Abstract
Background: Consumption of sugar-sweetened beverages has been shown to be associated with dyslipidemia, insulin resistance, fatty liver, diabetes, and cardiovascular disease. It has been proposed that adverse metabolic effects of chronic consumption of sugar-sweetened beverages are a consequence of increased circulating glucose and insulin excursions, ie, dietary glycemic index (GI).
Objective: We determined whether the greater adverse effects of fructose than of glucose consumption were associated with glucose and insulin exposures.
Design: The subjects were studied in a metabolic facility and consumed energy-balanced diets containing 55% of energy as complex carbohydrate for 2 wk (GI = 64). The subjects then consumed 25% of energy requirements as fructose- or glucose-sweetened beverages along with their usual ad libitum diets for 8 wk at home and then as part of energy-balanced diets for 2 wk at the metabolic facility (fructose GI = 38, glucose GI = 83). The 24-h glucose and insulin profiles and fasting plasma glycated albumin and fructosamine concentrations were measured 0, 2, 8, and 10 wk after beverage consumption.
Results: Consumption of fructose-sweetened beverages lowered glucose and insulin postmeal peaks and the 23-h area under the curve compared with the baseline diet and with the consumption of glucose-sweetened beverages (all P < 0.001, effect of sugar). Plasma glycated albumin concentrations were lower 10 wk after fructose than after glucose consumption (P < 0.01, effect of sugar), whereas fructosamine concentrations did not differ between groups.
Conclusion: The results suggest that the specific effects of fructose, but not of glucose and insulin excursions, contribute to the adverse effects of consuming sugar-sweetened beverages on lipids and insulin sensitivity. This study is registered at clinicaltrials.gov as NCT01165853.
INTRODUCTION
Consumption of sugar-sweetened beverages has been shown in epidemiologic studies to be associated with the development of dyslipidemia (1, 2), insulin resistance (3, 4), fatty liver (5, 6), type 2 diabetes (7–9), and cardiovascular disease (10, 11). We conducted a dietary intervention study during which older, overweight or obese men and women consumed either fructose- or glucose-sweetened beverages providing 25% of energy requirements for 10 wk. We reported that de novo lipogenesis, 23-h area under the curves (AUCs) for circulating triglycerides, and concentrations of fasting apolipoprotein B, LDL, small dense LDL, oxidized LDL, and postprandial remnant lipoprotein-triglycerides and cholesterol increased in the subjects who consumed fructose. In addition, visceral adipose deposition increased and insulin sensitivity decreased in these subjects. None of these adverse effects were observed in a group of subjects consuming glucose at 25% of the energy requirement, who gained the same amount of weight as the subjects consuming fructose (≈1.4 kg) (12). We have proposed that these adverse effects of fructose consumption are explained by its hepatic metabolism being independent of energy status, which leads to unregulated hepatic fructose uptake and increased lipogenesis (12–15). The resulting increase in the hepatic lipid supply leads to increased production and secretion of VLDL and is associated with decreased hepatic insulin sensitivity. Because the commonly consumed sugars sucrose and high-fructose corn syrup (HFCS) are composed of 50% to 55% fructose, these proposed mechanisms may explain the associations between sugar consumption and metabolic disease (1–11). However, it has also been suggested that the adverse effects associated with chronic consumption of sugar-sweetened beverages result from increased circulating glucose and insulin excursions [ie, glycemic index (GI)] (16–19). Mechanisms by which increased circulating glucose and insulin excursions are proposed to mediate the adverse effects associated with an increased risk of type 2 diabetes and cardiovascular disease have been reviewed (20) and include redistribution of metabolic substrates to adipose tissue; insulin resistance mediated by the direct effects of hyperglycemia, counterregulatory hormone secretion, and increased levels of postprandial free fatty acids; glucotoxicity; lipotoxicity; hyperglycemia-induced oxidative stress; and independent effects of hyperinsulinemia on blood pressure, lipids, inflammatory mediators and endothelial function.
The objective of this study was to investigate the relation between meal-induced glucose and insulin excursions and overall glucose exposure with the metabolic effects observed in subjects consuming glucose- and fructose-sweetened beverages (12). Therefore, we measured the 24-h profiles of glucose and insulin and fasting plasma concentrations of glycated albumin and fructosamine in samples collected during our previously reported study from the same subjects who consumed glucose- or fructose-sweetened beverages for 10 wk (12).
SUBJECTS AND METHODS
The participants were recruited through newspaper advertisements and underwent a complete blood count, a serum biochemistry panel, and a telephone and an in-person interview to obtain a medical history to assess eligibility. Inclusion criteria included age 40–72 y and a body mass index (BMI; in kg/m2) of 25–35 with a self-report of stable body weight during the prior 6 mo. Women were postmenopausal based on a self-report of no menstruation for ≥1 y. Exclusion criteria included diabetes (fasting glucose >125 mg/dL), renal or hepatic disease, fasting serum triglyceride concentrations >400 mg/dL, hypertension (>140/90 mm Hg), and surgery for weight loss. Individuals who smoked, reported exercise of >3.5 h/wk at a level more vigorous than walking, or used thyroid, lipid-lowering, glucose-lowering, antihypertensive, antidepressant, or weight-loss medications were also excluded. Diet-related exclusion criteria included habitual ingestion of more than one sugar-sweetened beverage daily or >2 alcoholic beverages/d. The University of California, Davis, Institutional Review Board approved the experimental protocol, and subjects provided informed consent to participate in the study. Thirty-nine subjects enrolled in the study and experimental groups were matched for sex, BMI, and fasting triglyceride and insulin concentrations. Subjects and University of California, Davis, Clinical and Translational Science Center's Clinical Research Center (CCRC) and technical personnel were blinded to the sugar assignments. Seven subjects (3 in the glucose group, 4 in the fructose group) did not complete the study because of an inability or unwillingness to comply with the protocol or because of personal or work-related conflicts. Seventeen subjects consuming fructose-sweetened beverages and 15 subjects consuming glucose-sweetened beverages completed the study, and the previously reported (12) lipid, adiposity, and insulin sensitivity results from this study were obtained from these same subjects.
As previously described (12), this was a parallel-arm, diet intervention study that consisted of 3 phases: 1) a 2-wk inpatient baseline period during which subjects consumed an energy-balanced diet, 2) an 8-wk outpatient intervention period during which subjects consumed glucose- (n = 15) or fructose-sweetened (n = 17) beverages providing 25% of daily energy requirements along with their usual ad libitum diet, and 3) a 2-wk inpatient intervention period during which subjects consumed 25% of daily energy requirements as their assigned sugar-sweetened beverage along with an energy-balanced diet. During the 2-wk baseline and intervention inpatient periods of the study, subjects resided in the CCRC and consumed energy-balanced [based on the Mifflin equation (21)] meals consisting of conventional foods. The baseline diet contained 55% of energy as mainly complex carbohydrate, 30% as fat, and 15% as protein and had a calculated GI of 64. The intervention inpatient meals were as identical as possible to those served during baseline, except that the carbohydrate was provided as 25% of energy as glucose- or fructose-sweetened beverages and 30% of energy as complex carbohydrate. The GIs of the glucose- and fructose-containing diets were 83 and 38, respectively. The timing of meal service and the energy distribution were as follows: breakfast (0900, 25%), lunch (1300, 35%), and dinner (1800, 40%). Sugars were provided to the subjects as 3 daily servings of glucose- or fructose-sweetened beverages flavored with an unsweetened drink mix (Kool-Aid; Kraft, Northfield, IL). During the 8-wk outpatient phase of the study, the subjects were instructed to drink 3 servings of sugar-sweetened beverages/d (one with each meal), to consume their usual diet, and to not consume other sugar-containing beverages, including fruit juice. The sugar-sweetened beverages contained a biomarker (riboflavin), which was measured fluorimetrically in urine samples collected at the time of beverage pickup to monitor compliance. These measurements indicated that the 2 groups of subjects were comparably compliant (12).
Estimates of food intake during the outpatient phase of the study were collected by 24-h recall (via telephone) with the use of the US Department of Agriculture's 5-step multiple pass method as described by Conway et al (22). The recalls were conducted on 3 random days per week before the study began and during intervention weeks 2 and 7. The same registered dietitian administered the recall to all subjects. The recalls were analyzed with Nutrition Data System for Research (version 2005; Minneapolis, MN). The results from all 3 prestudy recalls were averaged, as were the results from the 6 intervention recalls, except for reports that were judged by the dietitian to be outliers to the usual dietary pattern because of illness or other circumstances.
Twenty-four-hour serial blood collections were conducted during baseline (0 wk) and at 2, 8, and 10 wk of the intervention. The meals served were identical at all 3 intervention time points (2, 8, and 10 wk), and the intervention meals were matched as closely as possible to the baseline meals (0 wk), except for the substitution of 25% of energy from sugars in the sugar-sweetened beverages for the complex carbohydrate. The 24-h blood collections at baseline (0 wk) and at the end of the intervention (10 wk) were performed after subjects had consumed energy-balanced, weight-maintaining diets in the CCRC for 10 d. The 24-h blood collections performed after 2 and 8 wk of intervention were preceded by 2- and 8-wk periods of ad libitum food intake. The 24-h profile data were not obtained from one subject from the glucose group because of failure of the catheter to remain patent during the baseline trial.
On the days of serial blood sampling, an intravenous catheter was inserted into an arm vein by a registered nurse at 0730 and kept patent with a slow saline infusion. Three fasting blood samples were collected into tubes containing EDTA at 0800, 0830, and 0900. Thirty-three postprandial blood samples were collected at 30–60-min intervals from 0930 to 0800 the next morning (23, 24). An additional 3–6 mL blood was collected at the 3 fasting time points; this plasma was pooled and stored as multiple aliquots at −70°C.
Glucose concentrations were measured with an automated glucose analyzer (YSI Inc, Yellow Springs, OH) and insulin by radioimmunoassay (Millipore, St Charles, MO). The pooled fasting plasma samples were analyzed enzymatically (25) in the laboratory of Ernst Schaefer for glycated albumin and for fructosamine concentrations by using a Polychem Chemistry Analyzer (PolyMedCo Inc, Cortland Manor, NY).
The incremental 23-h AUC was calculated for glucose and insulin by using the trapezoidal method. The mean concentration of the 3 fasting samples was determined, and the net AUC was calculated by subtracting the AUC values below fasting from the AUC values above fasting levels. Glucose and insulin responses were also assessed by the mean amplitudes of the 3 postmeal peaks. Specifically, the peak postmeal value minus the premeal value was averaged for breakfast, lunch, and dinner for each subject. Statistical tests were performed with SAS 9.2 in a repeated-measure (RM) mixed procedures (PROC MIXED; SAS Institute, Cary, NC) model with time, sugar, sex, and number of metabolic syndrome risk factors (MSRFs) as factors. Insignificant 3-factor interactions were removed if they decreased the precision of the model. MSRFs were those defined by the American Heart Association/National Heart Lung and Blood Institute (26, 27). Outcomes with significant effects of sugar × time were further analyzed in sugar-specific RM models with time and sex as factors. Tukey's multiple comparison post hoc tests were used to compare values at 0 wk with the responses at 2, 8, and 10 wk. The mean GI and glycemic load (GL) of the intervention 24-h food intake recalls (≤6/subject) were compared with the GI and GL of the prestudy 24-h food intake recalls (≤3/subject) by Student's t test. The data are presented as means ± SEMs.
RESULTS
As previously reported, no significant differences in any of the baseline variables measured were observed between the 2 groups of subjects (12). The 32 subjects were aged 53.7 ± 1.4 y (range: 43–70 y) and had a BMI of 29.3 ± 0.5 (range: 25.0–34.4). The men had 27.3 ± 1.3% body fat (range: 12.6–32.3%), and the women had 41.7 ± 1.1% body fat (range: 31.0–47.2%) (12). The macronutrient composition and the GI and GL of the inpatient diet served during the 24-h blood sampling period are shown in Table 1. The macronutrient composition and the GI and GL of the diets consumed by the subjects during the prestudy week and during the outpatient intervention were estimated from 24-h food intake recalls (Table 2). The GI (P < 0.0001) and GL (P < 0.0001) of the outpatient diet increased significantly during the intervention in the subjects who consumed glucose. In the subjects who consumed fructose, the GI (P < 0.0001) and GL (P < 0.05) of the outpatient diet decreased significantly during the intervention. We previously reported that during the 8-wk outpatient period, when subjects consumed their usual ad libitum diet along with the sugar-sweetened beverages, no significant sugar- or sex-group differences in fat, sugar, or alcohol intake as a percentage of energy intake or in the amount of energy consumed relative to calculated energy requirements were observed, and both groups of subjects gained comparable amounts of weight, ≈1.4 kg (12).
TABLE 1.
Inpatient diet composition1
| Glucose |
Fructose |
|||
| Diet components | Baseline | Intervention | Baseline | Intervention |
| Energy (kcal) | 2390 ± 762 | 2390 ± 76 | 2398 ± 98 | 2398 ± 98 |
| Protein (g) | 90 ± 3 | 90 ± 3 | 91 ± 4 | 90 ± 4 |
| Total fat (g) | 80 ± 3 | 80 ± 3 | 80 ± 3 | 80 ± 3 |
| Available carbohydrate (g) | 329 ± 10 | 329 ± 10 | 330 ± 13 | 330 ± 13 |
| Complex carbohydrate (g) | 297 ± 9 | 146 ± 5 | 298 ± 12 | 146 ± 5 |
| Experimental beverage sugar (g) | 0 | 157 ± 5 | 0 | 158 ± 7 |
| Other added sugar (g) | 16 ± 3 | 12 ± 2 | 17 ± 3 | 13 ± 2 |
| Total fiber (g) | 21 ± 3 | 16 ± 1 | 21 ± 3 | 16 ± 1 |
| Glycemic index3 | 64 | 83 | 64 | 38 |
| Glycemic load3 | 200 ± 6 | 263 ± 8 | 200 ± 8 | 126 ± 5 |
Calculations were based on the diet consumed during the 24-h serial blood sampling period.
Mean ± SEM (all such values).
Calculations were based on the glucose standard.
TABLE 2.
Outpatient diet composition1
| Glucose |
Fructose |
|||
| Diet components | Baseline | Intervention | Baseline | Intervention |
| Energy (kcal) | 1891 ± 153 | 2602 ± 148 | 2066 ± 181 | 2534 ± 156 |
| Protein (g) | 73 ± 6 | 88 ± 6 | 81 ± 6 | 85 ± 6 |
| Total fat (g) | 67 ± 7 | 89 ± 7 | 78 ± 10 | 80 ± 7 |
| Available carbohydrate (g) | 235 ± 22 | 342 ± 23 | 239 ± 17 | 355 ± 20 |
| Complex carbohydrate (g) | 112 ± 11 | 111 ± 10 | 105 ± 12 | 115 ± 10 |
| Experimental beverage sugar (g) | 0 | 157 ± 5 | 0 | 158 ± 7 |
| Other added sugar (g) | 81 ± 19 | 56 ± 10 | 107 ± 12 | 64 ± 11 |
| Total fiber (g) | 22 ± 3 | 18 ± 2 | 19 ± 2 | 19 ± 2 |
| Glycemic index2 | 59 ± 1 | 81 ± 13 | 60 ± 2 | 45 ± 13 |
| Glycemic load2 | 167 ± 21 | 304 ± 173 | 160 ± 9 | 157 ± 114 |
All values are means ± SEMs. The composition of the ad libitum diet consumed during the outpatient periods was estimated from 24-h food recall interviews.
Calculations were based on the glucose standard.
Significantly different from baseline (Student's t test): 3P < 0.0001, 4P < 0.05.
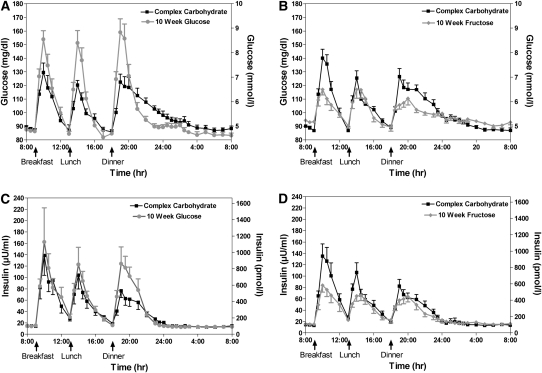
The 24-h profiles of glucose and insulin at baseline during the consumption of the complex carbohydrate diet and at 10 wk during consumption of 25% of energy requirements as glucose- or fructose-sweetened beverages are shown in Figure 1. The effects of glucose and fructose consumption on the mean amplitude of the postmeal peaks and 23-h AUCs of circulating glucose and insulin (Table 3) were markedly different (effect of sugar × time: P < 0.001, 4-factor RM PROC MIXED) and were not affected by sex or MSRF (sex × time, MSRF × time: P > 0.05 for all variables, 4-factor RM PROC MIXED). Compared with the consumption of complex carbohydrate at 0 wk, consumption of fructose lowered mean postmeal glucose (−32% to −47%; effect of time: P < 0.0001, 2-factor RM PROC MIXED) and insulin peaks (−30% to −37%; P < 0.01, effect of time, 2-factor RM PROC MIXED) and consumption of glucose resulted in increased mean postmeal glucose peaks (+61% to +75%; effect of time: P < 0.001, 2-factor RM PROC MIXED). The increases in mean postmeal insulin peaks induced by glucose consumption (+40% to +49%; effect of time: P < 0.01, 2-factor RM PROC MIXED) were significant at 2 wk. Similarly, compared with the consumption of complex carbohydrate, consumption of fructose lowered 23-h glucose (−59% to −80%; effect of time: P < 0.0001, 2-factor RM PROC MIXED) and insulin (−27% to −37%; effect of time: P < 0.01, 2-factor RM PROC MIXED) AUCs, and consumption of glucose increased 23-h insulin AUC (+31% to +46%; effect of time: P < 0.01, 2-factor RM PROC MIXED).
FIGURE 1.
Mean (±SEM) 24-h circulating glucose (A and B) and insulin (C and D) concentrations in subjects before and after consumption of glucose-sweetened (n = 14) or fructose-sweetened (n = 17) beverages for 10 wk.
TABLE 3.
Indexes of glucose and insulin exposure before and 2, 8, and 10 wk after consumption of glucose-sweetened (n = 14) or fructose-sweetened (n = 17) beverages1
| Variable and preceding diet | Complex carbohydrates, 0 wk (energy balance) | Sugar, 2 wk (ad libitum) | Sugar, 8 wk (ad libitum) | Sugar, 10 wk (energy balance) | P value (sugar × time)2 |
| Postmeal glucose peaks (mg/dL) | |||||
| Glucose (n = 14) | 45.1 ± 3.9 | 72.5 ± 8.13 | 73.0 ± 6.83 | 78.3 ± 7.54 | <0.0001 |
| Fructose (n = 17) | 48.2 ± 4.3 | 24.2 ± 2.63 | 28.8 ± 2.63 | 33.0 ± 3.63 | |
| 23-h Glucose AUC (mg/dL ⋅ 23 h) | |||||
| Glucose (n = 14) | 254.3 ± 20.1 | 247.4 ± 47.2 | 253.9 ± 43.7 | 342.4 ± 41.7 | <0.001 |
| Fructose (n = 17) | 308.1 ± 34.1 | 45.9 ± 22.55 | 100.3 ± 29.54 | 125.6 ± 29.84 | |
| Postmeal insulin peaks (μU/mL) | |||||
| Glucose (n = 14) | 94.7 ± 20.5 | 127.3 ± 24.46 | 119.3 ± 15.4 | 136.2 ± 36.3 | <0.0001 |
| Fructose (n = 17) | 97.6 ± 15.7 | 60.0 ± 8.63 | 66.1 ± 10.73 | 64.0 ± 8.26 | |
| 23-h Insulin AUC (μU/mL ⋅ 23 h) | |||||
| Glucose (n = 14) | 596.1 ± 118.7 | 699.6 ± 117.6 | 693.0 ± 118.7 | 757.5 ± 138.53 | <0.0001 |
| Fructose (n = 17) | 681.4 ± 112.6 | 426.1 ± 66.63 | 489.1 ± 85.83 | 480.4 ± 72.93 |
All values are means ± SEMs. AUC, area under the curve.
PROC MIXED (SAS Institute, Cary, NC) 4-factor (sugar, time, sex, and metabolic syndrome risk factors) repeated-measures analysis.
Significantly different from 0 wk [PROC MIXED 2-factor (time, sex) repeated-measures analysis, Tukey's multiple comparison tests]: 3P < 0.01, 4P < 0.001, 5P < 0.0001, 6P < 0.05.
The effects of glucose and fructose consumption on risk factors for metabolic disease that have been shown to be positively affected by low-GI diets are shown in Table 4 (28). As previously reported, fasting glucose concentrations were significantly greater in the subjects who consumed fructose than in those who consumed glucose (12). The effects of the 2 sugars on fasting insulin concentrations were not significantly different (12). We also previously reported that insulin sensitivity, as assessed with the glucose disposal test (29), decreased in subjects consuming fructose (−17%) and was unchanged in subjects consuming glucose (12). Glycated albumin values were differentially affected by the 2 sugars (Table 4; effect of sugar × time: P < 0.01, 4-factor RM PROC MIXED), with fructose tending to decrease levels (−3.3 ± 1.3%; P = 0.14, Tukey's test) and glucose tending to increase levels (+2.1 ± 1.3%; P = 0.38, Tukey's test) at 10 wk compared with baseline. No effects of fructose or glucose consumption on plasma fructosamine concentrations were observed.
TABLE 4.
Fasting plasma glucose, insulin, glycated albumin, and fructosamine concentrations and insulin sensitivity before and 2, 8, and 10 wk after consumption of glucose- or fructose-sweetened beverages1
| Variable and preceding diet | Complex carbohydrates, 0 wk (energy balance) | Sugar, 2 wk (ad libitum) | Sugar, 8 wk (ad libitum) | Sugar, 10 wk (energy balance) | P value (sugar × time) |
| Fasting glucose (mg/dL)2 | |||||
| Glucose (n = 15) | 87.6 ± 1.5 | 88.0 ± 1.9 | 89.8 ± 2.1 | 86.4 ± 1.3 | <0.00013 |
| Fructose (n = 17) | 88.7 ± 1.0 | 95.7 ± 2.14 | 94.6 ± 2.14 | 93.6 ± 1.35 | |
| Fasting insulin (μU/mL)2 | |||||
| Glucose (n = 15) | 15.0 ± 1.9 | 15.8 ± 1.6 | 16.4 ± 1.9 | 15.0 ± 1.6 | NS3 |
| Fructose (n = 17) | 14.0 ± 1.5 | 17.1 ± 2.0 | 16.3 ± 2.1 | 15.4 ± 1.7 | |
| Insulin sensitivity index (mmol 2H2O/insulin AUC)2 | |||||
| Glucose (n = 14) | 0.236 ± 0.036 | 0.210 ± 0.021 | <0.056 | ||
| Fructose (n = 17) | 0.254 ± 0.049 | 0.208 ± 0.0407 | |||
| Glycated albumin (%) | |||||
| Glucose (n = 15) | 13.9 ± 0.3 | 14.2 ± 0.3 | 14.1 ± 0.3 | 14.2 ± 0.4 | <0.013 |
| Fructose (n = 17) | 14.2 ± 0.3 | 14.1 ± 0.4 | 14.3 ± 0.3 | 13.8 ± 0.3 | |
| Fructosamine (μmol/L) | |||||
| Glucose (n = 15) | 211.6 ± 10.9 | 209.9 ± 13.4 | 204.7 ± 7.0 | 211.9 ± 9.4 | NS3 |
| Fructose (n = 17) | 219.1 ± 7.1 | 202.3 ± 6.1 | 204.0 ± 8.4 | 208.8 ± 8.9 |
All values are means ± SEMs. AUC, area under the curve.
Results previously reported in reference 12. Insulin AUC is calculated as μU/mL · 4 h.
PROC MIXED (SAS Institute, Cary, NC) 4-factor (sugar, time, sex, and metabolic syndrome risk factors) repeated-measures analysis.
Significantly different from 0 wk [PROC MIXED 2-factor (time, sex) repeated-measures analysis, Tukey's multiple comparison tests]: 4P < 0.01, 5P < 0.0001.
General linear model 3-factor (sugar, sex, metabolic syndrome risk factors) ANOVA on percentage difference, 10 compared with 0 wk.
Significantly different from 0 wk, P < 0.01 (paired t test).
DISCUSSION
We proposed that the dyslipidemia and decreased insulin sensitivity observed in subjects who consume fructose-sweetened beverages for 10 wk at 25% of energy requirements (12) are explained by the hepatic metabolism of fructose being independent of energy status, which leads to unregulated hepatic fructose uptake and lipogenesis, increased hepatic lipid and production/secretion of VLDLs, and decreased hepatic insulin sensitivity (14, 15). However, it has also been proposed that the adverse effects of chronic consumption of sugar-sweetened beverages (1–11) result from increased circulating glucose and insulin excursions, ie, GI (16–19). We investigated this by measuring the 24-h profiles of glucose and insulin and fasting plasma concentrations of glycated albumin and fructosamine in the same subjects who consumed glucose- or fructose sweetened beverages for 10 wk. The new results reported here indicate that glucose and insulin excursions and exposure are substantially decreased in subjects who consume fructose and significantly increased in subjects who consume glucose. Together with our previous report that the consumption of fructose produced many adverse metabolic effects compared with the consumption of glucose in these same subjects (12), these results do not support the hypothesis that increased circulating glucose and insulin excursions either mediate or contribute to the adverse effects of sugar-sweetened beverage consumption for 10-wk.
Furthermore, and in direct contrast with the hypothesis that meal-induced glucose and insulin excursions promote the development of metabolic disease, we proposed that the lowered meal-related glucose and insulin responses in subjects consuming fructose may contribute to the adverse effects initiated by the unregulated hepatic metabolism of fructose (12). Insulin increases lipoprotein lipase (LPL) expression and activity, and we reported that subjects who consumed fructose had a lower postprandial LPL activity than did subjects who consumed glucose (12). The lowered postprandial LPL activity likely delays postprandial triglyceride clearance and thus contributes to the marked increases in postprandial triglyceride concentrations observed in subjects consuming fructose (12). Much evidence suggests that high concentrations of postprandial triglycerides, resulting from either overproduction or delayed clearance, then initiate the sequence of lipoprotein changes that result in increased production of remnant lipoproteins and small dense LDL particles (30–32). In addition, we have also hypothesized that lowered postprandial LPL activity, in response to reduced postmeal insulin peaks in subjects consuming fructose, may lead to preferential deposition of fat in the visceral adipose tissue. LPL in subcutaneous adipose tissue is more responsive to the effects of insulin than is LPL in visceral adipose tissue (33). Therefore, increased insulin responses after consumption of glucose-sweetened beverages with meals may lead to greater LPL activity in subcutaneous adipose tissue and increased triglyceride uptake by subcutaneous adipose tissue. Conversely, decreased insulin responses to fructose consumption may lead to decreased insulin-mediated LPL activity in subcutaneous adipose tissue, which allows for greater triglyceride uptake by visceral adipose tissue (12, 15).
These results have important implications regarding the effects of dietary GI on the development of metabolic disease. GI is used to categorize carbohydrate-containing foods based on incremental AUCs for the glucose response after consumption of a standard amount of carbohydrate from a test food relative to that of a control food (glucose or white bread) (34). A low-GI diet can help to improve glycemic control in patients with diabetes (35), and clinical studies have shown a strong correlation between glycated hemoglobin concentrations and vascular complications of diabetes (36). In nondiabetic subjects, many studies suggest that high-GI diets promote metabolic disease relative to low-GI diets (28, 37). However, a substantial number of reports indicate that high-GI diets are not associated with an increase in risk factors for the development of cardiovascular disease (38), insulin resistance (39), or type 2 diabetes (40, 41). Furthermore, a recent study showed that consumption of diets enriched with test foods with comparable macronutrient and fiber compositions, but with different GLs and GIs, did not change markers for the metabolic syndrome (42). The results of the current study indicate that known risk factors of metabolic disease increased during consumption for 10 wk of a diet containing fructose-sweetened beverages that had a substantially lower GI (GI: 38–45) than did the diet consumed before the study began (GI: 60), and during the baseline period (GI: 64), and that resulted in the expected lowering of postprandial glucose and insulin responses. In contrast, metabolic disease risk factors remained unchanged during consumption for 10 wk of a diet containing glucose-sweetened beverages that had a substantially higher GI (GI: 81–83) than did the diet consumed before the study began (GI: 59), and during the baseline period (GI: 64), and that induced the expected increase in postprandial glucose and insulin responses.
In a recent review, it was concluded that for healthy and/or overweight subjects the importance of low-GI diets in relation to components of the metabolic syndrome has not been established, mainly because the diets frequently used in intervention studies not only have different GIs but also have different fiber, protein, and/or fat contents (43). Clearly, fructose needs to be added to this list of potentially confounding dietary components. The importance of this is illustrated in a recent investigation of the relation between GI, GL, fructose, and insulin resistance in 668 nondiabetic persons. A direct association was found between fructose intake and insulin resistance, whereas the association between GL and insulin resistance disappeared when the model was adjusted for fructose intake (44). It has been suggested that a fructose index would be more relevant to cardiovascular disease risk than is the GI (45).
A recent meta-analyses of observational studies investigating GI and disease risk reported that low-GI diets are independently associated with a reduced risk of coronary heart disease and type 2 diabetes (37). The nutritional factors included in the statistical model were alcohol, fiber, and dietary supplements, but not fructose (37). A recent meta-analysis of intervention studies investigating GI and markers of health concluded that consumption of low-GI diets is followed by favorable changes in the health markers (28). Again, the confounding effects of fiber were included, but those of fructose were not (28). The biochemical risk factors that were shown by a meta-analysis of the intervention studies to be positively and independently affected by low-GI diets were fasting blood glucose and insulin concentrations, insulin sensitivity, and fasting fructosamine concentrations (28). As we previously reported, consumption of fructose (the diet with the lowest GI) significantly increased fasting blood glucose concentrations and decreased insulin sensitivity compared with consumption of glucose (the high-GI diet) (12). Glycated albumin concentrations decreased significantly in subjects who consumed fructose compared with subjects who consumed glucose. This finding indicated that, whereas this outcome reflected the differential glucose exposure in these subjects, it did not accurately reflect the changes in several risk factors for metabolic disease.
It is important to note that this study was not designed to investigate the effects of sugar-sweetened beverage consumption as they are commonly consumed in this country, both with regard to the amount of sugar consumed and the types of sugars consumed. Self-reported intake data suggest that only 13% of the US population consumes ≥25% of energy from added sugars (46). Furthermore, sugar-sweetened beverages are not sweetened with pure glucose or pure fructose, but rather with HFCS (55% fructose, 45% glucose) and sucrose (50% fructose, 50% glucose). However, the current investigation of the effects of consuming glucose- and fructose-sweetened beverages allows for differentiation between the effects of increased glucose and insulin excursions and those of unregulated hepatic metabolism of fructose, which cannot be accomplished when beverages sweetened with HFCS or sucrose are consumed. The glucose component of these sugars results in increased postprandial glucose and insulin excursions, and the fructose component may lead to unregulated hepatic lipid production. Thus, it is not possible in most diet intervention studies involving HFCS or sucrose to determine whether the effects are mediated via changes in glucose and insulin excursions or are a consequence of hepatic fructose metabolism. However, a limitation of the current study was that it did not address the possibility that there is a synergistic relation between increased glucose and insulin excursions and unregulated hepatic fructose metabolism, which occurs when fructose and glucose are consumed in combination.
In conclusion, the results of this study provide strong evidence that increased circulating glucose and insulin excursions (GI) are not associated with the adverse effects of fructose-sweetened beverage consumption for 10 wk by older overweight and obese adults. Decreasing the GI by substituting fructose for complex carbohydrate decreased meal-associated glucose and insulin excursions and significantly increased several risk factors for metabolic disease. Increasing the GI by simply substituting glucose for complex carbohydrate increased meal-associated glucose and insulin excursions, but had little, if any, adverse effect on risk factors for metabolic disease. Thus, the adverse metabolic effects associated with consumption of sugar-sweetened beverages appear to result primarily from the fructose component of dietary sugars, rather than from the glucose and insulin excursion induced by the glucose component. The results also indicate the importance of adjusting and controlling for dietary fructose intake when investigating the effects of GI and GL on the development of metabolic disease.
Acknowledgments
We thank James Graham and Marinelle Nuñez (University of California, Davis) and Elaine Souza (US Department of Agriculture, Western Human Nutrition Research Center) for their excellent technical support, Nicole Mullen (University of California, Davis, Medical Center) and the nursing staff at the CCRC for their dedicated nursing support, and Janet Peerson (University of California, Davis) for her expert advice on the statistical analysis of the data.
The authors’ responsibilities were as follows—KLS: designed and conducted the research, performed the statistical analyses, and wrote and revised the manuscript; SCG and AAB: conducted the research; EJS, KN, CB, and J-MS: provided the essential reagents and labor; RGV: wrote and revised the manuscript; LB: designed the research; NLK: designed the research and provided essential labor; and PJH: designed the research and had primary responsibility for the final content. CB owns stock in and is an employee of KineMed Inc, Emeryville, CA. None of the other authors had a relevant conflict of interest to disclose.
REFERENCES
- 1.Bremer AA, Auinger P, Byrd RS. Relationship between insulin resistance-associated metabolic parameters and anthropometric measurements with sugar-sweetened beverage intake and physical activity levels in US adolescents: findings from the 1999-2004 National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med 2009;163:328–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welsh JA, Sharma A, Abramson JL, Vaccarino V, Gillespie C, Vos MB. Caloric sweetener consumption and dyslipidemia among US adults. JAMA 2010;303:1490–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bremer AA, Auinger P, Byrd RS. Sugar-sweetened beverage intake trends in US adolescents and their association with insulin resistance-related parameters. J Nutr Metab (Epub ahead of print 6 September 2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshida M, McKeown NM, Rogers G, et al. Surrogate markers of insulin resistance are associated with consumption of sugar-sweetened drinks and fruit juice in middle and older-aged adults. J Nutr 2007;137:2121–7 [DOI] [PubMed] [Google Scholar]
- 5.Assy N, Nasser G, Kamayse I, et al. Soft drink consumption linked with fatty liver in the absence of traditional risk factors. Can J Gastroenterol 2008;22:811–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouyang X, Cirillo P, Sautin Y, et al. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol 2008;48:993–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montonen J, Jarvinen R, Knekt P, Heliovaara M, Reunanen A. Consumption of sweetened beverages and intakes of fructose and glucose predict type 2 diabetes occurrence. J Nutr 2007;137:1447–54 [DOI] [PubMed] [Google Scholar]
- 8.Palmer JR, Boggs DA, Krishnan S, Hu FB, Singer M, Rosenberg L. Sugar-sweetened beverages and incidence of type 2 diabetes mellitus in African American women. Arch Intern Med 2008;168:1487–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004;292:927–34 [DOI] [PubMed] [Google Scholar]
- 10.Dhingra R, Sullivan L, Jacques PF, et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation 2007;116:480–8 [DOI] [PubMed] [Google Scholar]
- 11.Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr 2009;89:1037–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanhope KL, Schwarz JM, Keim NL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 2009;119:1322–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanhope KL, Havel PJ. Fructose consumption: considerations for future research on its effects on adipose distribution, lipid metabolism, and insulin sensitivity in humans. J Nutr 2009;139(6)1236S–41S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanhope KL, Havel PJ. Fructose consumption: potential mechanisms for its effects to increase visceral adiposity and induce dyslipidemia and insulin resistance. Curr Opin Lipidol 2008;19:16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanhope KL, Havel PJ. Fructose consumption: recent results and their potential implications. Ann N Y Acad Sci 2010;1190:15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding EL, Malik VS. Convergence of obesity and high glycemic diet on compounding diabetes and cardiovascular risks in modernizing China: an emerging public health dilemma. Global Health 2008;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrington S. The role of sugar-sweetened beverage consumption in adolescent obesity: a review of the literature. J Sch Nurs 2008;24:3–12 [DOI] [PubMed] [Google Scholar]
- 18.Hu FB, Malik VS. Sugar-sweetened beverages and risk of obesity and type 2 diabetes: epidemiologic evidence. Physiol Behav 2010;100:47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schernhammer ES, Hu FB, Giovannucci E, et al. Sugar-sweetened soft drink consumption and risk of pancreatic cancer in two prospective cohorts. Cancer Epidemiol Biomarkers Prev 2005;14:2098–105 [DOI] [PubMed] [Google Scholar]
- 20.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002;287:2414–23 [DOI] [PubMed] [Google Scholar]
- 21.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990;51:241–7 [DOI] [PubMed] [Google Scholar]
- 22.Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr 2003;77:1171–8 [DOI] [PubMed] [Google Scholar]
- 23.Havel PJ, Townsend R, Chaump L, Teff K. High-fat meals reduce 24-h circulating leptin concentrations in women. Diabetes 1999;48:334–41 [DOI] [PubMed] [Google Scholar]
- 24.Teff KL, Elliott SS, Tschop M, et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab 2004;89:2963–72 [DOI] [PubMed] [Google Scholar]
- 25.Kouzuma T, Usami T, Yamakoshi M, Takahashi M, Imamura S. An enzymatic method for the measurement of glycated albumin in biological samples. Clin Chim Acta 2002;324:61–71 [DOI] [PubMed] [Google Scholar]
- 26.Al-Daghri NM, Al-Attas OS, Al-Rubeaan K, Sallam R. Adipocytokine profile of type 2 diabetics in metabolic syndrome as defined by various criteria. Diabetes Metab Res Rev 2008;24:52–8 [DOI] [PubMed] [Google Scholar]
- 27.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–7 [DOI] [PubMed] [Google Scholar]
- 28.Livesey G, Taylor R, Hulshof T, Howlett J. Glycemic response and health–a systematic review and meta-analysis: relations between dietary glycemic properties and health outcomes. Am J Clin Nutr 2008;87:258S–68S [DOI] [PubMed] [Google Scholar]
- 29.Beysen C, Murphy EJ, McLaughlin T, et al. Whole-body glycolysis measured by the deuterated-glucose disposal test correlates highly with insulin resistance in vivo. Diabetes Care 2007;30:1143–9 [DOI] [PubMed] [Google Scholar]
- 30.Adiels M, Olofsson SO, Taskinen MR, Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol 2008;28:1225–36 [DOI] [PubMed] [Google Scholar]
- 31.Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res 2002;43:1363–79 [DOI] [PubMed] [Google Scholar]
- 32.Packard CJ, Shepherd J. Lipoprotein heterogeneity and apolipoprotein B metabolism. Arterioscler Thromb Vasc Biol 1997;17:3542–56 [DOI] [PubMed] [Google Scholar]
- 33.Fried SK, Russell CD, Grauso NL, Brolin RE. Lipoprotein lipase regulation by insulin and glucocorticoid in subcutaneous and omental adipose tissues of obese women and men. J Clin Invest 1993;92:2191–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolever TM, Jenkins DJ, Jenkins AL, Josse RG. The glycemic index: methodology and clinical implications. Am J Clin Nutr 1991;54:846–54 [DOI] [PubMed] [Google Scholar]
- 35.Thomas D, Elliott EJ. Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst Rev 2009;CD006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med 2004;141:421–31 [DOI] [PubMed] [Google Scholar]
- 37.Barclay AW, Petocz P, McMillan-Price J, et al. Glycemic index, glycemic load, and chronic disease risk—a meta-analysis of observational studies. Am J Clin Nutr 2008;87:627–37 [DOI] [PubMed] [Google Scholar]
- 38.Levitan EB, Mittleman MA, Hakansson N, Wolk A. Dietary glycemic index, dietary glycemic load, and cardiovascular disease in middle-aged and older Swedish men. Am J Clin Nutr 2007;85:1521–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liese AD, Schulz M, Fang F, et al. Dietary glycemic index and glycemic load, carbohydrate and fiber intake, and measures of insulin sensitivity, secretion, and adiposity in the Insulin Resistance Atherosclerosis Study. Diabetes Care 2005;28:2832–8 [DOI] [PubMed] [Google Scholar]
- 40.Meyer KA, Kushi LH, Jacobs DR, Jr, Slavin J, Sellers TA, Folsom AR. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr 2000;71:921–30 [DOI] [PubMed] [Google Scholar]
- 41.Stevens J, Ahn K. Juhaeri, Houston D, Steffan L, Couper D. Dietary fiber intake and glycemic index and incidence of diabetes in African-American and white adults: the ARIC study. Diabetes Care 2002;25:1715–21 [DOI] [PubMed] [Google Scholar]
- 42.Vrolix R, Mensink RP. Effects of glycemic load on metabolic risk markers in subjects at increased risk of developing metabolic syndrome. Am J Clin Nutr 2010;92:366–74 [DOI] [PubMed] [Google Scholar]
- 43.Vrolix R, van Meijl LE, Mensink RP. The metabolic syndrome in relation with the glycemic index and the glycemic load. Physiol Behav 2008;94:293–9 [DOI] [PubMed] [Google Scholar]
- 44.Dominguez Coello S, Cabrera de Leon A, Rodriguez Perez MC, et al. Association between glycemic index, glycemic load, and fructose with insulin resistance: the CDC of the Canary Islands study. Eur J Nutr 2010;49:505–12 [DOI] [PubMed] [Google Scholar]
- 45.Segal MS, Gollub E, Johnson RJ. Is the fructose index more relevant with regards to cardiovascular disease than the glycemic index? Eur J Nutr 2007;46:406–17 [DOI] [PubMed] [Google Scholar]
- 46.Marriott BP, Olsho L, Hadden L, Connor P. Intake of added sugars and selected nutrients in the United States, National Health and Nutrition Examination Survey (NHANES) 2003-2006. Crit Rev Food Sci Nutr 2010;50:228–58 [DOI] [PubMed] [Google Scholar]