Abstract
Background: Low concentrations of circulating vitamin D are common with obesity and may represent a potential mechanism explaining the elevated risk of certain cancers and cardiovascular outcomes observed in individuals who are overweight or obese.
Objective: The objective of this study was to investigate the effects of 12 mo of weight loss through caloric restriction, exercise intervention, or both on serum 25-hydroxyvitamin D [25(OH)D] concentrations.
Design: Overweight and obese postmenopausal women (n = 439) were randomly assigned to 1 of 4 groups: 1) diet modification (n = 118), 2) exercise (n = 117), 3) diet + exercise (n = 117), or 4) control (n = 87). The diet intervention was a group-based reduced-calorie program with a 10% weight-loss goal. The exercise intervention consisted of 45 min of moderate-to-vigorous intensity aerobic activity daily for 5 d/wk. Serum 25(OH)D concentrations were measured by using a competitive chemiluminescent immunoassay at baseline and 12 mo.
Results: No significant change in serum 25(OH)D was found between the intervention and control groups. Women who lost <5%, 5–9.9%, 10–14.9%, or ≥15% of baseline weight had mean increases in 25(OH)D of 2.1, 2.7, 3.3, and 7.7 ng/mL, respectively (P for trend = 0.002). Baseline vitamin D status did not modify the effect of the interventions on weight loss or body-composition changes at the 12-mo follow-up.
Conclusion: A greater degree of weight loss, achieved through either a reduced-calorie diet or increased exercise, is associated with increased circulating 25(OH)D concentrations. This trial is registered at clinicaltrials.gov as NCT00470119.
INTRODUCTION
Overweight and obesity are well-established risk factors for cardiovascular disease and several cancers (1); however, the underlying mechanisms have not yet been fully elucidated. Low concentrations of circulating vitamin D associated with obesity could account, in part, for the obesity-disease link.
Vitamin D receptors are found in >30 cell types (2), and vitamin D has diverse nonskeletal roles in addition to its function in maintaining bone health. Serum 25-hydroxyvitamin D [25(OH)D], the most widely accepted clinical indicator of vitamin D status, is inversely associated with obesity (3–5), whereas laboratory studies and epidemiologic investigations suggest that vitamin D could be protective against several types of cancer and certain cardiovascular outcomes (5–9). The Institute of Medicine recently concluded that there remains insufficient evidence to make definitive conclusions regarding a causal role for low vitamin D in the development of these nonskeletal health outcomes (10), but recommended that targeted research in this area should continue.
Less-than-optimal serum vitamin D concentrations are prevalent in the population, varying according to age, race, adiposity, geography, and other factors (4, 8, 11, 12). It is not clear whether individuals who are obese have lower concentrations of serum vitamin D than do their lean counterparts because of differences in dietary and/or supplement intakes, less sun exposure, reduced skin biosynthesis, genetic variation, or some intrinsic factor related to obesity, such as increased sequestering of fat-soluble vitamin D in adipose tissue. Furthermore, the degree to which serum concentrations are sensitive to change as a result of weight loss has not been determined. Vitamin D also has antiadipogenic properties (13), and limited research suggests that vitamin D may potentiate weight loss and improvements in metabolic markers (14–16); yet, whether weight loss through lifestyle intervention is influenced by vitamin D status is not known.
Given the high prevalence of obesity and its many chronic disease sequelae, a better understanding of the interrelations between obesity, vitamin D, and weight loss has important implications for understanding chronic disease risk. The purpose of this study was to investigate the effect of weight loss, achieved through caloric restriction and/or aerobic exercise, on serum 25(OH)D with 12 mo of follow-up in a population of overweight and obese postmenopausal women at a northern US latitude with low ultraviolet B exposure year-round (17). We hypothesized that a greater magnitude of weight loss would result in a greater rise in serum 25(OH)D. We also hypothesized that there would be a significant interaction between the study interventions and baseline vitamin D status, such that greater weight loss would be achieved in women with higher concentrations of baseline 25(OH)D.
SUBJECTS AND METHODS
Design overview
The Nutrition and Exercise in Women Study, conducted from 2005 to 2009, was a 12-mo randomized controlled trial that tested the effects of weight loss through caloric restriction and/or exercise on circulating hormones and other outcomes. The study procedures were reviewed and approved by the Fred Hutchinson Cancer Research Center Institutional Review Board in Seattle, WA, and all participants provided informed consent.
Participants
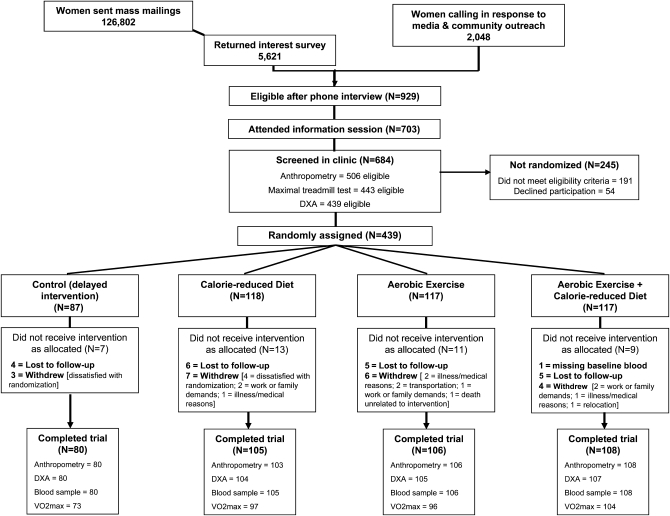
The participants were overweight or obese [body mass index (BMI; in kg/m2) ≥25.0, or ≥23.0 if Asian-American) postmenopausal women aged 50–75 y from the greater-Seattle area (latitude: 47.6° N). Women were recruited through media and mass mailings and underwent several screening activities (Figure 1). Specific exclusion criteria included >100 min/wk of moderate physical activity; diagnosed diabetes, fasting blood glucose ≥126 mg/dL or use of diabetes medications; use of postmenopausal hormones within 3 mo; history of other serious medical conditions; alcohol intake >2 drinks/d or current smoking; contraindication to participating in the study interventions for any reason (eg, abnormal exercise tolerance test); current or planned participation in another structured weight-loss program; use of weight-loss medications; or additional factors that might interfere with the measurement of outcomes or intervention success (eg, inability to attend facility-based sessions).
FIGURE 1.
Flow of participants through the Nutrition and Exercise in Women (NEW) Study. DXA, dual-energy X-ray absorptiometry; VO2max, maximal oxygen uptake.
Random assignment and interventions
Eligible women were randomly assigned to 1 of 4 study arms: 1) reduced-calorie dietary modification (n = 118); 2) moderate-to-vigorous intensity aerobic exercise (n = 117); 3) diet + exercise (n = 117); or 4) control (no intervention) (n = 87). Computerized random assignment was stratified according to BMI (≥ or <30) and the participants’ self-reported race-ethnicity. One participant randomly assigned to the diet + exercise intervention was excluded from this analysis because of missing baseline blood measures.
The 12-mo dietary intervention was a modification of the Diabetes Prevention Program (18) and Look AHEAD (Action for Health in Diabetes) (19) lifestyle behavior-change programs with goals of 1200–2000 kcal/d, <30% of daily energy intake from fat, and 10% weight loss. Participants met individually with a dietitian on ≥2 occasions, followed by weekly meetings in groups of 5 to 10 women for 6 mo. Thereafter, participants attended group meetings once per month and also had phone or E-mail contact. Women completed daily food logs for ≥6 mo or until they reached their weight-loss goal.
The exercise intervention progressed to 45 min of moderate-to-vigorous intensity exercise at a target heart rate of 70% to 85% observed maximum, 5 d/wk, by the seventh week. Participants attended 3 supervised sessions/wk at the study facility and exercised 2 d/wk at home. Participants recorded exercise mode, duration, peak heart rate, and perceived exertion at each session. Activities of ≥4 metabolic equivalents (METs) (20) were counted toward the prescribed target of 225 min exercise/wk.
Participants randomly assigned to diet + exercise received the diet intervention in separate sessions and were instructed not to discuss the diet during the supervised exercise sessions. The control group was requested not to change their diet or exercise habits for 12 mo. At study completion, the control subjects were offered 4 group nutrition classes and 8 wk of individualized facility-based exercise training.
Outcomes and follow-up
All study measures were obtained and analyzed by trained personnel who were blinded to the participants’ randomization status. Demographic information, medical history, dietary patterns (via 120-item self-administered food-frequency questionnaire; 21), supplement use, and physical activity patterns (via a modified interview-administered Minnesota Physical Activity Questionnaire; 22) were collected at baseline and 12 mo. At both time points, participants wore pedometers (Accusplit, Silicon Valley, CA) while awake for 7 consecutive days to determine an average daily step count.
Participants wore a hospital gown and no shoes during the anthropometric measurements. BMI was calculated from weight and height, measured to the nearest 0.1 kg and 0.1cm, respectively, with a balance-beam scale and stadiometer. Waist circumference was measured to the nearest 0.5 cm at the minimal waist. Body composition was measured by dual-energy X-ray absorptiometry with a whole-body scanner (GE Lunar, Madison, WI).
Fasting venous blood samples (50 mL) were collected during clinic visits before randomization and at 12 mo. Participants consumed no food or drink other than water for 12 h before sample collection and were requested not to exercise for 24 h preceding the blood draw. Blood was processed within 1 h of collection, and samples were stored at −70°C.
Serum 25(OH)D was quantified by direct, competitive chemiluminescent immunoassay by using the DiaSorin LIAISON 25-OH Vitamin D Total assay (23, 24) at Heartland Assays Inc (Ames, IA). Samples were analyzed in batches such that each participant's baseline and 12-mo samples were assayed simultaneously, the number of samples from each intervention group was approximately equal, participant randomization dates were similar, and sample order was random. The intra- and interassay CVs were 8.2% and 11.0%, respectively.
Statistical analysis
For the primary analysis, no change from baseline was assumed in cases of missing data. Because of its nonnormal distribution, serum vitamin D measures were log-transformed before further analysis, and these data are presented as geometric means (95% CIs) unless indicated otherwise. Generalized linear models and chi-square tests were used to test for differences in baseline characteristics across intervention groups, including season of randomization (March–May, June–August, September–November, and December–February). Pearson correlation coefficients were calculated to assess the relations between vitamin D intake, serum 25(OH)D, and other participant characteristics at baseline.
The mean changes in serum vitamin D and in vitamin D intake from dietary and supplement sources between baseline and 12 mo in the exercise, diet, and diet + exercise groups were computed and compared with the change in the control subjects by using the generalized estimating equations modification of linear regression to account for intraindividual correlation over time (25). One participant in the control group reported a 10,000-IU/d increase in vitamin D supplementation and was excluded from the 12-mo analyses.
The intervention effects were examined on the basis of the assigned treatment at randomization, regardless of adherence or study retention (ie, intent-to-treat). Adjustment for multiple comparisons was made by using Bonferroni correction (2-sided α = 0.05/3). To assess potential effect modification by baseline vitamin D status, these analyses were repeated after stratification according to baseline serum 25(OH)D <20 or ≥20 ng/mL. Subsequently, the effect of weight loss on serum vitamin D status was examined by using a stratified analysis (<5%, 5–9.9%, or ≥10% weight loss) (26, 27) that was performed within each intervention arm and after combining all intervention groups together. These analyses used available data without imputation for missing values and were adjusted for race-ethnicity, season of randomization, and total vitamin D intake (dietary sources + supplement use).
Finally, the effect of baseline vitamin D status on weight loss within each intervention group was assessed by including appropriate interaction terms in a generalized estimating equations model. These analyses were performed without imputation for missing data. The analyses were also repeated after stratifying the sample according to baseline vitamin D concentration (<20.0, 20.0–29.9, or ≥30.0 ng/mL) (10) and including only non-Hispanic white women to determine the degree to which skin pigmentation may have affected this study's findings. All statistical analyses were performed by using SAS software version 9.1 (SAS Institute, Cary, NC).
RESULTS
Participants
At 12 mo, 398 of the 438 participants completed physical exams and provided a blood sample, 397 underwent a dual-energy X-ray absorptiometry scan, and 371 completed a treadmill test; 39 did not complete the study (Figure 1). No significant differences were found in the relevant variables between groups at baseline, except for the percentage of daily calories consumed as fat (P = 0.02) (Table 1). Serum 25(OH)D was significantly different according to season of randomization [winter (December–February): 18.7 ng/mL; spring (March–May): 19.6 ng/mL; summer (June–August): 22.5 ng/mL; and fall (September–November): 21.1 ng/mL, P = 0.009]; however the frequency of randomization in each season did not vary significantly between groups (chi-square P = 0.29). The women consumed 6.1 ± 3.8 μg vitamin D (mean ± SD) from dietary sources. Approximately one-half (49.5%) of the women reported regular use of vitamin D supplements, with a mean intake of 531 IU/d (equivalent to 13.3 μg/d). Reported vitamin D intake from dietary sources or supplements did not change from baseline to 12 mo in any group compared with control subjects (Table 1).
TABLE 1.
Selected baseline characteristics of 438 randomly assigned women in the Nutrition and Exercise in Women (NEW) trial (Seattle, WA)1
| Variable | Control | Exercise | Diet | Diet + exercise |
| No. of subjects | 87 | 117 | 118 | 116 |
| Age (y) | 57.4 ± 4.42 | 58.1 ± 5.0 | 58.1 ± 5.9 | 58.0 ± 4.4 |
| Ethnicity (%) | ||||
| Non-Hispanic white | 85.1 | 83.8 | 85.6 | 85.3 |
| Non-Hispanic black | 6.9 | 12.8 | 7.6 | 4.3 |
| Hispanic | 3.5 | 1.7 | 1.7 | 4.3 |
| Other3 | 4.6 | 1.7 | 5.1 | 6.0 |
| Weight (kg) | 84.2 ± 12.5 | 83.7 ± 12.3 | 84.0 ± 11.8 | 82.6 ± 10.8 |
| BMI (kg/m2) | 30.7 ± 3.9 | 30.7 ± 3.7 | 31.1 ± 3.9 | 31.0 ± 4.3 |
| Waist circumference (cm) | 94.8 ± 10.2 | 95.1 ± 10.1 | 94.6 ± 10.2 | 93.7 ± 9.9 |
| Body fat (%) | 47.3 ± 4.4 | 47.3 ± 4.1 | 47.0 ± 4.3 | 47.4 ± 4.5 |
| Lean mass (%) | 48.5 ± 4.3 | 48.3 ± 3.9 | 48.7 ± 4.2 | 48.4 ± 4.4 |
| Pedometer steps (no./d)4 | 5605 ± 2334 | 5777 ± 2129 | 5539 ± 2257 | 5952 ± 2354 |
| Average dietary intake from FFQ | ||||
| Total energy (kcal/d)5 | 1988 ± 669 | 1986 ± 589 | 1884 ± 661 | 1894 ± 639 |
| Relative fat (% of energy)6 | 35.6 ± 6.9 | 33.6 ± 6.9 | 33.1 ± 6.3 | 35.3 ± 7.3 |
| Baseline dietary calcium intake (mg) | 1042 ± 556 | 1081 ± 550 | 1018 ± 500 | 1050 ± 482 |
| 12-mo Dietary calcium intake (mg) | 941 ± 475 | 1006 ± 394 | 1040 ± 465 | 1071 ± 442 |
| Regular users of calcium-containing supplements7 | ||||
| Baseline (% of users) | 47.4 | 40.2 | 48.7 | 47.8 |
| Daily intake in baseline users (mg/d) | 575 ± 449 | 612 ± 459 | 508 ± 423 | 625 ± 438 |
| 12 mo (% of users) | 41.4 | 31.6 | 35.6 | 39.6 |
| Daily intake among 12-mo users (mg/d) | 601 ± 464 | 541 ± 408 | 483 ± 364 | 588 ± 462 |
| Baseline dietary vitamin D intake (μg) | 6.2 ± 4.4 | 6.1 ± 3.8 | 5.9 ± 3.4 | 6.2 ± 3.8 |
| 12-mo Dietary vitamin D intake (μg) | 5.4 ± 3.4 | 5.4 ± 3.2 | 5.9 ± 3.7 | 6.4 ± 3.4 |
| Regular users of vitamin D–containing supplements7 | ||||
| Baseline (% of users) | 54.0 | 42.7 | 52.5 | 50.0 |
| Daily intake among baseline users (IU/d) | 447 ± 207 | 595 ± 401 | 540 ± 427 | 538 ± 333 |
| 12 mo (% of users) | 42.5 | 31.6 | 38.1 | 43.1 |
| Daily intake in 12-mo users (IU/d) | 820 ± 1592 | 850 ± 959 | 741 ± 955 | 738 ± 797 |
| Serum hydroxyvitamin D (ng/mL) | 22.7 ± 9.7 | 21.8 ± 9.0 | 22.0 ± 8.0 | 23.7 ± 9.0 |
FFQ, food-frequency questionnaire. Differences between the 4 arms were tested by using ANOVA. No significant differences were detected, except in daily calories (P = 0.02) consumed as fat.
Arithmetic mean ± SD (all such values).
Includes American Indian, Asian, and unknown.
7-d average.
Values derived from the FFQ were truncated: <600 kcal and >4000 kcal.
Percentage of energy from fat = (total calories from fat/total daily intake).
Self-reported regular use.
Intervention fidelity
Data on intervention adherence, weight loss, and body-composition changes in this trial were previously reported (28). The mean weight loss was 2.4% (P = 0.03) in the exercise group, 8.5% (P < 0.001) in the diet group, 10.8% (P < 0.001) in the diet + exercise group, and 0.8% in the control group. Women in all intervention groups had a significant decrease in waist circumference (exercise: P = 0.002; diet and diet + exercise: P < 0.001) and in percentage body fat (all P < 0.001) compared with control subjects. Lean mass decreased significantly in the diet group (P = 0.005), but was preserved in exercisers (both P > 0.10).
Women randomly assigned to exercise alone participated in moderate-to-vigorous activity for a mean (± SD) of 163.3 ± 70.6 min/wk, whereas women randomly assigned to diet + exercise participated for 171.5 ± 62.9 min/wk. Both groups had a significant increase in pedometer steps/d (+3202 steps/d and +4038 steps/d, respectively) compared with baseline. Women randomly assigned to diet + exercise had a greater increase in pedometer steps/d than did women assigned to exercise alone (P = 0.05). The percentage of daily energy from fat decreased in both the diet (−6.7%) and diet + exercise (−8.0%) groups. In both diet groups, women attended an average of 27 diet counseling sessions (86%).
Baseline associations
At baseline, the mean serum 25(OH)D concentration was 22.5 ng/mL (range: 4.1–57.0 ng/mL). Twelve percent of the participants were at risk of vitamin D deficiency (<12 ng/mL) (10), whereas only 2.7% had concentrations >40 ng/mL. Among African American participants, 45% (n = 15) had serum 25(OH)D concentrations <12 ng/mL, and only one had a baseline concentration >40 ng/mL. Baseline concentrations of serum 25(OH)D were significantly inversely correlated with BMI (r = −0.16, P < 0.01), waist circumference (r = −0.23, P < 0.01), and percentage body fat (r = −0.11, P < 0.01) and were positively associated with age (r = 0.17, P < 0.01), percentage lean mass (r = 0.12, P < 0.01), and vitamin D intake (r = 0.19 and r = 0.34 for dietary and supplement sources, respectively; both P < 0.01). Serum 25(OH)D was not significantly correlated with self-reported physical activity (r = −0.001) or average pedometer steps/d (r = 0.08) at baseline.
Intervention effects
Compared with control subjects, no significant change in serum vitamin D status was found in women in any of the intervention groups (Table 2), nor was there any effect modification according to baseline 25(OH)D status, except in the exercise group, in which serum 25(OH)D increased slightly more in women with baseline concentrations ≥20 ng/mL (+1.9 ng/mL) than in women with baseline concentrations <20 ng/mL (+2.5 ng/mL) (P for interaction = 0.04). Change in BMI and serum 25(OH)D were inversely correlated (r = −0.23; P < 0.001). Overall, the magnitude of weight loss was associated with the observed increase in 25(OH)D in a dose-dependent manner (Table 3). This trend was not statistically significant within individual intervention arms, but was significant when all women randomly assigned to intervention were combined together, even after adjustment for race-ethnicity, season of randomization, total vitamin D intake, and intervention arm. Women who lost <5%, 5–9.9%, 10–14.9%, or ≥15% of baseline weight had mean increases of 2.1, 2.7, 3.3, and 7.7 ng/mL, respectively (P for trend = 0.002). The rise in 25(OH)D was significantly greater (P = 0.0005) in women who lost ≥15% body weight over 12 mo than in control subjects. Similar analyses were repeated to examine the independent effect of fat loss and lean mass loss. The magnitude of fat loss (kg) was significantly associated with the rise in 25(OH)D (P = 0.035); the change in lean mass was not (P = 0.73).
TABLE 2.
Twelve-month intervention effects of diet, exercise, and diet + exercise on serum 25-hydroxyvitamin D [25(OH)D] concentrations in overweight and obese postmenopausal women1
| Control (n = 87) |
Exercise (n = 117) |
Diet (n = 118) |
Diet + exercise (n = 116) |
||||||||||||
| Baseline | 12 mo | Change2 | Baseline | 12 mo | Change2 | P3 | Baseline | 12 mo | Change2 | P3 | Baseline | 12 mo | Change2 | P3 | |
| ng/mL | ng/mL | ng/mL | ng/mL | ||||||||||||
| Serum 25(OH)D | 20.5 (18.6, 22.7)4 | 22.7 (20.5, 25.2) | 2.2 ± 10.7 | 19.8(18.3, 21.5) | 21.5(19.6, 23.5) | 1.7 ± 8.6 | 0.75 | 20.3(18.8, 21.9) | 23.1(21.4, 25.0) | 2.8 ± 13.8 | 0.44 | 22.0(20.5, 23.6) | 25.8(21.3, 27.8) | 3.8 ± 17.3 | 0.20 |
Missing 12-mo serum data were imputed by using the last observation carried forward method; changes in dietary and supplement intakes used all available data, without imputation for missing values.
Values are means ± SDs.
A general estimating equation model was used for the comparison of changes from baseline between the control and intervention groups, adjusted for race-ethnicity (white, other), season of random assignment (December–February, March–May, June–August, and September–November), and total (diet + supplement) vitamin D intake.
Geometric mean; 95% CI in parentheses (all such values).
TABLE 3.
Serum 25-hydroxyvitamin D [25(OH)D] concentrations at baseline and 12 mo, stratified by weight loss and intervention arm in overweight and obese postmenopausal women
| Serum 25(OH)D |
|||||||
| No. of subjects | Baseline1 | 12 mo1 | Change2 | P3 | |||
| ng/mL | |||||||
| Control | |||||||
| Weight loss | |||||||
| <5% | 67 | 20.9 (18.6, 23.4) | 23.0 (20.4, 25.9) | 2.7 ± 9.5 | Reference | ||
| 5–9.9% | 9 | 21.1 (17.0, 26.3) | 22.9 (19.6, 26.7) | 1.3 ± 7.2 | 0.99 | ||
| ≥10% | 3 | 21.2 (13.9, 32.4) | 32.4 (27.9, 37.4) | 9.4 ± 9.4 | 0.53 | ||
| P for trend4 | 0.43 | ||||||
| Exercise | |||||||
| Weight loss | |||||||
| <5% | 75 | 20.0 (18.1, 22.0) | 21.1 (18.8, 23.8) | 2.0 ± 6.1 | Reference | ||
| 5–9.9% | 26 | 19.4 (16.0, 23.5) | 22.3 (18.8, 26.6) | 2.7 ± 8.8 | 0.47 | ||
| ≥10% | 4 | 31.6 (27.5, 36.3) | 39.9 (33.9, 47.1) | 8.5 ± 5.9 | 0.56 | ||
| P for trend4 | 0.47 | ||||||
| Diet | |||||||
| Weight loss | |||||||
| <5% | 28 | 16.9 (14.2, 20.2) | 19.0 (15.8, 22.9) | 2.6 ± 6.7 | Reference | ||
| 5–9.9% | 27 | 20.6 (17.8, 23.9) | 22.5 (19.4, 26.2) | 2.1 ± 4.2 | 0.33 | ||
| ≥10% | 49 | 22.9 (20.7, 25.4) | 27.7 (25.2, 30.4) | 4.9 ± 6.4 | 0.03 | ||
| P for trend4 | 0.08 | ||||||
| Diet + exercise | |||||||
| Weight loss | |||||||
| <5% | 18 | 21.2 (17.8, 25.4) | 23.6 (20.1, 27.6) | 2.3 ± 5.1 | Reference | ||
| 5–9.9% | 21 | 20.7 (17.1, 25.1) | 23.8 (19.4, 29.2) | 3.5 ± 6.9 | 0.64 | ||
| ≥10% | 69 | 23.1 (21.1, 25.3) | 28.1 (25.7, 30.8) | 5.3 ± 7.3 | 0.14 | ||
| P for trend4 | 0.11 | ||||||
| All participants | |||||||
| Control subjects | 79 | 20.5 (18.6, 22.7) | 23.8 (21.2, 26.1) | 2.8 ± 9.3 | Reference | ||
| Gained weight | 43 | 19.6 (17.1, 22.5) | 21.3 (18.1, 25.0) | 2.3 ± 7.1 | 0.72 | ||
| Weight loss | |||||||
| <5% | 78 | 19.3 (17.5, 21.2) | 20.8 (18.7, 23.1) | 2.1 ± 5.4 | 0.35 | ||
| 5–9.9% | 74 | 20.2 (18.2, 22.3) | 22.8 (20.6, 25.2) | 2.7 ± 6.8 | 0.58 | ||
| 10–14.9% | 68 | 24.5 (22.4, 26.7) | 27.2 (24.9, 30.0) | 3.3 ± 6.1 | 0.93 | ||
| ≥15% | 54 | 21.8 (19.7, 24.2) | 29.5 (26.9, 32.3) | 7.7 ± 7.1 | 0.0005 | ||
| P for trend4 | 0.002 | ||||||
Values are geometric means; 95% CIs in parentheses.
Values are means ± SDs.
General estimating equation models adjusted for race-ethnicity, season of random assignment, total vitamin D intake, and intervention arm (in combined analysis).
Calculated across groups (excluding control subjects).
Body-composition changes according to baseline vitamin D status are shown in Table 4. Higher concentrations of 25(OH)D at baseline were not significantly associated with greater weight loss, a greater reduction in body fat, or the preservation of lean mass. Limiting the analyses to non-Hispanic whites tended to strengthen the observed associations but did not meaningfully alter any of the main findings (results not shown).
TABLE 4.
Mean 12-mo changes in body composition (weight, body fat, and lean mass) by baseline serum 25-hydroxyvitamin D [25(OH)D] status in postmenopausal women1
| Baseline 25(OH)D |
||||||||||
| <20.0 ng/mL |
20.0–29.9 ng/mL |
≥30.0 ng/mL |
||||||||
| No. of subjects | Change2 | Percentage change | No. of subjects | Change2 | Percentage change | No. of subjects | Change2 | Percentage change | P for trend4 | |
| kg | % | kg | % | kg | % | |||||
| Control | ||||||||||
| Weight | 31 | −0.9 ± 5.2 | −0.8 ± 5.9 | 33 | −0.8 ± 5.1 | −0.9 ± 5.6 | 15 | 0.6 ± 3.2 | 0.7 ± 3.5 | 0.11 |
| Body fat | 31 | 0.1 ± 3.5 | 0.6 ± 4.2 | 33 | −1.1 ± 4.2 | −0.9 ± 2.6 | 15 | 0.2 ± 2.8 | −0.1 ± 1.9 | 0.42 |
| Lean mass | 31 | −0.3 ± 1.5 | 0.3 ± 2.8 | 33 | 0.4 ± 2.0 | 1.0 ± 2.4 | 15 | 0.0 ± 1.9 | −0.3 ± 2.3 | 0.06 |
| Exercise | ||||||||||
| Weight | 53 | −2.0 ± 3.3 | −2.2 ± 3.6 | 32 | −2.3 ± 4.8 | −2.8 ± 5.8 | 20 | −2.9 ± 4.0 | −3.4 ± 4.6 | 0.38 |
| Body fat | 53 | −2.0 ± 2.7 | −1.4 ± 2.1 | 32 | −2.7 ± 4.5 | −2.2 ± 3.5 | 20 | −2.6 ± 3.6 | −1.9 ± 3.1 | 0.14 |
| Lean mass | 53 | 0.3 ± 2.0 | 1.7 ± 2.2 | 32 | 0.6 ± 2.0 | 2.2 ± 3.4 | 20 | −0.1 ± 2.0 | 1.9 ± 3.3 | 0.09 |
| Diet | ||||||||||
| Weight | 41 | −7.0 ± 7.1 | −8.2 ± 7.7 | 48 | −9.4 ± 5.8 | −11.4 ± 7.4 | 15 | −7.3 ± 4.9 | −9.5 ± 6.2 | 0.90 |
| Body fat | 41 | −6.0 ± 6.3 | −3.7 ± 4.2 | 48 | −8.0 ± 5.4 | −5.6 ± 5.2 | 15 | −6.6 ± 4.7 | −4.8 ± 4.0 | 0.12 |
| Lean mass | 41 | −0.8 ± 1.5 | 3.6 ± 4.5 | 48 | −1.1 ± 1.9 | 5.4 ± 5.0 | 15 | −0.4 ± 1.6 | 4.9 ± 4.1 | 0.17 |
| Diet + exercise | ||||||||||
| Weight | 40 | −10.2 ± 6.2 | −11.9 ± 7.0 | 43 | −11.3 ± 7.1 | −11.3 ± 7.1 | 25 | −9.1 ± 4.1 | −11.6 ± 5.1 | 0.53 |
| Body fat | 40 | −9.0 ± 5.7 | −5.6 ± 4.6 | 43 | −8.5 ± 5.6 | −6.3 ± 5.0 | 25 | −9.0 ± 3.8 | −7.3 ± 4.0 | 0.45 |
| Lean mass | 40 | −1.0 ± 2.3 | 5.3 ± 4.6 | 43 | −0.5 ± 1.7 | 6.0 ± 5.0 | 25 | 0.3 ± 1.4 | 7.4 ±3.9 | 0.18 |
Analysis based on available data without imputation for missing values.
Values are arithmetic means ± SDs.
Calculated as 12-mo change (%) − baseline (%).
P for trend across serum 25(OH)D groups, adjusted for race-ethnicity (white, other), season of random assignment, and total (diet + supplement) vitamin D intake.
DISCUSSION
One of the mechanisms believed to result in low vitamin D associated with obesity is decreased bioavailability (29), although this remains controversial (30). Our observation that greater adiposity is associated with lower serum 25(OH)D is consistent with this pathway and suggests that weight loss could lead to increased 25(OH)D concentrations through decreased peripheral sequestration. Although our findings indicate that 12 mo of weight loss achieved through caloric restriction and/or aerobic exercise interventions did not significantly increase serum 25(OH)D relative to values in the control subjects, greater weight loss was associated with increased 25(OH)D in a dose-dependent manner, independent of changes in dietary vitamin D intake. The correlation between BMI change and serum 25(OH)D was −0.23, which was similar to the inverse correlation (r = −0.27, P = 0.013) between change in BMI and 25(OH)D reported by Reinehr et al (31) in a study of obese children who underwent a 1-y lifestyle-based weight-loss program.
The effect of weight reduction on circulating vitamin D has been examined in a few other, but small, studies. Riedt et al (32) randomly assigned 47 overweight postmenopausal women to receive either 1 g/d (n = 24) or 1.7 g/d (n = 23) of supplemental calcium while undergoing a 6-mo weight-loss program. The average weight loss was similar in both groups (−9.3± 3.9% body weight) and was associated with a modest but nonsignificant increase in 25(OH)D (11.9 ±29.2 and 6.9 ±22.3 ng/mL, respectively) compared with baseline. Tzotzas et al (33) measured serum 25(OH)D after weight loss induced by 20 wk of a low-calorie diet in 26 of 37 women who completed the intervention. The mean weight loss was 9.7% of body weight, and mean serum 25(OH)D increased from 15.4 to 18.3 ng/mL (+2.9 ng/mL; P < 0.05). Similarly, the mean concentration of serum 25(OH)D increased 2.9 ng/mL (from 30.3 to 33.2 ng/mL) in 43 perimenopausal women who underwent a 12-wk weight-reduction program that achieved a mean weight loss of 11.5% initial body weight (34).
In comparison with these previous trials, the major strengths of the current study included its large sample size, excellent intervention adherence and study retention, and long duration. Our findings were consistent with those of previous studies (31–34); women who lost 10–14.9% of body weight over 12 mo had an increase in serum 25(OH)D of 2.9 ng/mL. However, we also had sufficient power to examine the subgroup of women who lost ≥15% body weight. In this group, serum 25(OH)D increased, on average, by 7.7 ng/mL, which suggests that there may be a threshold of weight loss that is important in terms of affecting serum vitamin D concentrations. Changes in circulating 25(OH)D have also been documented after larger weight loss achieved through bariatric surgery (35), but are likely confounded by the malabsorptive state that is a common consequence of bypass surgeries. Decreased concentrations of vitamin D have been reported with weight loss induced by lipase inhibitors such as orlistat (36–39), which suggests that resulting weight loss is insufficient to counterbalance the direct drug effect. To our knowledge, the effects of other pharmacologic agents for weight loss on circulating vitamin D concentrations have not been investigated. Whether greater amounts of weight loss (ie, >15%) achieved through diet and exercise will result in further increases in 25(OH)D remains unknown.
Whether vitamin D status influences successful weight loss also remains unknown. In a small study of 60 overweight or obese young women (age 20–35 y) receiving a hypocaloric diet, women with baseline 25(OH)D concentrations ≥20 ng/dL had a greater loss of body fat than did women with baseline concentrations <20 ng/dL (14), whereas Zitterman et al (15) reported no difference in 12-mo weight loss in 200 overweight men and women aged 18–70 y (n = 165 completed) who were randomly assigned to receive daily vitamin D (3320 IU) or placebo (−5.6% and −6.7%, respectively; P = 0.248) while undergoing a weight-loss program. In our study, the magnitude of weight loss within each intervention arm was not significantly affected by baseline concentrations of 25(OH)D. However, the mean concentrations of 25(OH)D were low overall, and the range of serum concentrations may have been inadequate to detect an effect. Whether vitamin D supplementation can influence weight loss, and whether the same dose of vitamin D will achieve similar changes in serum and stored concentrations of vitamin D across all levels of adiposity, should be investigated in future randomized clinical trials.
Vitamin D has direct effects on muscle and has been associated with greater strength and physical function (40, 41), which could lead to better exercise adherence. Although 25(OH)D was positively associated with lean mass at baseline, it was not correlated with pedometer steps/d, minutes of moderate-to-vigorous activity, or any measure of adherence in either of the groups receiving the exercise intervention. Baseline vitamin D status also did not have any effect on the preservation of lean mass during weight loss.
Serum 25(OH)D concentrations are lower in African Americans and other populations with darker skin pigmentation than in light-skinned populations (42). We did not observe a significant interaction effect of race in this study; unfortunately, our sample of nonwhite women was not large enough for a stratified analysis. Limiting the analysis to non-Hispanic whites strengthened the observed relations but did not meaningfully change any of our results. Further studies to examine the effect of weight loss on serum vitamin D in nonwhite populations are warranted.
Sun exposure was not assessed in this trial; therefore, potential changes in ultraviolet B exposure could not be ascertained. A cohort of breast cancer survivors living in the Seattle area showed little seasonal variation in 25(OH)D (43), whereas we observed modestly higher 25(OH)D concentrations in women randomly assigned in summer months than in winter months (22.5 and 18.7 ng/mL). Because follow-up measures were made at 12 mo, we anticipated that these differences were unlikely to have affected our results. Nevertheless, future studies should collect detailed sun exposure data, including time spent outdoors at particular times of the day, use of sunscreen, and type of clothing worn.
Vitamin D physiologically functions as a hormone, and its regulatory pathways and metabolism are complex. Although greater adiposity is associated with lower concentrations of circulating vitamin D, our findings suggest that lifestyle-based weight loss of 5% to 10% body weight is associated with a modest increase in serum 25(OH)D. However, baseline vitamin D status had little effect on the achievement of weight loss in a sample of overweight and obese postmenopausal women. Ongoing research to better understand the role of vitamin D in pathways influencing energy balance may lead to a clearer understanding of optimal vitamin D concentrations for promoting health in human populations.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—CM and AM: had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the analysis; CM, AM, KEF-S, C-YW, CMA, and GLB: study concept and design; AK, CB, KLC, CRD, GLB, and AM: acquisition of data; CM, LX, CRD, MLN, CMA, and AM: analysis and interpretation of data; CM: drafting of the manuscript; II, AK, KLC, CY-W, CMA, LL, KR, RWJ, GLB, and AM: critical revision of the manuscript for important intellectual content; LX and CM: statistical analysis; AM: obtained funding; CB and LX: administrative, technical, or material support; and AM: supervised the study. None of the authors disclosed a conflict of interest.
REFERENCES
- 1.IARC Working Group on Evaluation of Cancer-Prevention Strategies Weight control and physical activity. Vol 6 Lyon, France: IARC Press, 2002 [Google Scholar]
- 2.Gouni-Berthold I, Krone W, Berthold HK. Vitamin D and cardiovascular disease. Curr Vasc Pharmacol 2009;7:414–22 [DOI] [PubMed] [Google Scholar]
- 3.Beydoun MA, Boueiz A, Shroff MR, et al. Associations among 25-hydroxyvitamin D, diet quality, and metabolic disturbance differ by adiposity in adults in the United States. J Clin Endocrinol Metab 2010;95:3814–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brock K, Huang WY, Fraser DR, et al. Low vitamin D status is associated with physical inactivity, obesity and low vitamin D intake in a large US sample of healthy middle-aged men and women. J Steroid Biochem Mol Biol 2010;121:462–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng S, Massaro JM, Fox CS, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes 2010;59:242–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin L, Grandi N, Raum E, et al. Meta-analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther 2009;30:113–25 [DOI] [PubMed] [Google Scholar]
- 7.Yin L, Grandi N, Raum E, et al. Meta-analysis: serum vitamin D and breast cancer risk. Eur J Cancer 2010;46:2196–205 [DOI] [PubMed] [Google Scholar]
- 8.Fiscella K, Franks P. Vitamin D, race, and cardiovascular mortality: findings from a national US sample. Ann Fam Med 2010;8:11–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med 2008;168:1340–9 [DOI] [PubMed] [Google Scholar]
- 10.Ross C, Taylor CL, Yaktine AL. et al. Dietary Reference Intakes for calcium and vitamin D. Washington, DC: National Academy of Sciences, 2010 [PubMed] [Google Scholar]
- 11.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 2008;87(suppl):1080S–6S [DOI] [PubMed] [Google Scholar]
- 12.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81 [DOI] [PubMed] [Google Scholar]
- 13.Rayalam S, Della-Fera MA, Ambati S, et al. Enhanced effects of 1,25(OH)(2)D(3) plus genistein on adipogenesis and apoptosis in 3T3-L1 adipocytes. Obesity (Silver Spring) 2008;16:539–46 [DOI] [PubMed] [Google Scholar]
- 14.Ortega RM, Lopez-Sobaler AM, Aparicio A, et al. Vitamin D status modification by two slightly hypocaloric diets in young overweight/obese women. Int J Vitam Nutr Res 2009;79:71–8 [DOI] [PubMed] [Google Scholar]
- 15.Zittermann A, Frisch S, Berthold HK, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr 2009;89:1321–7 [DOI] [PubMed] [Google Scholar]
- 16.Nagpal J, Pande JN, Bhartia A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabet Med 2009;26:19–27 [DOI] [PubMed] [Google Scholar]
- 17.Eide MJ, Weinstock MA. Association of UV index, latitude, and melanoma incidence in nonwhite populations–US Surveillance, Epidemiology, and End Results (SEER) Program, 1992 to 2001. Arch Dermatol 2005;141:477–81 [DOI] [PubMed] [Google Scholar]
- 18.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan DH, Espeland MA, Foster GD, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003;24:610–28 [DOI] [PubMed] [Google Scholar]
- 20.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32:S498–504 [DOI] [PubMed] [Google Scholar]
- 21.Patterson RE, Kristal AR, Tinker LF, et al. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol 1999;9:178–87 [DOI] [PubMed] [Google Scholar]
- 22.Taylor HL, Jacobs DR, Jr, Schucker B, et al. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 1978;31:741–55 [DOI] [PubMed] [Google Scholar]
- 23.Ersfeld DL, Rao DS, Body JJ, et al. Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON automated analyzer. Clin Biochem 2004;37:867–74 [DOI] [PubMed] [Google Scholar]
- 24.Wagner D, Hanwell HE, Vieth R. An evaluation of automated methods for measurement of serum 25-hydroxyvitamin D. Clin Biochem 2009;42:1549–56 [DOI] [PubMed] [Google Scholar]
- 25.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986;42:121–30 [PubMed] [Google Scholar]
- 26.National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults–the evidence report. Obes Res 1998;6(suppl 2):51S–209S [PubMed] [Google Scholar]
- 27.Christian JG, Tsai AG, Bessesen DH. Interpreting weight losses from lifestyle modification trials: using categorical data. Int J Obes (Lond) 2010;34:207–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foster-Schubert KE, Alfano CM, Duggan C, et al. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese post-menopausal women. Obesity (Silver Spring) (Epub ahead of print 14 April 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000;72:690–3 [DOI] [PubMed] [Google Scholar]
- 30.Heaney RP, Recker RR, Grote J, Horst RL, Armas LA. Vitamin D3 is more potent than vitamin D2 in humans. J Clin Endocrinol Metab 2011;96:E447–52 [DOI] [PubMed] [Google Scholar]
- 31.Reinehr T, de Sousa G, Alexy U, Kersting M, Andler W. Vitamin D status and parathyroid hormone in obese children before and after weight loss. Eur J Endocrinol 2007;157:225–32 [DOI] [PubMed] [Google Scholar]
- 32.Riedt CS, Cifuentes M, Stahl T, et al. Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/day calcium intake. J Bone Miner Res 2005;20:455–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tzotzas T, Papadopoulou FG, Tziomalos K, et al. Rising serum 25-hydroxy-vitamin D levels after weight loss in obese women correlate with improvement in insulin resistance. J Clin Endocrinol Metab 2010;95:4251–7 [DOI] [PubMed] [Google Scholar]
- 34.Holecki M, Zahorska-Markiewicz B, Janowska J, et al. The influence of weight loss on serum osteoprotegerin concentration in obese perimenopausal women. Obesity (Silver Spring) 2007;15:1925–9 [DOI] [PubMed] [Google Scholar]
- 35.Lin E, Armstrong-Moore D, Liang Z, et al. Contribution of adipose tissue to plasma 25-hydroxyvitamin D concentrations during weight loss following gastric bypass surgery. Obesity (Silver Spring) 2011;19:588–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sjostrom L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet 1998;352:167–72 [DOI] [PubMed] [Google Scholar]
- 37.Hollander PA, Elbein SC, Hirsch IB, et al. Role of orlistat in the treatment of obese patients with type 2 diabetes. A 1-year randomized double-blind study. Diabetes Care 1998;21:1288–94 [DOI] [PubMed] [Google Scholar]
- 38.Finer N, James WP, Kopelman PG, Lean ME, Williams G. One-year treatment of obesity: a randomized, double-blind, placebo-controlled, multicentre study of orlistat, a gastrointestinal lipase inhibitor. Int J Obes Relat Metab Disord 2000;24:306–13 [DOI] [PubMed] [Google Scholar]
- 39.Hauptman J, Lucas C, Boldrin MN, Collins H, Segal KR. Orlistat in the long-term treatment of obesity in primary care settings. Arch Fam Med 2000;9:160–7 [DOI] [PubMed] [Google Scholar]
- 40.Gerdhem P, Ringsberg KA, Obrant KJ, Akesson K. Association between 25-hydroxy vitamin D levels, physical activity, muscle strength and fractures in the prospective population-based OPRA Study of Elderly Women. Osteoporos Int 2005;16:1425–31 [DOI] [PubMed] [Google Scholar]
- 41.Wilhelm-Leen ER, Hall YN, Deboer IH, Chertow GM. Vitamin D deficiency and frailty in older Americans. J Intern Med 2010;268:171–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Looker AC, Pfeiffer CM, Lacher DA, et al. Serum 25-hydroxyvitamin D status of the US population: 1988-1994 compared with 2000-2004. Am J Clin Nutr 2008;88:1519–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neuhouser ML, Sorensen B, Hollis BW, et al. Vitamin D insufficiency in a multiethnic cohort of breast cancer survivors. Am J Clin Nutr 2008;88:133–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.