTO, MC4R, and TMEM18 were positively associated with prepregnancy weight. Maternal allele score was inversely associated with GWG in the first 18 wk of pregnancy (−14.46 g/wk per allele; 95% CI: −24.75, −4.17 g/wk per allele) but was not associated with other periods of GWG. Offspring allele score and maternal and offspring individual SNPs were not associated with GWG in any period or with birth weight or postnatal weight retention.
Conclusions: Our findings suggest that neither maternal nor fetal adiposity-related genetic variants are associated with greater GWG. The inverse association of maternal allele score with GWG in the first 18 wk requires replication.
INTRODUCTION
Variation in gestational weight gain (GWG) is associated with a wide range of perinatal outcomes (1, 2) and later health outcomes in the offspring (3–5) and mothers (6). It is important to elucidate the factors that determine variation in GWG to better understand the mechanisms that link it with health outcomes, but relatively little is known about this. On average, healthy women gain 10–12 kg during pregnancy, with ≈55% of this gain being maternal tissue, 15–20% being placenta and amniotic fluid, and 20–25% being fetal tissue (2, 7, 8). Rates of deposition of maternal fat parallel GWG (9, 10) and women gain between 2 and 6 kg fat during pregnancy (11). These substantive novel maternal fat depots are accumulated relatively constantly from the start of pregnancy to the middle of the second trimester, when the rate of accumulation flattens; as a result, most maternal fat accumulation has occurred by the end of the second trimester (7, 11). Maternal factors related to ease of fat deposition may be an important determinant of GWG.
Human fetuses have a much greater proportion of fat than do any other mammal, with ≈15% of a human at birth being fat (compared with <1% for most other mammals) (2, 12). In contrast with maternal fat, fetal fat accumulation becomes most notable from the middle of the second trimester of pregnancy, with a gain of ≈2.5–4.0 g/d between 28 and 40 wk gestation in healthy singleton fetuses (2). Fetal fat accumulation is influenced by intrauterine characteristics, such as maternal diabetes in pregnancy (13).
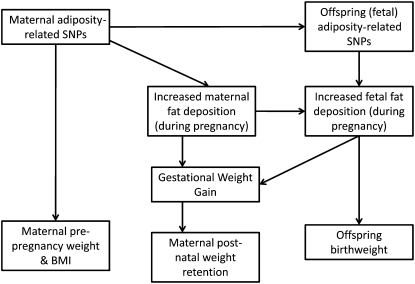
Given the relevance of maternal and fetal fat to overall GWG, it is conceivable that genetic variants that are known to be associated with adiposity might be associated with GWG. Many genetic variants have been shown to be associated with variation in weight and body mass index (BMI) in humans, with evidence that these associations are driven by greater fat mass (14–16). To our knowledge only one study to date has examined the association of these variants with GWG. In a small study of just 960 women, maternal adiposity-related genetic variants were not associated with GWG based on 2 measurements (the difference between final clinic weight measurement and maternal self-reported prepregnancy weight) (17). The aim of this study was to examine the association with GWG of a BMI-risk allele score derived from 4 single nucleotide polymorphisms (SNPs) that have been found in genome-wide association studies (GWAS) to be robustly associated with BMI in European populations (14–16). We also examined associations of this score with prepregnancy maternal BMI, postnatal maternal weight retention, and birth weight and in secondary analyses examined associations of each individual SNP with our outcomes. The 4 SNPs (rs9939609 in FTO, rs17782313 near MC4R, rs6548238 near TMEM18, and rs10938397 near GNPDA2) were chosen because they had the strongest associations with adiposity in previous GWAS (16). SNPs discovered since these 4 SNPs were discovered have weaker associations and would have insufficient statistical power in our study. The pathways via which we hypothesize that these variants might influence our outcomes are outlined in Figure 1.
FIGURE 1.
Summary of how maternal and fetal (offspring) adiposity genotypes might influence gestational weight gain. SNPs, single nucleotide polymorphisms.
SUBJECTS AND METHODS
Participants
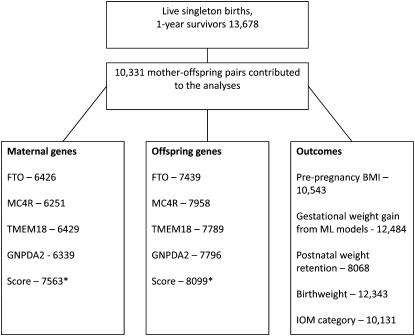
The Avon Longitudinal Study of Parents and Children (ALSPAC) is a prospective population-based birth cohort study that recruited 14,541 pregnant women resident in Avon, United Kingdom, with expected dates of delivery between 1 April 1991 and 31 December 1992 (http://www.alspac.bris.ac.uk) (18). There were 13,678 mother-offspring pairs from singleton live births who survived to ≥1 y of age; only singleton pregnancies are considered in this article. Of these women, 94% gave consent for abstraction of data from their obstetric records. There are different amounts of missing data for each of the exposures and outcomes used in this study. The numbers available (from the 13,678) with data on each exposure and outcome used in this study are presented in Figure 2. For each main association analysis, we included maximal data available and the numbers included in each analysis are presented in the tables. Our assumption in these analyses is that data are missing at random (ie, that there is no interaction between having missing data and the exposure in its association with outcome). Ethical approval for all aspects of data collection was obtained from the ALSPAC Law and Ethics Committee (Institutional Review Board 00003312) and the Local Research Ethics Committee. Participants provided written informed consent to participate in the study, including consent for genetic information to be obtained and information from medical records to be abstracted.
FIGURE 2.
Numbers of subjects with data on each of the key exposure and outcome variables. The eligible cohort consisted of 13,678 mother-offspring pairs, in whom there was a live singleton birth with the infant still alive at 1 y of age. In the main association tables, the numbers for each association are provided. *This score reflects the number of eligible subjects with data available for at least one of the single nucleotide polymorphisms (SNPs; irrespective of which SNP); thus, this number is greater than the number for any one SNP. IOM, Institute of Medicine; ML, multilevel.
Gestational weight gain and other obstetric/perinatal data
Six trained research midwives abstracted data from obstetric medical records. Mean values of abstracted data were similar for each midwife, and repeat data entry checks demonstrated error rates consistently <1%. Obstetric data abstractions included every measurement of weight entered into the medical records (weight was measured at all antenatal clinic visits in the United Kingdom at the time of data collection for this study) and the corresponding gestational age and date at the time of the weight measurement. At enrollment, the mothers were asked to record their immediate prepregnancy weight and height. Maternal self-report of prepregnancy weight, measured weight at the first antenatal clinic, and predicted (from our multilevel models; see Statistical analysis) weight at gestational age 0 were highly correlated (both Pearson's correlation coefficients: > 0.95; P < 0.0001).
In addition to deriving GWG variables from multilevel models, we also allocated women to categories of weight gain attained (less than recommended, recommended, or more than recommended) based on the revised (2009) Institute of Medicine (IOM) categories (Table 1) (2). To derive these categories we used actual weight measurements from the obstetric notes (ie, not predicted measures from our multilevel models) and subtracted the first from the last weight measurement in pregnancy to derive absolute weight gain. Prepregnancy BMI was based on the mother's self-reported weight before pregnancy (results were identical for predicted prepregnancy weight with the use of multilevel models).
TABLE 1.
Institute of Medicine–recommended levels of gestational weight gain1
| Prepregnancy BMI (in kg/m2) | Range of recommended absolute weight gain |
| kg | |
| Underweight (<18.5) | 12.5–18 |
| Normal weight (18.5–24.9) | 11.5–16 |
| Overweight (25–29.9) | 7–11.5 |
| Obese (≥30) | 5–9 |
Based on the levels recommended in reference 2.
Postnatal maternal weight was obtained from a questionnaire, sent to mothers at ≈8 wk after the birth of the infant, in which mothers were asked to report their weight at the time of questionnaire completion. Weeks since delivery were derived from the date that the mother completed the questionnaire and the known date of birth of their child. We calculated postnatal weight retention as maternal self-report of postnatal weight minus her report of prenatal weight. Birth weight and gestational age (in completed weeks) were obtained from obstetric/perinatal records at the time of birth.
Our expectation was that genetic variants would not be directly associated with maternal characteristics that were not a consequence of GWG or prepregnancy weight (19), but we examined this expectation. Maternal age, parity, mode of delivery (cesarean or vaginal delivery), and the child's sex were obtained from the obstetric records. On the basis of the questionnaire responses, the highest parental occupation was used to allocate the children to family social class groups [classes I (professional/managerial) to V (unskilled manual workers)] by using the 1991 British Office of Population and Census Statistics classification. Mothers were asked about their smoking throughout pregnancy on several occasions, and these data were used to generate a categorical variable: never smoked, smoked before pregnancy or in the first trimester and then stopped, or smoked throughout pregnancy.
Genotyping
All genotyping was performed by KBioscience (http://www.kbioscience.co.uk). SNPs were genotyped by using KASPar chemistry, which is a competitive allele-specific polymerase chain reaction SNP genotyping system that uses FRET quencher cassette oligos. Blind duplicates, plate-identifying blank wells, and Hardy-Weinberg equilibrium tests were used as quality-control tests.
Statistical analysis
For our main analyses, we combined the 4 SNPs into an at-risk allele score. To derive this allele score, each SNP was coded so that the allele associated with greater BMI was coded 1 and the other as 0, and each individual was assigned a score of 0, 1, or 2 for each SNP. Participants (mothers or offspring) contributed to the score if they had data on at least one of the SNPs (irrespective of which SNP); therefore, the number contributing to the analyses with the score is greater than the number with any one SNP because “missingness” for each SNP does not overlap (eg, 6426 mothers have complete data on FTO, and an additional 1081 had data on MC4R who did not have FTO). The simplest version of the gene score was calculated by summing the number of at-risk alleles across all relevant SNPs for each individual and then dividing by the number of SNPs for which data were available. An alternative approach of weighting each SNP score by the magnitude of its association (obtained from previous GWAS; 16)) with BMI yielded very similar findings, and results obtained by using the simpler unweighted score are presented here. This sum of at-risk alleles approach has been used in many other genetic association studies and is valid when using genetic variants that are not in linkage disequilibrium with one another (20–22). This score provides the greatest statistical power, but it may mask SNP-specific associations; therefore, we also examined the associations of each of the 4 SNPs separately with all outcomes in secondary analyses.
In all analyses, a per-allele additive genetic model was examined. We examined associations separately for maternal and fetal genotype and then mutually adjusted one for the other. (Associations of offspring genotype with maternal prepregnancy weight or BMI are not presented because there would be no reason for fetal genotype to affect maternal prepregnancy size.) The mutual adjustment is important because of the strong association between maternal and offspring genotype and our interest in establishing whether any associations were primarily driven by maternal genetic variants (independent of offspring genotype) and vice-versa. The ways in which maternal and offspring genotypes can influence the key outcomes examined here are shown in Figure 1.
Multilevel models of GWG
All of the multilevel models and assessment of genetic associations with GWG derived from the multilevel models were conducted in the statistical package MLwiN version 2.10 (www.cmm.bristol.ac.uk/MLwiN/index.shtml). All pregnancy weight measurements (median number of repeat measurements per woman: 10; interquartile range: 8, 11) were used to develop a linear spline random-effects model with 2 knots. Full details of this statistical modeling are provided in supplemental materials (see supplemental materials under “Supplemental data” in the online issue) and were reported previously (5). This modeling confirmed that there were 3 distinct periods of GWG: 0–18, 19–28, and ≥29 wk. The average predicted maternal weight by gestational age from these models is shown in Figure 3. From these models, there were 4 maternal outcomes that we assessed in relation to maternal and offspring genes: weight at 0 wk gestation (referred to as “prepregnancy weight”) and weight change from 0 to 18 wk (period 1), from 19 to 28 wk (period 2), and from 29 wk to delivery (period 3).
FIGURE 3.
Mean gestational weight by gestational age derived from the multilevel models. n = 12,484.
Genetic differences in the GWG trajectories derived from the multilevel models were estimated by including a statistical interaction between gestational age and genotype (each SNP and the allelic score for mothers and offspring) in the multilevel model and estimating whether there were differences between genotypes in terms of the constant term (representing prepregnancy weight) and each of the slopes for the different periods of GWG. Thus, the associated P values test the null hypothesis of no difference in prepregnancy weight or GWG in each period per allele, and the coefficients represent the mean difference in birth length or growth rate per allele. In all analyses, the results were adjusted for baseline weight (constant in the multilevel model) and GWG in the earlier (but not subsequent) period.
Associations with IOM categories of GWG, postnatal weight retention, and birth weight
All associations with IOM categories, postnatal weight retention, and birth weight were conducted in the statistical package StataMP (version 11). In addition to the associations of genetic variants with GWG derived from repeat measurements within each women, we examined associations of genotype (allelic scores and individual SNPs) with women categorized according to IOM recommendations of GWG (Table 1) and also with postnatal weight retention (continuous variable; in kg) and birth weight (continuous variable; in g). Ordinal logistic regression was used to examine genetic associations with IOM categories and linear regression to examine associations with birth weight and postnatal weight retention.
The associations of maternal genetic variants/allele score with prepregnancy weight and BMI are anticipated because the variants we examined are known from GWAS to be associated with BMI, and our cohort has contributed as a replication sample to some of the published GWAS; for these we felt it appropriate to consider α = 0.05 as indicating statistical significance because they are testing an established hypothesis in our cohort. For the main analyses of the allele score with GWG, birth weight and postnatal weight retention, there were 12 tests, and a P value <0.004 (Bonferroni correction of 0.05) was considered to indicate statistical significance. Our secondary analyses with each individual SNP added a further 48 tests, and for these we correct for 60 tests (12 + 48), and a P value <0.0008 was considered to indicate statistical significance. For our examination of whether genetic variants were associated with potential confounding factors (where our a priori hypothesis was that they would not be), we also applied a Bonferroni correction giving a P value <0.0009. Given the differences between each of these sets of analyses (main analyses, secondary analyses, testing the assumption of no association with confounders), we believe that it is appropriate to consider each separately.
RESULTS
Characteristics of the mothers included in our sample (ie, those who contributed to any of the association analyses) are presented in Table 2. The respective genotyping call rates were >95% for all maternal and offspring SNPs. The risk allele distribution and Hardy-Weinberg equilibrium (HWE) P values are given in Table 3. There was no evidence of departure from HWE. For each of rs9939609, rs17782313, rs6548238, and rs10938397, 52%, 63%, 73%, and 52% of the mothers and offspring had the same genotype, respectively. The median of both maternal and offspring risk allele scores was 4 (interquartile range: 3–5).
TABLE 2.
Characteristics of participants1
| Characteristic | Eligible subjects with data | Value |
| Prepregnancy BMI, self-reported (kg/m2) | 8886 | 22.9 ± 3.82 |
| Prepregnancy weight (predicted) models (kg) | 10,331 | 60.8 ± 12.4 |
| Period (g/wk) | ||
| 1: 0–18 wk | 10,331 | 310 ± 173 |
| 2: 19–28 wk | 10,331 | 536 ± 175 |
| 3: ≥29 wk | 10,331 | 467 ± 196 |
| Overall weight gain in pregnancy (kg) | 10,035 | 12.3 ± 4.8 |
| Postnatal weight retention (kg) | 6996 | 3.64 (1.00, 6.36)3 |
| Manual social class (%) | 8914 | 18.2 |
| Maternal age (y) | 10,331 | 28.3 ± 4.9 |
| Parity, ≥3 including this pregnancy (%) | 9729 | 5.7 |
| Cesarean delivery (%) | 9856 | 10.2 |
| No smoking in pregnancy (%) | 9864 | 75.9 |
| Never breastfed (%) | 9467 | 20.7 |
| Offspring birth weight (g) | 10,224 | 3475.2 ± 480.3 |
| Male offspring (%) | 10,331 | 51.2 |
n = 10,331 mother-offspring pairs.
Mean ± SD (all such values).
Median; interquartile range in parentheses.
TABLE 3.
Allele frequency and Hardy-Weinberg equilibrium (HWE) P value for each adiposity-related single nucleotide polymorphism (SNP)
| No. (%) in each genotype group |
|||||
| SNP | Homozygous for nonrisk allele | Heterozygous | Homozygous for risk allele | HWE P value1 | |
| Maternal | |||||
| FTO | T/A | 2317 (36.1) | 3097 (8.2) | 1012 (15.7) | 0.67 |
| MC4R | T/C | 3641 (57.5) | 2258 (37.0) | 352 (5.5) | 0.94 |
| TMEM18 | C/C | 4464 (69.4) | 1787 (27.8) | 178 (2.8) | 0.96 |
| GNPDA2 | A/G | 2022 (31.9) | 3152 (49.7) | 1165 (18.4) | 0.30 |
| Offspring | |||||
| FTO | T/A | 2728 (36.7) | 3535 (47.5) | 1176 (15.8) | 0.58 |
| MC4R | T/C | 4576 (58.3) | 2942 (36.1) | 440 (5.6) | 0.25 |
| TMEM18 | C/C | 5445 (69.9) | 2124 (27.3) | 220 (2.8) | 0.46 |
| GNPDA2 | A/G | 2534 (32.5) | 3833 (49.2) | 1429 (18.3) | 0.76 |
Chi-square test.
Association of genotype with potential covariables
The association of each SNP (maternal and offspring) with maternal and offspring characteristics (social class, maternal age, parity, mode of delivery, maternal smoking in pregnancy, breastfeeding, and offspring sex) are shown elsewhere (see supplemental Table 1 under “Supplemental data” in the online issue). No statistical evidence of any associations at the P < 0.05 level after Bonferroni correction (<0.0009 Bonferroni equivalent of <0.05) was observed.
Associations of maternal and offspring allele score and individual SNPs with maternal BMI, GWG, postnatal weight retention, and offspring birth weight
The associations of maternal and offspring risk allele scores with all outcomes are shown in Table 4. The maternal risk allele score was positively associated with prepregnancy BMI and weight. Maternal risk allele score was inversely associated with GWG in the first (0–18 wk) gestational period, even when multiple testing was taken into account (mean difference: −6.77 g/wk per allele; 95% CI: −11.01, −2.53 g/wk per allele; P = 0.002). With adjustment for offspring genotype, the point estimate actually increased (−14.46 g/wk per allele; 95% CI: −24.75, −4.17 g/wk per allele; P = 0.006). However, because the sample size was smaller when both maternal and offspring allele scores were included in the model, the P value was smaller and was not statistically significant once we had corrected for multiple testing (P < 0.004 equivalent to <0.05 with Bonferroni correction). The offspring risk allele score was not associated with GWG in any period. Neither maternal nor offspring risk allele scores were associated with IOM categories of GWG, postnatal weight retention, or birth weight.
TABLE 4.
Associations of adiposity risk allele scores (maternal and offspring) with prepregnancy BMI for each gestational weight gain (GWG) multilevel model variable, postnatal weight, and Institute of Medicine (IOM) characteristics
| Exposure = maternal risk allele score |
Exposure = offspring risk allele score |
|||||||
| Unadjusted |
Adjusted for offspring allele score |
Unadjusted |
Adjusted for maternal allele score |
|||||
| Mean difference per risk allele score (95% CI) | P value1 | Mean difference per risk allele score (95% CI) | P value1 | Mean difference per risk allele score (95% CI) | P value1 | Mean difference per risk allele score (95% CI) | P value1 | |
| Prepregnancy BMI (kg/m2) | 0.11 (0.05, 0.16) | <0.001 (6520) | — | — | — | — | — | — |
| Prepregnancy weight (g) | 450.06 (275.25, 624.86) | <0.001 (7563) | — | — | — | — | — | — |
| GWG in first period (g/wk)2 | −6.77 (−11.01, −2.53) | 0.002 (7563) | −14.46 (−24.75, −4.17) | 0.006 (2324) | −6.87 (−12.15, −1.60) | 0.01 (8099) | 1.93 (−8.29, 12.14) | 0.71 (2324) |
| GWG in second period (g/wk)2 | −2.52 (−5.66, 0.62) | 0.12 (7563) | 2.36 (−5.32, 10.03) | 0.55 (2324) | −2.12 (−5.99, 1.74) | 0.28 (8099) | −1.88 (−9.55, 5.79) | 0.63 (2324) |
| GWG in third period (g/wk)2 | −0.02 (−3.45, 3.06) | 0.91 (7563) | 6.98 (−1.16, 15.12) | 0.09 (2324) | 0.56 (−3.41, 4.54) | 0.78 (8099) | 6.04 (−4.19, 2.10) | 0.15 (2324) |
| Postnatal weight retention (g)3 | 21.46 (−54.62, 97.55) | 0.58 (5124) | 64.22 (−57.39, 185.83) | 0.38 (3729) | −16.56 (−105.45, 72.32) | 0.72 (5601) | −101.85 (−227.74, 24.05) | 0.11 (3729) |
| Birth weight (g)4 | 1.65 (−4.77, 8.07) | 0.61 (7486) | −3.35 (−13.89, 7.19) | 0.53 (5276) | 3.28 (−4.44, 11.00) | 0.41 (8014) | 2.20 (−8.45, 12.85) | 0.69 (5276) |
| Odds ratio per increase in IOM category5 | 1.01 (0.98, 1.03) | 0.70 (6336) | 1.04 (0.99, 1.09) | 0.09 (4549) | 1.01 (0.98, 1.04) | 0.50 (6835) | 1.00 (0.95, 1.04) | 0.83 (4549) |
Values derived with t tests for all results unless otherwise tested. The number included in the analyses is in parentheses.
First period = 0–18 wk gestation, second period = 19–28 wk gestation, third period = ≥29 wk gestation; the results are adjusted for prepregnancy weight and GWG in the earlier period but not for GWG in the subsequent periods.
Calculated as the difference between post- and prepregnancy weights and adjusted for weeks since birth.
Adjusted for offspring sex.
Note that estimates in this row are odds ratios; Wald tests were used to derive the P values.
Associations of maternal and offspring genotypes with prepregnancy weight and BMI, GWG, postnatal weight retention, and birth weight for each individual SNP are presented elsewhere (see supplemental Tables 2–5 under “Supplemental data” in the online issue). Maternal FTO and TMEM18 were positively associated with prepregnancy weight and BMI, and MC4R was positively associated with prepregnancy weight. There were no statistically significant associations of any individual SNP (maternal or offspring) with GWG by period of pregnancy, GWG assessed by IOM categories, birth weight, or postnatal weight retention when multiple testing was taken into account (Bonferroni corrected P < 0.0008 equivalent to < 0.05).
When all analyses were repeated only for those with complete mother-offspring genotype data (n = 5331 with available genotype data for allele scores and 4732–5059 for each SNP; n = 2324–5276 included in the association analyses), they did not differ from those presented here (results available from authors). When the analyses were restricted to only those who were experiencing their first pregnancy (n = 2038–3338 included in the association analyses), the results did not differ markedly from those presented here (results available from authors).
DISCUSSION
In this population, a maternal BMI-risk allele score and individual SNPs in FTO and MC4R were positively associated with prepregnancy weight and BMI; TMEM18 was also associated with prepregnancy weight. These findings are consistent with GWAS, which showed these variants to be associated with greater weight and BMI (14–16). No evidence of associations of maternal or offspring genotypes with greater GWG, postnatal weight retention, or birth weight were observed. Maternal allele score was inversely associated with GWG in the first 18 wk of pregnancy. Because pregnancy-related maternal fat deposition, which strongly correlates with GWG (Pearson's correlations ≈0.8), occurs largely in the first and second trimesters (9, 10), this finding is surprising, may be due to chance, and requires replication in additional large cohort studies. However, we are unaware of studies that have repeat measurements of gestational weight that would allow specification of different periods of GWG, together with maternal and offspring DNA, and that would therefore be able to act as replication samples.
To our knowledge only one previous study has examined these associations (17). Our study adds to this previous publication by being larger (the previous study included only 960 women), by examining fetal as well as maternal adiposity genetic variants (the previous study examined maternal variants only), and by using repeat measurements of maternal weight to characterize GWG across pregnancy in detail (the previous study used the simple difference between the final clinic measure and the mothers reported prepregnancy weight). Despite key differences between the studies, both found no association of maternal adiposity genetic variants with greater GWG. The previous study also examined maternal diabetes genetic variants with GWG and found that women carrying 1 or 2 alleles of the type 2 diabetes–associated SNP in KCNQ1 had greater GWG (17). Recent GWAS have identified a large number of type 2 diabetes genes, some of which have been shown to be associated with gestational diabetes. We plan in a future study to examine these in relation to GWG and other outcomes considered here.
We hypothesized that variation in maternal and/or offspring SNPs that are associated with greater adiposity in GWAS may also be associated with greater GWG. Our results, and those of one previous study (17), do not support this hypothesis. Several possible reasons could explain this. First, our study may have lacked statistical power to detect any associations. In general, we found expected positive associations with prepregnancy weight/BMI. A post hoc power calculation indicates the minimal effect sizes that we would be able to detect, with 90% power and an α of 5%, for the associations of maternal allele score with GWG in the first, second, and third periods of gestation given the fixed sample sizes that we have in this study are 5–6 g/wk for each 1-SD increase in the allele score. Similar power calculations for the offspring allele score give minimal effect sizes of 6–7 g/wk for all 3 periods of GWG. We had 90% power at an α of 5% to detect a 14- and 18-g difference in birth weight per 1-SD increase in the mother's and offspring's allele score, respectively. The equivalent minimal effects for postnatal retrained weight were 0.14 and 0.18 kg for the mother's and offspring's allele scores, respectively.
Second, it is possible that whereas these genetic variants are associated with mean differences in weight/BMI between individuals in the population, they are not related to rates of weight gain in general or at specific times, such as during pregnancy. A recent analysis in the same cohort used in this study found that a risk allele score, composed of the 4 SNPs examined in this publication and 4 additional SNPs, was unrelated to birth weight, positively associated with greater weight gain in infancy, but less strongly with weight gain in later childhood (23). These findings suggest that the SNPs that have been associated with adult weight/BMI may have their effect largely because of promoting weight gain during infancy. Whereas these associations with weight change require further replication in large studies, if they are replicated it may be that maternal weight/BMI-related SNPs have little influence on GWG because they have little effect on change in weight/BMI in the mother during adulthood in general (including when she is pregnant), because they only relate to weight change during the infant period. Similarly, the lack of association with birth weight may mean that offspring (fetal) BMI SNPs have little influence on GWG because they have little influence on fetal growth. This may be because fetal growth and fat deposition is primarily determined by maternal supply of nutrients or that any effect of these variants on fetal fat deposition is too small to be detected as an effect on total GWG. Further examination of this might be undertaken by examining the association of these variants with more direct measurements of fetal adiposity, such as those obtained via antenatal ultrasound scan or detailed neonatal body-composition assessment.
Third, GWG may be more importantly influenced by environmental factors and different genetic and biological pathways, rather than by general adiposity-related genetic variants. Because GWG is a complex phenotype that has contributions from the growing fetus, placenta, amniotic fluid, maternal plasma expansion, and maternal fat deposition, many biological and lifestyle characteristics are likely to influence it. These would include maternal dietary changes during pregnancy and quitting smoking, which is associated with increases in weight (24) and with physiologic responses to pregnancy and pregnancy complications, such as preeclampsia. GWAS of GWG would allow exploration of alternative pathways that influence this phenotype.
The main strength of this study is the availability of both maternal and offspring genotype and the detailed measures of weight during pregnancy which allowed us to study associations with timing of GWG. Prepregnancy weight was estimated from multilevel models and prepregnancy BMI was based on self-reported height and weight. However, associations were similar for both measures and self-reported and predicted weight were highly correlated.
In summary, we found no associations between maternal or offspring variation in SNPs that were found to be associated with BMI, with greater GWG. The inverse association of maternal allele score with GWG in the first 18 wk of pregnancy requires replication. Our findings suggest that mechanisms other than maternal or genetic variants related to greater adiposity determine GWG. Given the composite nature of GWG, including maternal increase in fat stores, maternal plasma volume expansion, the growing fetus, placenta, and amniotic fluid, analyses of genome-wide data from fetus and mother with this outcome might identify the greatest contribution from any of these different factors.
Supplementary Material
Acknowledgments
We are grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the entire ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
The authors’ responsibilities were as follows—DAL: obtained funding for the study and wrote the first draft of the manuscript; DAL and KT: developed the study aims and analysis protocol; KT and AF: supervised the statistical analyses; and CM-W and TP: contributed to the analyses. All authors contributed to the final version. None of the authors declared a conflict of interest.
REFERENCES
- 1.Viswanthan M, Siega-Riz AM, Moos M-K, et al. Outcomes of maternal weight gain. Evidence Report/Technology Assessment no. 168. AHRQ publication no. 08-E009 ed. Rockville, MD: Agency for Healthcare Research and Quality, 2008 [Google Scholar]
- 2.Rasmussen KM, Yaktine AL, eds. Committee to reexamine IOM pregnancy weight guidelines. Weight gain during pregnancy: reexamining the guidelines. Washington, DC: National Academies Press, 2009 [PubMed] [Google Scholar]
- 3.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol 2007;196:322–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mamun AA, O'Callaghan M, Callaway L, Williams G, Najman J, Lawlor DA. Associations of gestational weight gains with offspring body mass index and blood pressure at 21 years: evidence from a birth cohort study. Circulation 2009;119:1720–7 [DOI] [PubMed] [Google Scholar]
- 5.Fraser A, Tilling K, Macdonald-Wallis C, et al. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation 2010;121:2557–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet 2006;368:1164–70 [DOI] [PubMed] [Google Scholar]
- 7.Pitkin RM. Nutritional support in obstetrics and gynecology. Clin Obstet Gynecol 1976;19:489–513 [DOI] [PubMed] [Google Scholar]
- 8.Chesley LC. Weight changes and water balance in normal and toxic pregnancy. Am J Obstet Gynecol 1944;48:565 [Google Scholar]
- 9.Butte NF, Ellis KJ, Wong WW, Hopkinson JM, Smith EO. Composition of gestational weight gain impacts maternal fat retention and infant birth weight. Am J Obstet Gynecol 2003;189:1423–32 [DOI] [PubMed] [Google Scholar]
- 10.Lederman SA, Paxton A, Heymsfield SB, Wang J, Thornton J, Pierson RN., Jr Body fat and water changes during pregnancy in women with different body weight and weight gain. Obstet Gynecol 1997;90:483–8 [DOI] [PubMed] [Google Scholar]
- 11.Nelson SM, Matthews P, Poston L. Maternal metabolism and obesity: modifiable determinants of pregnancy outcome. Hum Reprod Update 2010;16:255–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Widdowson EM. Chemical composition of newly born mammals. Nature 1950;166:626–8 [DOI] [PubMed] [Google Scholar]
- 13.Freinkel N. Banting Lecture 1980. Of pregnancy and progeny. Diabetes 1980;29:1023–35 [DOI] [PubMed] [Google Scholar]
- 14.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loos RJ, Lindgren CM, Li S, et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet 2008;40:768–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willer CJ, Speliotes EK, Loos RJ, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet 2009;41:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stuebe AM, Lyon H, Herring AH, et al. Obesity and diabetes genetic variants associated with gestational weight gain. Am J Obstet Gynecol 2010;203:283.e1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golding J, Pembrey M, Jones R. ALSPAC–the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol 2001;15:74–87 [DOI] [PubMed] [Google Scholar]
- 19.Davey Smith G, Lawlor DA, Harbord R, Timpson N, Day I, Ebrahim S. Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. PLoS Med 2008;4:e352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weedon MN, McCarthy MI, Hitman G, et al. Combining information from common type 2 diabetes risk polymorphisms improves disease prediction. PLoS Med 2006;3:e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meigs JB, Shrader P, Sullivan LM, et al. Genotype score in addition to common risk factors for prediction of type 2 diabetes. N Engl J Med 2008;359:2208–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans DM, Visscher PM, Wray NR. Harnessing the information contained within genome-wide association studies to improve individual prediction of complex disease risk. Hum Mol Genet 2009;18:3525–31 [DOI] [PubMed] [Google Scholar]
- 23.Elks CE, Loos RJ, Sharp SJ, et al. Genetic markers of adult obesity risk are associated with greater early infancy weight gain and growth. PLoS Med 2010;7:e1000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freathy RM, Kazeem GR, Morris RW, et al. Genetic variation at CHRNA5-CHRNA3-CHRNB4 interacts with smoking status to influence BMI. Int J Epidemiol (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.