Abstract
Background: Indexes of diet quality have been shown to be associated with decreased risk of mortality in several countries. It remains unclear if the Alternative Healthy Eating Index (AHEI), designed to provide dietary guidelines to combat major chronic diseases, is related to mortality risk.
Objective: We aimed to examine the association between adherence to the AHEI and cause-specific mortality over 18 y of follow-up in a British working population.
Design: Analyses are based on 7319 participants (mean age: 49.5 y; range: 39–63 y; 30.3% women) from the Whitehall II Study. Cox proportional hazards regression models were performed to analyze associations of the AHEI (scored on the basis of intake of 9 components: vegetables, fruit, nuts and soy, white or red meat, trans fat, polyunsaturated or saturated fat, fiber, multivitamin use, and alcohol) with mortality risk.
Results: After potential confounders were controlled for, participants in the top compared with the bottom third of the AHEI score showed 25% lower all-cause mortality [hazard ratio (HR): 0.76; 95% CI: 0.61, 0.95] and >40% lower mortality from cardiovascular disease (CVD; HR: 0.58; 95% CI: 0.37, 0.91). Consumption of nuts and soy and moderate alcohol intake appeared to be the most important independent contributors to decreased mortality risk. The AHEI was not associated with cancer mortality or noncancer/non-CVD mortality.
Conclusion: Our findings suggest that the encouragement of adherence to the AHEI dietary recommendations constitutes a valid and clear public health recommendation that would decrease the risk of premature death from CVD.
INTRODUCTION
Several studies have examined the association between diet and mortality through consideration of an overall diet approach (1). One line of research has attempted to identify “a posteriori” eating patterns with the use of methods such as cluster analysis (2), decreased rank regression (3), or principal components analysis (4). Another approach has exploited validated diet quality indexes (5–11) that have the advantage that they are based on existing knowledge on “healthy eating,” which has allowed comparisons between studies, and has provided clear food guideline recommendations (1). However, very few studies have investigated whether adherence to a specific set of dietary guidelines is associated with a decreased risk of mortality from all causes and from cardiovascular disease (CVD). This is especially pertinent in the British context, where rates of CVD mortality continue to exceed those of most other Western nations (http://www.who.int/research/en/).
The Alternative Healthy Eating Index (AHEI) is a particularly relevant target for mortality research because it was originally designed to provide dietary guidelines with the specific intention to combat major chronic diseases (12). High scores on this index have been shown to be associated with decreased risk of CVD (13) and type 2 diabetes (12) in a US population, and previous findings from the British Whitehall II study (14) suggest that adherence to the AHEI is related to an ≈2-fold higher odds of reversal of the metabolic syndrome, a condition known to be a strong predictor of cardiovascular morbidity and mortality (15). Surprisingly, there has been little attempt to directly examine the AHEI–mortality association and hence validate its presumed efficacy as a public health intervention strategy. To our knowledge, the only previous study on the AHEI and mortality was limited to deaths from colorectal cancer (16) and we are not aware of prior investigations that have examined the long-term effect of the AHEI on overall mortality and cause-specific mortality, especially in a large, middle-aged population.
The present report sought to examine the association between adherence to the AHEI and mortality risk over 18 y of follow-up of >7000 British civil servants, the Whitehall II cohort study. A further aim was to investigate which components of the AHEI contributed most to any decrease in mortality rates in this population.
SUBJECTS AND METHODS
Participants and design
The target population for the Whitehall II study was all London-based office staff, aged 35–55 y, who worked in 20 civil service departments (17). Baseline screening (phase 1: 1985–1988, n = 10,308) involved a clinical examination and a self-administered questionnaire. Subsequent phases of data collection have alternated between a clinical examination alongside a questionnaire survey and a postal questionnaire alone. Phase 3 (1991–1993) is considered the baseline for the purpose of this study, because it was the first assessment of the AHEI. The University College London Ethics Committee approved the study. After the subjects were given a complete description of the study, written informed consent was obtained from all participants.
Baseline examination
Dietary intake at phase 3 was assessed with the use of a semiquantitative food-frequency questionnaire (FFQ) with 127 food items, as described previously (2, 14, 18). The selected frequency category for each food item was converted to a daily intake. Nutrient intakes were computed by multiplication of the consumption frequency for each food by its nutrient content (for specified portions), and then the nutrient contributions from all foods were summed. Frequency of consumption for multivitamin supplements was also collected. Nutrient values were calculated with the use of a computerized system developed for the Whitehall II dietary data. Information on this can be found in the supplemental Appendix materials under “Supplemental data” in the online issue.
The AHEI (12) was scored on the basis of the intake levels of 9 components. The original components of the index include vegetables, fruit, nuts and soy, the ratio of white (seafood and poultry) to red meat, cereal fiber, trans fat, the ratio of polyunsaturated fatty acids to saturated fatty acids, long-term multivitamin use (<5 or ≥5 y), and alcohol consumption. Because cereal fiber was not available in our nutrient data set, we adapted the score by the replacement of it with total fiber. Each component had the potential to contribute 0–10 points to the total score, with the exception of multivitamin use, which contributed either 2.5 or 7.5 points (see Tables S1 and S2 under “Supplemental data” in the online issue). All the component scores were summed to obtain a total AHEI score, which ranged from 2.5 to 87.5; higher scores corresponded to a healthier diet (Table S1 under “Supplemental data” in the online issue). Correlations between AHEI components and with total AHEI score are shown in Table S3 under “Supplemental data” in the online issue.
Covariates were measured at phase 3. Sociodemographic variables consisted of age; sex; ethnicity (white, South Asian,black); marital status (married or cohabiting, single, divorced, widowed); and occupational position with the use of current (or last for retired participants) British civil service employment grade, defined on the basis of salary and grouped into 3 categories: high (senior administrators)/intermediate (executives, professionals, and technical staff)/low (clerical and office support staff) grades.
Health behaviors included in the analysis were smoking (current/former/nonsmoker), total energy intake estimated from the FFQ, and physical activity assessed via questionnaire data and categorized into 3 groups (high, intermediate, low) according to frequency of participation in “vigorous” (eg, running, hard swimming, playing squash), “moderately energetic” (eg, dancing, cycling, leisurely swimming), and “mildly energetic” physical activity.
Baseline health status (phase 3) was ascertained with the use of a number of measures: prevalence of coronary heart diseases (CHD) on the basis of clinically verified events, which included nonfatal myocardial infarction and definite angina; self-reported stroke or transient ischemic attack; diabetes (diagnosed with the use of the World Health Organization definition); hypertension (systolic or diastolic blood pressure ≥ 140 or ≥ 90 mm Hg, respectively, or use of hypertensive drugs); dyslipidemia (LDL cholesterol ≥ 4.1 mmol/L or use of lipid-lowering drugs); body mass index (BMI; in kg/m2) [underweight, BMI < 20; normal (reference), BMI ≥20 to <25; overweight, BMI ≥25 to <30 ; obese, BMI ≥30]; metabolic syndrome defined with the use of the National Cholesterol Education Program criteria (19); and inflammatory markers, interleukin-6 and C-reactive protein, measured from serum stored at −70°C; procedures are detailed elsewhere (20).
Mortality follow-up
The study is linked to the National Health Services death and electronic patient records with the use of the National Health Services identification number assigned to all British citizens. A total of 10,297 participants (99.9%) were successfully traced and have been followed through these registers. Mortality data (median: 17.7 y, range: 0.08–18.4), which included the cause of death, were available through the National Health Services Central Registry until 31 January 2010. Death certificates were coded with the use of the 9th or 10th revision of the International Classification of Diseases (ICD) (http://www.who.int/classifications/icd/en/). We analyzed all-cause mortality and mortality from specific causes, such as CVD (ICD-9 codes 390.0–458.9 and ICD-10 codes I00–I99) and cancer (ICD-9 140.0–209.9 and ICD-10 C00–C97). Noncancer/non-CVD mortality includes all deaths that remain that are not classified as cancer or CVD, and included deaths from diseases of the respiratory system (most common other cause of death), digestive system, or nervous system; injuries; poisoning; and external causes.
Statistical methods
We generated survival curves with the use of actuarial life table methods with Wilcoxon tests to compare survival between AHEI tertile groups, as shown in Figure 1. Associations between tertiles (thirds) of the AHEI score distribution and the risk of mortality and cause-specific mortality were determined by Cox proportional hazards regression and were adjusted for sociodemographic factors, health behaviors, and health factors. The proportional hazards assumption was verified by the addition of a time-dependent variable to the model and was shown not to be violated. Interactions between the AHEI and covariates on all-cause mortality were tested and were not shown to be statistically significant at P < 0.05. A set of analyses that corresponded was also performed to examine the separate association between each AHEI component score (standardized by the use of the z score) and mortality risk.
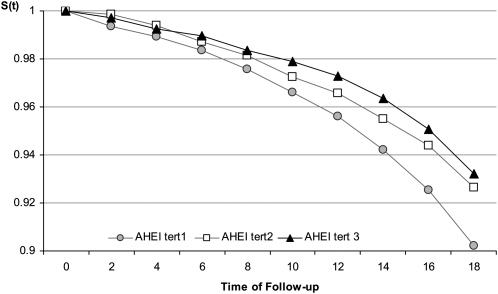
FIGURE 1.
Survival distribution over 18 y of follow-up as a function of Alternative Healthy Eating Index (AHEI) tertiles (tert) for the 7319 Whitehall II participants. Tertile 1—mean ± SD: 36.5 ± 6.3; median (range): 37.5 (13.5−44.5). Tertile 2—mean ± SD: 50.6 ± 3.1; median (range): 50.5 (45.5−55.5). Tertile 3—mean ± SD: 63.3 ± 5.3; median (range): 62.5 (56.5−85.5). Survival curves were generated with the use of actuarial life table methods with Wilcoxon tests to compare survival between AHEI tertile groups. S(t), survival function.
To assess whether the overall AHEI score predicts mortality better than the sum of its 9 components, we compared the goodness-of-fit of 2 nested models [model 1: Y = βt* (total AHEI score) compared with model 2: Y= Σ (βi* component i)] with the use of a likelihood ratio test. Further analyses were performed to examine the contribution of AHEI components to the association between the AHEI and mortality risk. For each component (component i), we computed a modified AHEI score on the basis of the total AHEI score without the component i (modified AHEI score i = total AHEI score − score of the component i). Separate Cox proportional hazards regression models were performed, in which each component, adjusted for the modified AHEI score that corresponded to it, was included. These analyses allowed us to examine whether some specific AHEI components would independently predict all-cause mortality risk. Similar analyses were conducted for CVD mortality. In these analyses, adjusted for potential confounders, all component scores and modified AHEI scores were standardized with the use of z scores (mean = 0, SD = 1). Analyses were conducted with the use of SAS software, version 9.2 (SAS Institute, Cary, NC).
RESULTS
The analyses were carried out on 7319 participants who had complete data on mortality status, dietary assessment, and other covariates. Compared with these participants, who were included in the present analyses, those excluded were slightly older, more likely to be women, and of lower occupational grade.
Over the 18-y follow-up, 534 participants (7.3%) died. Analyses of cause of death (available for 7312 participants) showed that the majority of participants died of cancer (49.1%, n = 259) or CVD (26.8%, n = 141). Among the 141 CVD deaths, 52.5% were caused by CHD (n = 74) and 19.9% by stroke (n = 28). Of the 134 deaths that remained, 127 were classified as noncancer/non-CVD death (for 7 deaths, information on cause of death was missing). Comparison of characteristics of the participants by vital status is shown in Table 1.
TABLE 1.
Characteristics of participants according to survival status over 18 y of follow-up
| Covariates measured at phase 3 | Alive (n = 6785) | Deceased (n = 534) | P value1 |
| Age (y) | 49.3 ± 6.02 | 52.9 ± 5.9 | <0.001 |
| Female sex (%) | 30.3 | 30.3 | 0.98 |
| Married/cohabiting (%) | 77.3 | 73.8 | 0.06 |
| Ethnicity (%) | 0.74 | ||
| White | 91.8 | 90.8 | |
| South Asian | 5.0 | 5.6 | |
| Black | 3.2 | 3.6 | |
| Occupational grade (%) | <0.001 | ||
| Low | 15.0 | 21.1 | |
| Intermediate | 45.8 | 42.9 | |
| High | 39.2 | 36.0 | |
| Smoking habit (%) | <0.001 | ||
| Nonsmoker | 51.4 | 40.5 | |
| Former smoker | 34.6 | 36.5 | |
| Current smoker | 14.0 | 23.0 | |
| Total energy intake (kcal/d) | 2101 ± 625 | 2052 ± 630 | 0.08 |
| Physical activity (%) | |||
| Low | 12.7 | 16.7 | 0.006 |
| Intermediate | 54.0 | 47.9 | |
| High | 33.4 | 35.4 | |
| BMI (%) | <0.001 | ||
| Underweight (<20 kg/m2) | 4.5 | 3.9 | |
| Normal (≥20 to <25 kg/m2) | 49.5 | 42.7 | |
| Overweight (≥25 to <30 kg/m2) | 37.3 | 38.8 | |
| Obese (≥30 kg/m2) | 8.7 | 14.6 | |
| C-reactive protein tertiles (%)3 | <0.001 | ||
| Highest tertile | 34.1 | 22.9 | |
| Intermediate tertile | 33.6 | 29.1 | |
| Lowest tertile | 32.2 | 48.0 | |
| Interleukin-6 tertiles (%)3 | <0.001 | ||
| Highest tertile | 34.4 | 19.8 | |
| Intermediate tertile | 33.6 | 30.5 | |
| Lowest tertile | 32.0 | 49.7 | |
| Metabolic syndrome (%) | 9.3 | 17.2 | <0.001 |
| History of ischemic vascular diseases (%) | 2.5 | 7.1 | <0.001 |
| Dyslipidemia | 58.5 | 65.7 | 0.001 |
| Hypertension | 17.9 | 27.9 | <0.001 |
| Type 2 diabetes | 1.1 | 2.8 | <0.001 |
Chi-square and Student's t tests (for age and total energy intake) were applied to compare characteristics of 7319 participants as a function of mortality status.
Mean ± SD (all such values).
The number of subjects with missing values for C-reactive protein and interleukin-6 were 355 and 402, respectively.
The survival curves diverged as a function of the AHEI tertiles across the entire follow-up period, with the highest mortality seen among participants in the bottom tertile and the lowest mortality among those in the top tertile, as shown in Figure 1. The age- and sex-adjusted hazard ratios (HR) for the top and the intermediate tertile of the AHEI compared with the lowest tertile were 0.65 (95% CI: 0.53, 0.80) and 0.74 (95% CI: 0.60, 0.90), respectively.
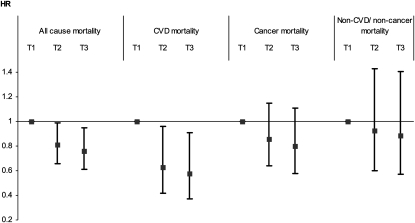
To identify potential confounders or mediators of these relationships, factors associated with AHEI tertiles were identified (Table 2). The multivariable-adjusted Cox proportional hazards models showed the association between AHEI tertiles and risk of all-cause mortality to be robust to adjustment for covariates. After sex, age, ethnic group, marital status, occupational grade, smoking habits, physical activity, and total energy intake were controlled for, to be in the higher tertiles of the AHEI was associated with a decreased risk of all-cause mortality (intermediate compared with bottom tertile HR: 0.79, 95% CI: 0.64, 0.97; top compared with bottom tertile HR: 0.73, 95% CI: 0.58, 0.91) and CVD mortality (intermediate compared with bottom tertile HR: 0.64, 95% CI: 0.42, 0.95; top compared with bottom tertile HR: 0.58, 95% CI: 0.37, 0.89) (results not shown). Further adjustment for BMI categories, inflammatory markers (C-reactive protein and interleukin-6), metabolic syndrome, prevalence of CVD, dyslipidemia, hypertension, and type 2 diabetes did not attenuate these results much (Figure 2). Participants in the highest tertile of the AHEI score had ≈25% lower risk of all-cause mortality (HR: 0.76, 95% CI: 0.61, 0.95) and 40% lower risk of CVD mortality (HR: 0.58, 95% CI: 0.37, 0.91) compared with those in the lowest tertile. Adherence to the AHEI was not associated with cancer mortality (HR: 0.80, 95% CI: 0.58, 1.11) or noncancer/non-CVD deaths (HR: 0.89, 95% CI: 0.57, 1.41) (Figure 2).
TABLE 2.
Cross-sectional associations between baseline characteristics and tertiles of Alternative Healthy Eating Index (AHEI) score
| AHEI tertiles |
||||
| Baseline characteristics | Low (n = 2458) | Intermediate (n = 2477) | High (n = 2384) | P value1 |
| AHEI score | 36.6 ± 6.32 | 50.6 ± 3.1 | 63.3 ± 5.3 | |
| Age (y) | 49.4 ± 6.0 | 49.4 ± 6.1 | 49.8 ± 6.0 | 0.08 |
| Female sex (%) | 24.2 | 28.9 | 38.1 | <0.001 |
| Living alone (%) | 75.4 | 78.7 | 77.1 | 0.02 |
| White (%) | 94.5 | 91.9 | 88.7 | <0.001 |
| High-grade occupational position (%) | 33.2 | 41.8 | 42.0 | <0.001 |
| Current smoker (%) | 20.4 | 13.9 | 9.3 | <0.001 |
| Total energy intake (kcal/d) | 1922 ± 583 | 2137 ± 628.4 | 2236 ± 622 | <0.001 |
| Low physical activity (%) | 14.7 | 12.1 | 12.0 | 0.01 |
| Overweight (%)3 | 38.5 | 37.5 | 36.3 | 0.59 |
| Obese (%)4 | 9.5 | 8.8 | 9.0 | |
| C-reactive protein in highest tertile (%)5 | 38.1 | 32.4 | 29.4 | <0.001 |
| Interleukin-6 in highest tertile (%)5 | 38.0 | 31.9 | 29.7 | <0.001 |
| Metabolic syndrome (%) | 11.3 | 9.0 | 9.3 | 0.02 |
| History of cardiovascular disease (%) | 2.4 | 2.7 | 3.4 | 0.10 |
| Dyslipidemia (%) | 62.0 | 59.0 | 56.1 | <0.001 |
| Hypertension (%) | 19.1 | 17.8 | 19.1 | 0.37 |
| Type 2 diabetes (%) | 1.2 | 1.0 | 1.4 | 0.49 |
| AHEI component scores (points) | ||||
| Vegetables | 3.7 ± 2.4 | 5.6 ± 2.6 | 7.4 ± 2.4 | <0.001 |
| Fruit | 3.7 ± 2.5 | 6.1 ± 2.7 | 8.1 ± 2.2 | <0.001 |
| Nuts and soy | 1.9 ± 2.5 | 3.0 ± 2.9 | 4.6 ± 3.1 | <0.001 |
| Ratio of white to red meat | 3.6 ± 2.4 | 5.1 ± 2.6 | 6.6 ± 2.5 | <0.001 |
| Total fiber | 5.5 ± 3.2 | 8.1 ± 2.6 | 9.3 ± 1.6 | <0.001 |
| trans Fat | 7.6 ± 3.1 | 8.4 ± 2.7 | 9.3 ± 1.9 | <0.001 |
| Ratio of PUFAs to SFAs | 3.8 ± 2.4 | 5.3 ± 2.6 | 6.7 ± 2.4 | <0.001 |
| Duration of multivitamin use | 3.4 ± 1.9 | 4.1 ± 2.4 | 5.1 ± 2.5 | <0.001 |
| Alcohol | 3.2 ± 3.3 | 4.7 ± 3.6 | 6.2 ± 3.6 | <0.001 |
Chi-square test and Fisher's F statistic (for age and total energy intake) were applied to compare characteristics of 7319 participants as a function of tertiles of AHEI score. PUFAs, polyunsaturated fatty acids; SFAs, saturated fatty acids.
Mean ± SD (all such values).
BMI (in kg/m2) ≥25 and <30.
BMI ≥30.
The number of subjects with missing values for C-reactive protein and interleukin-6 were 355 and 402, respectively.
FIGURE 2.
Associations between Alternative Healthy Eating Index (AHEI) tertiles (T) and all-cause and cause-specific mortality over 18 y of follow-up for the 7319 Whitehall II participants. Cox proportional hazards models were adjusted for sex, age, ethnic group, marital status, occupational grade, smoking habits, total energy intake, physical activity, BMI categories, concentrations of inflammatory markers (C-reactive protein and interleukin-6) categorized in tertiles, metabolic syndrome status, prevalence of cardiovascular disease (CVD), dyslipidemia, hypertension, and prevalence of type 2 diabetes status at baseline. For 7 participants, data on cause of death were missing.
The associations between each AHEI component and mortality risk are shown in Table S4 under "Supplemental data" in the online issue. After adjustment for potential confounders, only 4 of the 9 components (nuts and soy, ratio of white to red meat, total fiber, and alcohol) were significantly associated with all-cause mortality risk. A decreased risk of CVD mortality was observed with consumption of nuts and soy and with moderate alcohol consumption.
To examine whether the effect of the total AHEI score on mortality is as strong as the sum of its separate component effects, we first compared a fully adjusted model (model 1), in which total AHEI score was included, with a second model (model 2), in which all of the 9 components of the AHEI score were included separately. Results of the likelihood ratio test indicated that the total AHEI score did not predict mortality risk as well as did the use of each component separately (P = 0.007), as shown in Table S5 under “Supplemental data” in the online issue. In other words, the coefficients for effects of the 9 components that comprised the total AHEI score were not all equal. Model 2 also showed that consumption of nuts and soy, total fiber, and moderate alcohol, and, to a lesser extent, the ratio of white to red meat, remained associated with a decreased risk of all-cause mortality, after adjustment for other AHEI components (Table S5 under “Supplemental data” in the online issue). No association was observed between other components of the AHEI and all-cause mortality risk.
Further analyses were performed to identify which of the AHEI components contributed most to the decreased mortality risk associated with adherence to the AHEI. Cox regression models were performed separately for each component and were adjusted for a modified total AHEI score that excluded the component considered in the analysis. Of the 9 components, consumption of nuts and soy and moderate alcohol intake were significantly associated with a decreased risk of all-cause mortality, independent of the modified AHEI score and after potential confounders were controlled for (Table 3). The observed attenuation of the association between the AHEI computed without the alcohol component and mortality risk suggested that this component makes a major contribution to the association between the AHEI and the risk of both all-cause mortality and CVD mortality. In addition, when the AHEI was computed without the nuts and soy component, a similar attenuation of the association between the modified AHEI and CVD mortality was observed, which highlights the importance of this component in the AHEI in the assessment of CVD mortality risk.
TABLE 3.
Association between Alternative Healthy Eating Index (AHEI) components and all-cause mortality risk and cardiovascular disease (CVD) mortality risk1
| Association between AHEI components and all-cause mortality risk (534 deaths/7319 participants) |
Association between AHEI components and CVD mortality risk (141 CVD deaths/6926 participants) |
|||||||||||
| Effect of AHEI component z score |
Effect of AHEI z score computed without the component |
Effect of AHEI component z score |
Effect of AHEI z score computed without the component |
|||||||||
| AHEI components | HR2 | 95% CI | P value | HR3 | 95% CI | P value | HR2 | 95% CI | P value | HR3 | 95% CI | P value |
| Vegetables | 1.06 | 0.96, 1.16 | 0.27 | 0.83 | 0.76, 0.92 | <0.001 | 0.96 | 0.80, 1.16 | 0.70 | 0.79 | 0.66, 0.96 | 0.02 |
| Fruit | 1.02 | 0.92, 1.12 | 0.75 | 0.85 | 0.77, 0.94 | 0.001 | 1.06 | 0.88, 1.29 | 0.53 | 0.75 | 0.62, 0.91 | 0.003 |
| Nuts and soy | 0.90 | 0.82, 0.99 | 0.03 | 0.89 | 0.81, 0.98 | 0.01 | 0.80 | 0.65, 0.95 | 0.01 | 0.85 | 0.71, 1.01 | 0.07 |
| Ratio of white to red meat | 0.94 | 0.86, 1.03 | 0.19 | 0.88 | 0.80, 0.97 | 0.01 | 0.95 | 0.80, 1.14 | 0.61 | 0.79 | 0.66, 0.96 | 0.02 |
| Total fiber | 0.93 | 0.84, 1.04 | 0.21 | 0.89 | 0.81, 0.98 | 0.02 | 0.91 | 0.74, 1.12 | 0.36 | 0.82 | 0.67, 0.99 | 0.04 |
| trans Fat | 1.07 | 0.98, 1.18 | 0.14 | 0.83 | 0.81, 0.91 | <0.001 | 1.08 | 0.89, 1.30 | 0.44 | 0.75 | 0.62, 0.90 | 0.003 |
| Ratio of PUFAs to SFAs | 1.03 | 0.94, 1.13 | 0.52 | 0.84 | 0.76, 0.93 | <0.001 | 1.02 | 0.86, 1.22 | 0.79 | 0.76 | 0.63, 0.92 | 0.006 |
| Duration of multivitamin use | 0.99 | 0.90, 1.08 | 0.80 | 0.86 | 0.78, 0.94 | 0.002 | 1.03 | 0.87, 1.23 | 0.71 | 0.77 | 0.64, 0.92 | 0.005 |
| Moderate alcohol | 0.84 | 0.77, 0.92 | <0.001 | 0.92 | 0.83, 1.00 | 0.06 | 0.80 | 0.67, 0.96 | 0.01 | 0.84 | 0.70, 1.00 | 0.06 |
| Total AHEI score4 | — | — | — | 0.86 | 0.78, 0.94 | 0.001 | — | — | — | 0.78 | 0.65, 0.94 | 0.008 |
Cox regression models were performed separately for each component and were adjusted for a modified total AHEI score that excluded the component considered in the analysis and for sex, age, ethnicity, occupational grade, marital status, smoking status, total energy intake, physical activity, BMI categories, prevalent CVD, type 2 diabetes, hypertension, dyslipidemia, metabolic syndrome, and inflammatory markers. HR, hazard ratio; PUFAs, polyunsaturated fatty acids; SFAs, saturated fatty acids.
HR of mortality associated with each increase of 1 SD of component score. Cox proportional hazards regression models were performed.
HR of mortality associated with each increase of 1 SD of total AHEI score.
Each AHEI component contributed from 0 to 10 points to the total AHEI score (See Table S1 and Appendix under “Supplemental data” in the online issue), except for the multivitamin component, which was dichotomous and contributed either 2.5 points (for nonuse) or 7.5 points (for use). A score of 10 indicates that the recommendations were fully met, whereas a score of 0 represents the less healthy dietary behavior. Intermediate intakes were scored proportionately between 0 and 10.
DISCUSSION
The results from a large British occupational cohort suggest that adherence to the AHEI is associated with a decreased risk of mortality over an 18-y follow-up. After a wide range of sociodemographic factors, health behaviors, and health status measures (which included major cardiovascular and metabolic risk factors and inflammatory markers) were controlled for, participants in the highest third of the AHEI score had ≈25% lower risk of death from all causes and >40% lower risk of death from CVD, compared with those in the lowest third. There was evidence that the 9 components of the AHEI did not predict mortality equally. Of these components, alcohol, nuts and soy, total fiber, and, to a lesser extent, ratio of white to red meat were associated with a decreased risk of mortality independent of other components. Our analyses highlight particularly the major role of the alcohol and nuts and soy components in the association between the AHEI and decreased risk of mortality. Our findings are consistent with those of several studies that have investigated other diet-quality scores and mortality. The majority of these investigations focused on the Mediterranean diet score, which measures adherence to a traditional Greek Mediterranean type of diet (11) characterized by high intake of fruit, vegetables, cereal, potatoes, and fish; high ratio of polyunsaturated fatty acids to saturated fatty acids; low intake of meat (white and red) and dairy products; and moderate alcohol consumption. Those studies support an inverse association between Mediterranean diet score and all-cause, CHD, and cancer mortality (21). Although the Mediterranean diet has similarities to the AHEI, the use of the latter in a British population can be justified by the closer match between traditional British and US diets. Indeed, a previous report from the present cohort showed no evidence of beneficial effects of the “Mediterranean-like” diet pattern identified by cluster analysis (2).
Our findings emphasize the importance of a high intake of nuts and soy, a high ratio of white to red meat, a high intake of fiber, and moderate alcohol intake in the decrease of all-cause mortality. These findings are in agreement with results from other studies that have shown a decreased risk of all-cause mortality in relation to higher dietary intakes of fiber (22). The protective effect of moderate alcohol consumption on all-cause and CVD mortality, shown to be independent of other AHEI components, is also consistent with results of a meta-analysis that confirmed the beneficial effect of moderate drinking in terms of survival (23). With regard to meat consumption, the high ratio of white to red meat shown to be associated with a decreased risk of all-cause but not CVD mortality in our study is in accordance with the findings of a recent study carried out among more than half a million Americans (24), in which high consumption of red meat was associated with an increased risk of all-cause and CVD mortality, whereas high consumption of white meat was associated with a decreased risk of all-cause mortality but with an increased risk of CVD mortality.
Some AHEI components were not shown to be associated with mortality risk. This was the case, for example, for the trans fat component, for which healthy rates were unexpectedly observed in the lowest AHEI tertile. In 1980, mean intake of trans fat in the Nurses’ Health Study was estimated to be 2.2% of energy intake (25), compared with 0.4% in the present study (results not shown). The original cutoffs we used (≤0.05% of total energy intake), although considered to be ideal in the original score, may not be appropriate in this British cohort and may explain the absence of association in our study. Similarly, the high consumption of fruit and vegetables, even among participants in the lowest tertile of the AHEI scores, shows a ceiling effect, which may explain why the fruit and vegetable components were not associated with mortality. We observed a protective effect on both all-cause and CVD mortality for consumption of nuts and soy products. These are the 2 important sources of protein in vegetarian diets (12), and the intake of dietary fiber (the component most strongly correlated with fruit and vegetable consumption in our study) may partially account for the beneficial effect of a diet rich in fruit and vegetables (12).
In this report we also sought to examine which components contributed most to the association observed between overall diet and mortality. The attenuation of the association between modified AHEI score computed without the alcohol component and mortality risk highlights evidence that moderate alcohol intake substantially contributes to the decrease in mortality associated with the AHEI score. Similarly, our study highlighted the high contribution of the nuts and soy component to the AHEI-CVD mortality relation.
Our study was limited by the assessment of dietary intake. We used a semiquantitative FFQ that covers only specific foods and is recognized to be less precise than dietary assessment by diary records. However, we have shown previously that nutrient intake estimated by the FFQ method is well correlated with biomarker concentrations and with intake estimates from the generally more accurate 7-d diary in this study (18). To measure overall diet we decided to derive dietary patterns theoretically by the application of an “a priori” approach. Because the AHEI was originally designed for American populations, the cutoff used for some components, such as the one for trans fat, might not be optimal to assess the association between trans fat intake and health outcomes in a British population and should be reevaluated. However, compared with the use of indexes such as the Mediterranean diet score (in which the cutoffs used for 7 of its 9 items are defined in accordance with the median of the food/nutrient intakes in the population studied) or other approaches (in which dietary patterns are derived “a posteriori” with the use of data-driven methods), the AHEI provides clear and concrete guidelines, which allows comparison between studies.
The extent to which our results are generalizable is an important consideration. Whitehall II study participants are mainly white, office-based civil servants who worked in London at study baseline (17). Because low socioeconomic status has been shown to be related to high-risk health behavior (which includes diet) and a higher rate of mortality in this cohort, the results may represent an underestimation of the association between AHEI and mortality risk reported in the general population.
To the best of our knowledge, this study is the first to provide epidemiologic evidence that adherence to dietary recommendations of the AHEI may decrease the long-term risk of all-cause and CVD mortality. Our findings emphasize the benefit of adherence to the AHEI and suggest that encouragement of the consumption of nuts and soy products and white meat instead of red meat, a high fiber intake, and moderate alcohol consumption may have a significant benefit in terms of a decrease in all-cause and cardiovascular mortality. Our analyses underline the importance and usefulness of observational studies to evaluate the benefit of the introduction of dietary recommendations into public health promotion packages.
Supplementary Material
Acknowledgments
We thank all participating men and women in the Whitehall II Study; all participating Civil Service departments and their welfare, personnel, and establishment officers; the Occupational Health and Safety Agency; and the Council of Civil Service Unions. The Whitehall II Study team comprises research scientists, statisticians, study coordinators, nurses, data managers, administrative assistants, and data entry staff, who make the study possible.
The authors’ responsibilities were as follows—TNA, JEF, EJB, JH, MGM, AS-M, MJS and MK: designed the research; TNA and MK: conducted the research; TNA: analyzed the data and performed statistical analysis; TNA and MK: drafted the manuscript; TNA: had primary responsibility for final content; and JEF, CB, EJB, JH, MGM, AS-M, KR, and MJS: critically revised the manuscript for important intellectual content. The sponsors were not influential in the study design, data collection, analysis, interpretation of results, or writing of the manuscript. None of the authors declared a conflict of interest.
REFERENCES
- 1.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 2002;13:3–9 [DOI] [PubMed] [Google Scholar]
- 2.Brunner EJ, Mosdol A, Witte DR, et al. Dietary patterns and 15-y risks of major coronary events, diabetes, and mortality. Am J Clin Nutr 2008;87:1414–21 [DOI] [PubMed] [Google Scholar]
- 3.Heroux M, Janssen I, Lam M, et al. Dietary patterns and the risk of mortality: impact of cardiorespiratory fitness. Int J Epidemiol 2010;39:197–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heidemann C, Schulze MB, Franco OH, van Dam RM, Mantzoros CS, Hu FB. Dietary patterns and risk of mortality from cardiovascular disease, cancer, and all causes in a prospective cohort of women. Circulation 2008;118:230–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008;168:713–20 [DOI] [PubMed] [Google Scholar]
- 6.Huijbregts P, Feskens E, Rasanen L, et al. Dietary pattern and 20 year mortality in elderly men in Finland, Italy, and The Netherlands: longitudinal cohort study. BMJ 1997;315:13–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kant AK, Schatzkin A, Graubard BI, Schairer C. A prospective study of diet quality and mortality in women. JAMA 2000;283:2109–15 [DOI] [PubMed] [Google Scholar]
- 8.Mitrou PN, Kipnis V, Thiebaut AC, et al. Mediterranean dietary pattern and prediction of all-cause mortality in a US population: results from the NIH-AARP Diet and Health Study. Arch Intern Med 2007;167:2461–8 [DOI] [PubMed] [Google Scholar]
- 9.Sjogren P, Becker W, Warensjo E, et al. Mediterranean and carbohydrate-restricted diets and mortality among elderly men: a cohort study in Sweden. Am J Clin Nutr 2010;92:967–74 [DOI] [PubMed] [Google Scholar]
- 10.Trichopoulou A, Bamia C, Trichopoulos D. Mediterranean diet and survival among patients with coronary heart disease in Greece. Arch Intern Med 2005;165:929–35 [DOI] [PubMed] [Google Scholar]
- 11.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 2003;348:2599–608 [DOI] [PubMed] [Google Scholar]
- 12.McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr 2002;76:1261–71 [DOI] [PubMed] [Google Scholar]
- 13.Fung TT, McCullough M, van Dam RM, Hu FB. A prospective study of overall diet quality and risk of type 2 diabetes in women. Diabetes Care 2007;30:1753–7 [DOI] [PubMed] [Google Scholar]
- 14.Akbaraly TN, Singh-Manoux A, Tabak AG, et al. Overall diet history and reversibility of the metabolic syndrome over 5 years: the Whitehall II prospective cohort study. Diabetes Care 2010;33:2339–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sundstrom J, Riserus U, Byberg L, Zethelius B, Lithell H, Lind L. Clinical value of the metabolic syndrome for long term prediction of total and cardiovascular mortality: prospective, population based cohort study. BMJ 2006;332:878–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reedy J, Mitrou PN, Krebs-Smith SM, et al. Index-based dietary patterns and risk of colorectal cancer: the NIH-AARP Diet and Health Study. Am J Epidemiol 2008;168:38–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marmot M, Brunner E. Cohort profile: the Whitehall II study. Int J Epidemiol 2005;34:251–6 [DOI] [PubMed] [Google Scholar]
- 18.Brunner E, Stallone D, Juneja M, Bingham S, Marmot M. Dietary assessment in Whitehall II: comparison of 7 d diet diary and food-frequency questionnaire and validity against biomarkers. Br J Nutr 2001;86:405–14 [DOI] [PubMed] [Google Scholar]
- 19.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97 [DOI] [PubMed] [Google Scholar]
- 20.Elovainio M, Ferrie JE, Singh-Manoux A, et al. Organisational justice and markers of inflammation: the Whitehall II study. Occup Environ Med 2010;67:78–83 [DOI] [PubMed] [Google Scholar]
- 21.Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr 2010;92:1189–96 [DOI] [PubMed] [Google Scholar]
- 22.Streppel MT, Ocke MC, Boshuizen HC, Kok FJ, Kromhout D. Dietary fiber intake in relation to coronary heart disease and all-cause mortality over 40 y: the Zutphen Study. Am J Clin Nutr 2008;88:1119–25 [DOI] [PubMed] [Google Scholar]
- 23.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med 2006;166:2437–45 [DOI] [PubMed] [Google Scholar]
- 24.Sinha R, Cross AJ, Graubard BI, Leitzmann MF, Schatzkin A. Meat intake and mortality: a prospective study of over half a million people. Arch Intern Med 2009;169:562–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu FB, Stampfer MJ, Manson JE, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med 1997;337:1491–9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.