Abstract
Background: A small number of relatively small studies have found greater gestational weight gain to be associated with greater offspring body mass index (BMI; in kg/m2), but whether this association is caused by intrauterine mechanisms or by shared genetic and environmental risk factors for adiposity is unclear.
Objective: The objective was to examine the association of greater maternal weight gain (MWG; postnatal weight minus weight at the first antenatal clinic assessment) with greater offspring BMI and to explore whether any observed association is explained by intrauterine mechanisms.
Design: This was a prospective cohort study that used record linkage data (n = 146,894 individuals from 136,050 families). To compare the within-sibling and between-nonsibling associations, we used fixed- and between-cluster linear regression models.
Results: Associations of MWG with later offspring BMI differed by the mother's early-pregnancy overweight or obesity status (P for interaction <0.0001). MWG was positively associated with BMI at a mean age of 18 y in the offspring of normal-weight women but only between unrelated men (0.07; 95% CI: 0.06, 0.07) per 1-kg greater MWG; no within-sibling association (0.00; 95% CI: −0.02, 0.02) per 1-kg greater MWG was found. In contrast, in overweight and obese women we found a within-sibling association (0.06; 95% CI: 0.01, 0.12) and an association between unrelated men (0.02; 95% CI: 0.01, 0.03) per 1-kg greater MWG.
Conclusion: In normal-weight mothers, most of the association between MWG and later offspring BMI is explained by shared familial (genetic and early environmental) characteristics, whereas evidence indicates a contribution of intrauterine mechanisms in overweight and obese women.
INTRODUCTION
There is increasing interest in the hypothesis that greater adiposity in women during pregnancy programs their offspring to greater adiposity at birth and into later life (1–5). The suggestion is that mothers with greater amounts of fat during pregnancy (either as a result of being more adipose at the start or as a result of gaining more fat during pregnancy) deliver greater concentrations of glucose and fatty acids to the developing fetus, because these nutrients easily cross the placenta (6, 7). This results in an increased fetal secretion of insulin and consequent increased growth and is also thought to result in permanent changes to the pancreatic islet cells, hypothalamus, and adipose tissue, which results in greater adiposity throughout life (6, 7).
It is important to establish whether this developmental-overnutrition explanation for the association of maternal early-pregnancy body mass index (BMI; in kg/m2) and gestational weight gain (GWG) with BMI and other measures of adiposity in offspring later in life is correct (8). Evidence from animal studies supports this hypothesis (7, 9). In humans, several studies have shown that maternal BMI in early pregnancy and greater GWG are positively associated with offspring birth weight (10, 11) and with BMI and fat mass in offspring later in life (3, 12–14). However, these associations might be explained by shared genetic variants or lifestyles between mother and offspring that are related to greater weight gain and adiposity. Furthermore, studies that have related GWG to later adiposity in offspring may have a poor proxy for greater acquisition of maternal fat during pregnancy, because GWG includes contributions from offspring birth weight, placenta, amniotic fluid, and maternal plasma expansion as well as weight/fat gained solely by the mother.
One way of controlling for shared familial characteristics is to make within-sibling comparisons, because maternal characteristics such as genetic make-up and socioeconomic background are fixed and are therefore fully controlled for, regardless of whether they are measured in a study (15). This approach has been used to examine the causal intrauterine effect of greater GWG on birth weight (4); however, to our knowledge it has not been used to examine offspring BMI later in life. The aim of this study was to examine the association of maternal weight gain (MWG; postnatal weight minus weight at the first antenatal clinic visit) in pregnancy with offspring BMI at a mean age of 18 y in a large Swedish record linkage study. We compared within-sibling associations with associations between nonsiblings to explore the extent to which associations were driven by shared maternal-offspring characteristics and by intrauterine mechanisms. We also explored whether both within- and between-sibling associations differ depending on whether the mother is of normal weight or overweight/obese at the start of pregnancy. This work builds on a previous publication of ours conducted in the same cohort, which illustrated an important intrauterine effect of maternal diabetes in pregnancy on later offspring BMI, but not of variation in early-pregnancy BMI (16).
SUBJECTS AND METHODS
Study participants and data sources
The study consisted of all men born in Sweden between 1973 and 1988 who were still alive and completed their conscription medical examination during 1990–2005 (n = 390,108). We used data from the mandatory national conscription examination for offspring BMI and hence included men only, because only men complete this examination. Date of birth of the index participant, together with mother's age at birth and the parent's unique identity numbers (used to generate a family ID for the purpose of identifying full siblings), were extracted from the Swedish Multi-Generation Register. A linkage was made between these data and the Swedish Medical Birth Register (17, 18), the Swedish Military Service Conscription Register, and the Population and Housing Censuses of 1990.
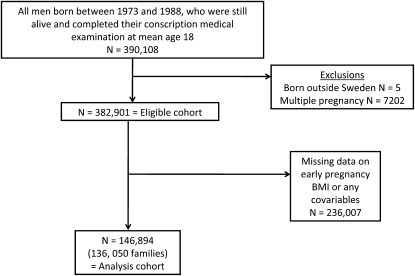
We excluded anyone born outside of Sweden, multiple births, and anyone with missing data on any variables included in this study. After these exclusions, the study population consisted of 146,894 men from 136,050 families. The derivation of the eligible and analysis cohorts is shown in Figure 1. The Regional Ethics Committee, Stockholm, Sweden, approved this study.
FIGURE 1.
Sampling frame, eligible cohort, and number of subjects included in the analyses.
Exposures and covariables
All of the data that we used in this study were obtained from routine data registers that were linked to each other as described above. We had no direct access to any medical records or survey data. Our descriptions of these data were taken from the manuals that accompany the registers. Data on maternal weight and height at the first antenatal clinic assessment (which took place at ≈10 wk gestation), birth weight, parity, and diabetes in pregnancy were measured by midwives, obstetricians, or other physicians as part of normal clinical practice. These data were taken directly from the obstetric records and entered into the Medical Birth Register.
MWG, which had been calculated by subtracting the weight at the first antenatal clinic assessment (at ≈10 wk gestation) from the weight measured shortly after delivery (within the first 12 h of delivery), was entered in the Medical Birth Register. The exact dates for these measurements are unknown. The assessment of MWG that we used here differs from that of GWG used in previous publications, in which GWG was calculated from measurements that were collected during pregnancy and, hence, includes weight gained from the developing fetus, placenta, and amniotic fluid as well as that gained by the mother. Thus, although our results could not be directly compared with the results from previous studies, our method of calculation is arguably a better measure of the true exposure (ie, greater fat gain by the mother in pregnancy).
“Thus, although our results could not be directly compared with the results from previous studies, our method of calculation is arguably a better measure of the true exposure (ie, greater fat gain by the mother in pregnancy).”
According to the Medical Birth Register manual, gestational age at birth was assessed from the first day of the last menstrual period for 80% of the cohort, with ultrasound scan results being used alone or in combination with last menstrual period in the remainder of the cohort. Highest maternal education (4 categories: primary and lower secondary only, upper secondary only, postsecondary, or university education) was obtained from the individual-level responses of each mother to the 1990 census.
Outcome
During the years covered by this study, it was a legal requirement that all Swedish males undergo the Swedish military service conscription examination; there were almost no exclusions to this requirement. Only individuals with severe mental retardation, being hospitalized for severe psychiatric morbidity, or imprisoned for severe criminality were exempt. At the conscription units across Sweden, height and weight were measured by trained personnel using standard procedures while the men were wearing underclothes and no shoes.
Statistical analyses
We compared distributions of characteristics between men who were included in the study and those who were excluded because of missing data using chi-square-, t, and F tests as appropriate. To compare the within-sibling and between–unrelated family associations, we used fixed and between cluster linear regression models with the xtreg command in Stata (version 11; StataCorp, College Station, TX) (18). This approach, which is equivalent to a mixed linear model, runs 2 regression models simultaneously: the within-sibling fixed effect model and the between-clusters (unrelated families in this study) model. The random effect was then obtained as the weighted average of the regression coefficients from these 2 models. For all models, our main outcome was offspring BMI at age 18 y; we also examined birth weight as an outcome. The exposure of interest was MWG. The equations for the models are provided in Appendix A.
The fixed-effect regression coefficient provides the within-sibling association. This coefficient represents the association of MWG with offspring BMI after control for fixed maternal characteristics (eg, socioeconomic background, lifestyle, and genes). A positive association would support an intrauterine effect, because it would suggest that the sibling exposed to greater maternal MWG while in utero (with all fixed maternal characteristics controlled for) would have a higher BMI than would the sibling who was in utero when the mother gained less weight during pregnancy. The between-cluster regression coefficient represents the between-nonsiblings effect. This coefficient represents the association of MWG with offspring BMI between unrelated individuals. The estimate still uses data from all participants, but relates the mean offspring BMI within a cluster (family) to mean exposure within clusters (family); the clusters are independent of each other. A Hausman statistic was used to compare these 2 models (19). The finding of consistent within-sibling and between-unrelated cluster coefficients and of an association of MWG with offspring BMI suggests that the association is importantly driven by intrauterine mechanisms. If it is found that the within-sibling and between-unrelated-individuals associations are consistent with each other and both of these are positive, this suggests that the association is importantly driven by intrauterine mechanisms. The random-effects regression coefficient (overall association) is then obtained as the weighted average of the within-sibling and between-cluster (nonsibling) effects, each coefficient weighted by the inverse of its variance (19). This latter association represents the overall association between MWG and offspring BMI at mean age 18 y, taking family clustering into account in the estimation of 95% CIs, but does not provide control for fixed maternal effects.
In the basic model we adjusted only for year of birth (model 1). We then additionally adjusted for potential confounding by maternal age at birth, parity, diabetes during pregnancy, and education (model 2). Finally, we adjusted for gestational age (a potential confounder) in the association with birth weight and for both gestational age and birth weight (potential mediators) in the association with offspring BMI as the outcome (model 3). We explored whether there was any evidence of interaction between maternal early-pregnancy BMI [categorized as normal weight (BMI <24.9) or overweight/obese (BMI ≥25)] and MWG in their associations with offspring birth weight and BMI by including interaction terms in the regression models. Overweight/obese women were combined into one category because only 2% of the women were obese. Finally, we undertook 3 sensitivity analyses in which we restricted analyses to 1) those offspring born at term: from >37 to ≤41 completed weeks, 2) those born with a healthy weight (2500–4000 g), and 3) those brothers who were born within 3 y of each other. The first analysis was conducted to examine whether any association of MWG with offspring birth weight was driven by preterm births, the second analysis was conducted to examine whether any association was driven by low– or high–birth weight individuals, and the third analysis was conducted because background family socioeconomic status and lifestyles may differ between siblings who are born some years apart.
RESULTS
The 146,894 men included in the analyses belonged to 136,050 families: 46,066 men had at least one brother within the cohort. Characteristics of the cohort and differences between those included and those excluded because of missing data are shown in Table 1. The differences between those included and excluded were small with respect to effect sizes; however, the large sample size meant that most of these small differences were statistically significant. For example, the mean MWG was 14.1 kg in those included and 14.2 kg in those excluded; the mean early-pregnancy BMI was 21.9 in those included and 22.0 in those excluded. The mean BMI at conscription in the sons of those included was 22.9 and in those excluded was 22.6, but all P values for these differences were <0.001. In normal-weight women, the mean (±SD) MWG was 14.2 ± 4.2 kg, which did not differ markedly from the MWG in overweight/obese women (13.4 ± 4.8 kg).
TABLE 1.
Characteristics of men included in the analyses (n = 146,894) and of those excluded because of some missing data
| Characteristic | Number with data in excluded category | Excluded men1 | Included men | P value2 |
| Maternal characteristic | 77,259 | — | — | — |
| Maternal early-pregnancy BMI categories [n (%)] | — | — | — | — |
| Normal | — | 64,971 (84.1) | 125,748 (85.6) | <0.001 |
| Overweight | — | 10,450 (13.5) | 18,297 (12.5) | — |
| Obese | — | 1838 (2.4) | 2849 (1.9) | — |
| Mean early-pregnancy BMI (kg/m2) | 77,259 | 22.0 ± 3.33 | 21.9 ± 3.1 | 0.001 |
| Weight gain in pregnancy (kg) | 77,259 | 14.2 ± 4.5 | 14.1 ± 4.3 | <0.001 |
| Maternal diabetes in pregnancy [n (%)] | 236,007 | — | — | — |
| No | — | 234,674 (99.4) | 146,129 (99.5) | 0.07 |
| Yes | — | 1333 (0.6) | 765 (0.5) | |
| Height (cm) | 83,207 | 165.7 ± 5.8 | 166.1 ± 5.7 | <0.001 |
| Age at birth (y) | 236,007 | 27.6 ± 5.1 | 28.0 ± 5.0 | <0.001 |
| Parity [n (%)] | 235,986 | — | — | — |
| 1 | — | 95,461 (40.5) | 63,267 (43.1) | <0.001 |
| 2 | — | 88,176 (37.4) | 52,384 (35.7) | — |
| 3 | — | 38,130 (16.2) | 23,501 (16.0) | — |
| 4 | — | 10,278 (4.4) | 5753 (3.9) | — |
| 5 | — | 2672 (1.1) | 1431 (1.0) | — |
| ≥6 | — | 1269 (0.5) | 558 (0.4) | — |
| Highest educational level achieved [n (%)] | — | — | — | — |
| Primary or lower secondary | 234,300 | 54,670 (23.3) | 26,159 (17.8) | <0.001 |
| Upper secondary | — | 116,276 (49.6) | 75,763 (51.6) | — |
| Postsecondary | — | 62,835 (26.8) | 44,689 (30.4) | — |
| University | — | 519 (0.2) | 283 (0.2) | — |
| Son's characteristics | ||||
| Birth weight (kg) | 234,544 | 3673 ± 555 | 3610 ± 530 | <0.001 |
| Gestational age (d) | 234,824 | 279.2 ± 12.8 | 279.1 ± 11.4 | <0.001 |
| Age at conscription (y) | 236,007 | 17.88 ± 0.56 | 17.79 ± 0.49 | <0.001 |
| Weight at conscription (kg) | 137,164 | 73.4 ± 12.5 | 74.5 ± 13.1 | <0.001 |
| Height at conscription (cm) | 137,164 | 180.1 ± 6.6 | 180.4 ± 6.5 | <0.001 |
| BMI at conscription (kg/m2) | 137,164 | 22.6 ± 3.5 | 22.9 ± 3.7 | <0.001 |
| BMI category at conscription [n (%)] | 137,164 | — | — | <0.001 |
| Normal | — | 111,370 (81.2) | 115,833 (78.9) | — |
| Overweight | — | 20,025 (14.6) | 23,553 (16.0) | — |
| Obese | — | 5769 (4.2) | 7508 (5.1) | — |
Excluded because of some missing data.
P values derived from t tests for comparison of 2 means and from chi-square tests for comparison of categories between the 2 groups.
Mean ± SD (all such values).
Scatter plots were inspected between continuously assessed variables, which did not suggest any nonlinear associations. The correlations between maternal and offspring measurements are shown in Table 2. Maternal early-pregnancy BMI was weakly inversely correlated with MWG; it was weakly positively associated with birth weight and moderately positively associated with offspring BMI at a mean age of 18 y. MWG was moderately positively correlated with offspring birth weight and weakly positively correlated with offspring BMI at a mean age of 18 y. MWG was weakly positively correlated with gestational age, which was moderately positively correlated with birth weight but very weakly correlated with later BMI.
TABLE 2.
Pearson's correlation coefficients for anthropometric data of mothers and their sons (n = 146,894 Swedish mother-son groups)1
| Mothers |
Sons |
||||||||
| Height2 | Weight2 | BMI2 | Absolute weight gain3 | Gestational age | Birth weight | Weight4 | Height4 | BMI4 | |
| Mothers | |||||||||
| Height2 | 1 | ||||||||
| Weight2 | 0.38 | 1 | |||||||
| BMI2 | −0.12 | 0.85 | 1 | ||||||
| Absolute weight gain3 | 0.10 | −0.01 | −0.06 | 1 | |||||
| Gestational age | 0.06 | 0.07 | 0.04 | 0.13 | 1 | ||||
| Sons | |||||||||
| Birth weight | 0.17 | 0.22 | 0.15 | 0.24 | 0.55 | 1 | |||
| Weight4 | 0.17 | 0.32 | 0.25 | 0.09 | 0.03 | 0.18 | 1 | ||
| Height4 | 0.46 | 0.23 | 0.02 | 0.08 | 0.05 | 0.27 | 0.39 | 1 | |
| BMI4 | −0.02 | 0.25 | 0.27 | 0.06 | 0.01 | 0.08 | 0.91 | −0.03 | 1 |
P < 0.001 (t test) for all variables.
Measured at the first antenatal clinic visit.
Difference between maternal weight measured after delivery of the infant and placenta and maternal weight at the first antenatal clinic visit.
Measured at the conscription examination when the son was 18 y of age on average.
We found strong statistical evidence that associations between maternal MWG and both birth weight and later offspring BMI differed by mothers’ early-pregnancy BMI category (both P values for interaction <0.0001); therefore, all analyses are presented separately for women who were normal weight and those who were overweight or obese.
The overall within-sibling and between-nonsibling associations of MWG with offspring birth weight in normal-weight and overweight/obese mothers are shown in Table 3. Greater MWG was associated with higher birth weight within siblings and between nonsiblings in both women who were normal weight and those who were overweight/obese, but associations were stronger in normal-weight mothers than in overweight/obese mothers. In both normal-weight and overweight/obese mothers, the association between nonsiblings was stronger than that within siblings, with statistical evidence that these 2 coefficients differed from each other. Adjustment for most confounders did not substantively affect any of the associations, with the exception of adjustment for gestational age, which resulted in attenuation of all associations, particularly those within siblings (although with positive associations remaining).
TABLE 3.
Associations of maternal gestational weight gain with birth weight, within sibling groups and between unrelated individuals, stratified by whether mothers were normal weight or overweight or obese in early pregnancy1
| Mean difference in offspring birth weight (95% CI) per 1-kg greater gestational weight gain |
|||||
| Maternal BMI and model | n | Overall | Within siblings | Between nonsiblings | P value2 |
| g | |||||
| Normal weight [BMI (kg/m2) <25]3 | |||||
| Model 1 | 125,748 | 35.01 (34.34, 35.67) | 27.71 (25.00, 30.41) | 35.54 (34.86, 36.22) | <0.001 |
| Model 2 | 125,748 | 36.66 (36.00, 37.31) | 29.10 (26.38, 31.81) | 37.07 (36.39, 37.73) | <0.001 |
| Model 3 | 125,748 | 26.90 (26.33, 27.46) | 19.92 (17.54, 22.31) | 27.23 (26.65, 27.81) | <0.001 |
| Overweight [BMI (kg/m2) ≥25]3 | |||||
| Model 1 | 21,146 | 21.37 (19.82, 22.92) | 14.99 (8.02, 21.94) | 21.98 (20.39, 23.57) | 0.04 |
| Model 2 | 21,146 | 23.61 (22.07, 25.15) | 18.29 (11.35, 25.23) | 24.05 (22.47, 25.63) | 0.08 |
| Model 3 | 21,146 | 17.17 (15.84, 18.50) | 8.87 (2.78, 14.96) | 17.72 (16.35, 19.08) | 0.003 |
The null value is 0. Model 1 was adjusted for year of birth. Model 2 was adjusted as for model 1 plus maternal age at birth, parity, diabetes in pregnancy, and education. Model 3 was adjusted as for model 2 plus gestational age.
Obtained by using the Hausman test, testing the null hypothesis that the within-sibling and between-nonsibling associations were identical.
There was strong statistical evidence that all associations differed between women who were normal weight and those who were overweight (P for interaction <0.0001 for all).
The associations of MWG with offspring BMI, stratified by maternal overweight/obesity status in early pregnancy, are shown in Table 4. In normal-weight women, a positive overall association was found between greater MWG and greater offspring BMI at age 18 y, which did not seem to be importantly mediated by birth weight or gestational age, because adjustment for these factors resulted in very little attenuation compared with that after adjustment for potential confounding factors (model 3 compared with model 2). In normal-weight women, we found no evidence of an association of MWG with greater offspring BMI at age 18 y within siblings; the overall association was due to the between-nonsibling association. In contrast, a positive association was found between MWG and offspring BMI within siblings in overweight/obese mothers. In overweight/obese women, this within-sibling association appeared stronger than that between nonsiblings, but there was no strong statistical evidence that the 2 differed from each other.
TABLE 4.
Associations of maternal gestational weight gain with offspring BMI at a mean age of 18 y within sibling groups and between unrelated individuals, stratified by whether the mothers were normal weight or overweight or obese in early pregnancy1
| Mean difference in offspring BMI (95% CI) per 1-kg greater gestational weight gain |
||||||
| Maternal BMI and model | n | Overall | Within siblings | Between nonsiblings | P value2 | |
| kg/m2 | ||||||
| Normal weight [BMI (kg/m2) <25]3 | ||||||
| Model 1 | 125,748 | 0.08 (0.07, 0.08) | 0.01 (−0.01, 0.03) | 0.08 (0.08, 0.09) | <0.001 | |
| Model 2 | 125,748 | 0.06 (0.06, 0.07) | 0.00 (−0.02, 0.02) | 0.07 (0.06, 0.07) | <0.001 | |
| Model 3 | 125,748 | 0.06 (0.06, 0.07) | 0.00 (−0.02, 0.02) | 0.07 (0.06, 0.07) | <0.001 | |
| Overweight [BMI (kg/m2) ≥25]3 | ||||||
| Model 1 | 21,146 | 0.03 (0.02, 0.04) | 0.07 (0.01, 0.13) | 0.03 (0.02, 0.04) | 0.18 | |
| Model 2 | 21,146 | 0.03 (0.01, 0.04) | 0.07 (0.01, 0.13) | 0.03 (0.02, 0.04) | 0.18 | |
| Model 3 | 21,146 | 0.02 (0.01, 0.03) | 0.06 (0.01, 0.12) | 0.02 (0.01, 0.03) | 0.16 | |
The null value is 0. Model 1 was adjusted for year of birth. Model 2 was adjusted as for model 1 plus maternal age at birth, parity, diabetes in pregnancy, and education. Model 3 was adjusted as for model 2 plus birth weight and gestational age.
Obtained by using the Hausman test, testing the null hypothesis that the within-sibling and between-nonsibling associations were identical.
There was strong statistical evidence that all associations differed between women who were normal weight and those who were overweight (P for interaction < 0.0001 for all).
In sensitivity analyses in which we excluded pre- and postterm births (n = 107,816 included in the analyses), associations with birth weight in all models were essentially the same as those for model 3 in Table 3; associations with BMI at age 18 y did not substantively differ from any of those shown for any model in Table 4. When we excluded men with a low or high birth weight, none of the results differed substantively from those presented. Similarly, when we restricted the analyses to only those for brothers born within 3 y of each other (n = 144,663 included in the analyses), none of the results differed substantively from those shown in Tables 3 and 4.
DISCUSSION
To our knowledge, this was the first study to examine the association of MWG with later offspring BMI within siblings and to compare these findings with associations between unrelated individuals from the same cohort. Our findings suggest that, in normal-weight women, the positive association of MWG with later offspring BMI is driven largely by shared familial (genetic and/or environment) risk factors for BMI; however, in women who are overweight or obese in early pregnancy, greater MWG appears to be associated with greater later offspring BMI via intrauterine mechanisms and shared familial characteristics.
Several mechanisms could explain the association of MWG with later offspring BMI. First, the association could be mediated by the effect of MWG on birth weight. Birth weight contributes directly to GWG (as measured in other studies; 3, 12–14), and greater maternal fat deposition during pregnancy may result in developmental overnutrition of the fetus and, hence, greater birth weight. Birth weight is positively correlated with later size (including later BMI, fat mass, and lean mass); therefore, the association of GWG or MWG with later offspring BMI may largely reflect the association of GWG/MWG with birth weight. However, our measure of MWG will not be directly influenced by birth weight, and, consistent with previous studies that did not use a within-sibling analysis (3, 12–14), we found little evidence that the positive association of MWG with later offspring BMI was mediated by its association with birth weight or gestational age. Second, shared familial genetic and environmental characteristics (eg, socioeconomic status and associated diet and physical activity behaviors) may link greater MWG in pregnancy and greater offspring BMI in later life. We found in normal-weight women that these characteristics largely explained the association of MWG with offspring BMI, because once these shared characteristics were controlled for in the within-sibling analyses, no association between MWG and offspring BMI was found. Third, intrauterine mechanisms may program offspring to have a greater BMI when there has been greater MWG. Our results suggest that, in overweight/obese women, intrauterine mechanisms play some part in the positive association of MWG with greater BMI in offspring later in life, because a within-sibling association was observed in these women. Such an association cannot be explained by maternal genetic variation or background socioeconomic status or behaviors that are likely to be similar for siblings born close in age to each other (as in our study).
The main strengths of this study were its large sample size and its ability to examine associations within siblings in addition to unrelated, nonsibling control subjects. This made it possible to explore the extent to which associations were explained by familial (genetic or early environmental) characteristics as opposed to intrauterine effects. We were not able to assess GWG because no measure of weight in late pregnancy was available. This importantly limited the extent to which we could compare our results with those of other studies that assessed GWG. It also limited the extent to which our findings can be used to inform guidelines, such as those of the US Institute of Medicine (2) regarding the monitoring of weight during pregnancy. On the other hand, with respect to exploring the etiologic question of whether the developing fetus of women who gain more fat during pregnancy is exposed to greater concentrations of circulating glucose and fatty acids that then result in programming of greater adiposity in later life, a measure of MWG (as used here) that excludes birth weight, placental weight, and amniotic fluid might serve as a better proxy of the key exposure than does GWG. Given that previous studies have not used a measure of MWG, it would be useful to explore whether our findings can be replicated in future studies. A more direct assessment of maternal fat acquisition in pregnancy, obtained through imaging, would also be valuable in future studies. The date at which the mothers first attended the antenatal clinic was not recorded in the register; therefore, it was impossible to know the gestational age of the mothers at the time that early-pregnancy BMI was assessed. Most of the mothers were likely at ≈10 wk gestation on the basis of clinical practice at the time. Indeed, weight was not assessed if women presented late for their first antenatal clinic assessment; thus, a considerable amount of data on early-pregnancy BMI and MWG were missing. Despite these missing data, the mean values for the characteristics of those subjects included and those excluded were very similar (Table 1). Because this study was conducted in male offspring only and of a population from Northern Europe, the findings may not necessarily be generalizable to women or individuals from other geographic regions.
In conclusion, our study suggests that, in a general European population, both intrauterine mechanisms and shared familial characteristics explain the positive association of MWG with birth weight. MWG is also positively associated with offspring BMI later in life, but our results suggest that this finding is largely explained by shared familial characteristics in normal-weight mothers and that only in women who are overweight or obese in early pregnancy is there evidence that greater MWG is associated with a greater BMI in offspring later in life via intrauterine mechanisms. Given the obesity epidemic and the greater number of women who are overweight or obese at the beginning of their pregnancy, relative to the number in earlier decades (20), evidence that intrauterine mechanisms contribute to a greater BMI in offspring later in life has important public health implications.
Acknowledgments
We are indebted to Eva Carlström who helped prepare the data for this study.
The authors’ responsibilities were as follows—DAL: had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, conceived of the study idea, developed the analysis plan, conducted the statistical analyses, and wrote the first draft of the manuscript; PL and NL: contributed to the data collection, advised on the data variables, and made critical comments on the first draft and revisions of the manuscript; and AF: contributed to the study conception and made critical comments on the first draft and revisions of the manuscript. None of the funders were involved in the design, implementation, analysis, or interpretation of these data. No conflicts of interest were declared.
APPENDIX A
Details of regression models used in main analyses
The models used in our analyses are as follows:
Fixed-effect (within-sibling) regression:
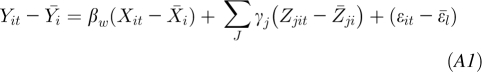 |
Between-sibling regression:
Random-effects regression:
This regression is obtained as the weighted average of the regression coefficients from the fixed effect and between-sibling models. The random-effects model is expressed as follows:
where Yit is the outcome (offspring BMI at age 18 y) in sibling t of family i; 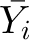 is the mean outcome (offspring BMI at age 18 y) of family i; α is the constant/intercept; βw is the within-sibling regression coefficient of the association of the main exposure with outcome; βb is the between-sibling regression coefficient of the association of the main exposure with outcome; βr is the random-effects regression coefficient giving the overall association of the main exposure with outcome having accounted for clustering within families; Xit is the exposure (maternal MWG) for sibling t of family i;
is the mean outcome (offspring BMI at age 18 y) of family i; α is the constant/intercept; βw is the within-sibling regression coefficient of the association of the main exposure with outcome; βb is the between-sibling regression coefficient of the association of the main exposure with outcome; βr is the random-effects regression coefficient giving the overall association of the main exposure with outcome having accounted for clustering within families; Xit is the exposure (maternal MWG) for sibling t of family i; 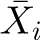 is the mean exposure (maternal MWG) for family i; Zjit j = 1, … j is the jth covariable included in the model when the covariables for sibling t of family i are controlled for;
is the mean exposure (maternal MWG) for family i; Zjit j = 1, … j is the jth covariable included in the model when the covariables for sibling t of family i are controlled for; 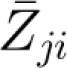 is the mean of the jth covariable included in the model; ϵit is the error term (residual variation in outcome not explained by the exposure or covariables) for sibling t of family i;
is the mean of the jth covariable included in the model; ϵit is the error term (residual variation in outcome not explained by the exposure or covariables) for sibling t of family i;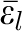 is the mean error term for family i; and vi terms are the unobserved cluster-specific effects that are fixed within clusters (sibling groups).
is the mean error term for family i; and vi terms are the unobserved cluster-specific effects that are fixed within clusters (sibling groups).
REFERENCES
- 1.Viswanthan M, Siega-Riz AM, Moos M-K, et al. Outcomes of maternal weight gain. Rockville, MD: Agency for Healthcare Research and Quality, 2008. (Evidence Report/Technology Assessment no. 168. AHRQ Publication no. 08-E009.) [Google Scholar]
- 2.Rasmussen KM, Yaktine AL. Weight gain during pregnancy: reexamining the guidelines. Committee to reexamine IOM pregnancy weight guidelines. Washington, DC: US Institute of Medicine and National Research Council, 2009 [PubMed] [Google Scholar]
- 3.Fraser A, Tilling K, Macdonald-Wallis C, et al. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation 2010;121:2557–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludwig DS, Currie J. The association between pregnancy weight gain and birthweight: a within-family comparison. Lancet 2010;376:984–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halfon N, Lu MC. Gestational weight gain and birthweight. Lancet 2010;376:937–8 [DOI] [PubMed] [Google Scholar]
- 6.Oken E, Gillman MW. Fetal origins of obesity. Obes Res 2003;11:496–506 [DOI] [PubMed] [Google Scholar]
- 7.Taylor PD, Poston L. Developmental programming of obesity in mammals. Exp Physiol 2007;92:287–98 [DOI] [PubMed] [Google Scholar]
- 8.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet 2002;360:473–82 [DOI] [PubMed] [Google Scholar]
- 9.Levin BE, Govek E. Gestational obesity accentuates obesity in obesity-prone progeny. Am J Physiol 1998;275:R1374–9 [DOI] [PubMed] [Google Scholar]
- 10.Eastman NJ, Jackson E. Weight relationships in pregnancy. I. The bearing of maternal weight gain and pre-pregnancy weight on birth weight in full term pregnancies. Obstet Gynecol Surv 1968;23:1003–25 [PubMed] [Google Scholar]
- 11.Simpson JW, Lawless RW, Mitchell AC. Responsibility of the obstetrician to the fetus. II. Influence of prepregnancy weight and pregnancy weight gain on birthweight. Obstet Gynecol 1975;45:481–7 [PubMed] [Google Scholar]
- 12.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol 2007;196:322–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreira P, Padez C, Mourão-Carvalhal I, Rosado V. Maternal weight gain during pregnancy and overweight in Portuguese children. Int J Obes (Lond) 2007;31:608–14 [DOI] [PubMed] [Google Scholar]
- 14.Mamun AA, O'Callaghan M, Callaway L, Williams G, Najman J, Lawlor DA. Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of age: evidence from a birth cohort study. Circulation 2009;119:1720–7 [DOI] [PubMed] [Google Scholar]
- 15.Rutter M. Proceedings from observed correlation to causal inference: the use of natural experiments. Perspect Psychol Sci 2007;2:377–95 [DOI] [PubMed] [Google Scholar]
- 16.Lawlor DA, Lichtenstein P, Långström N. Maternal diabetes in pregnancy programmes greater offspring adiposity into early adulthood: sibling study in a prospective cohort of 280,866 men from 248,293 families. Circulation 2011;123:258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centre for Epidemiology The Swedish Medical Birth Register—a summary of content and quality. Stockholm, Sweden: Swedish Centre for Epidemiology, 2003 [Google Scholar]
- 18.Cnattingius S, Ericson A, Gunnarskog J, Kallen B. A quality study of a medical birth registry. Scand J Soc Med 1990;18:143–8 [DOI] [PubMed] [Google Scholar]
- 19.Mann V, De Stavola BL, Leon DA. Separating within and between effects in family studies: an application to the study of blood pressure in children. Stat Med 2004;23:2745–56 [DOI] [PubMed] [Google Scholar]
- 20.Heslehurst N, Ells LJ, Simpson H, Batterham A, Wilkinson J, Summerbell CD. Trends in maternal obesity incidence rates, demographic predictors, and health inequalities in 36,821 women over a 15-year period. BJOG 2007;114:187–94 [DOI] [PubMed] [Google Scholar]