Abstract
A roundtable to discuss the measurement of folate status biomarkers in NHANES took place in July 2010. NHANES has measured serum folate since 1974 and red blood cell (RBC) folate since 1978 with the use of several different measurement procedures. Data on serum 5-methyltetrahydrofolate (5MTHF) and folic acid (FA) concentrations in persons aged ≥60 y are available in NHANES 1999–2002. The roundtable reviewed data that showed that folate concentrations from the Bio-Rad Quantaphase II procedure (Bio-Rad Laboratories, Hercules, CA; used in NHANES 1991–1994 and NHANES 1999–2006) were, on average, 29% lower for serum and 45% lower for RBC than were those from the microbiological assay (MA), which was used in NHANES 2007–2010. Roundtable experts agreed that these differences required a data adjustment for time-trend analyses. The roundtable reviewed the possible use of an isotope-dilution liquid chromatography–tandem mass spectrometry (LC-MS/MS) measurement procedure for future NHANES and agreed that the close agreement between the MA and LC-MS/MS results for serum folate supported conversion to the LC-MS/MS procedure. However, for RBC folate, the MA gave 25% higher concentrations than did the LC-MS/MS procedure. The roundtable agreed that the use of the LC-MS/MS procedure to measure RBC folate is premature at this time. The roundtable reviewed the reference materials available or under development at the National Institute of Standards and Technology and recognized the challenges related to, and the scientific need for, these materials. They noted the need for a commutability study for the available reference materials for serum 5MTHF and FA.
INTRODUCTION
The NHANES program is a valuable source of data on the US population's nutritional and health status. NHANES I (1974–1975) was the first NHANES to measure serum folate concentrations (1). NHANES II began measuring red blood cell (RBC) folate concentrations in 1978–1980. NHANES has continued the measurement of both biomarkers through 2010 and will measure them in 2011–2012.
The US Food and Drug Administration partnered with the National Center for Health Statistics of the Centers for Disease Control and Prevention to convene expert panels in 1983–1984 and 1994 to review folate biomarker data from NHANES II (1976–1980) and NHANES III (1988–1994) (2, 3). Interest in the assessment of folate status increased with the 1996 implementation of folic acid (FA) fortification of cereal grains in the United States (4). NHANES data documented substantial elevations in serum and RBC folate concentrations after fortification (1, 5, 6). In the postfortification era, public health concerns have shifted from inadequate intakes to potential adverse effects associated with excessive FA intakes from fortified foods and dietary supplements (7–10).
The National Center for Health Statistics and the Office of Dietary Supplements of the National Institutes of Health convened a roundtable panel on 15–16 July 2010, in Rockville, MD, to review measurement issues for folate and vitamin B-12 status biomarkers in NHANES that have arisen since the 1994 expert panel review. In this article, we summarize the roundtable's review of 1) the quality of the folate-related measurement procedures that NHANES has used or that are available for future surveys, 2) the quality of the reference measurement procedures and reference materials currently available or under development, and 3) public health considerations for the choice of which, if any, folate-related biomarkers to measure in future NHANES. Other articles in this supplement provide background information on biomarker measurement and use and the roundtable's review of vitamin B-12 status biomarkers (1, 11–16). In a separate, introductory article, we describe how the individual articles within this supplement packet fit within the overall roundtable process (17). We also describe the relevance of the roundtable review and dialogue to clinical and research settings outside the NHANES context.
THE ROUNDTABLE
The roundtable panel included 23 experts in folate and vitamin B-12 assessment, epidemiology, clinical laboratory science, and biostatistics. The roundtable also had 10 scientists from government agencies that conduct and fund folate biomarker measurements in NHANES and develop relevant reference methods and materials.
The roundtable reviewed 3 biomarkers of folate status that NHANES has used: serum and RBC folate and total homocysteine. Because of the increasing interest in folate vitamer measurement (8, 10, 18, 19), the panel also considered these biomarkers’ usefulness for future NHANES. Because vitamin B-12 status is the predominant nutrient determinant of high total homocysteine concentrations among the US elderly (13, 20–22), the article on vitamin B-12 status measurements in NHANES describes the roundtable's review of total homocysteine measurements in NHANES (16).
The roundtable reviewed folate-related measurement quality and public health issues in the context of the NHANES mission and capabilities. Roundtable members did not consider broader research and clinical application issues or the differences between the measurement procedures that research, clinical, and commercial laboratories use. The roundtable did identify and discuss the scientific issues involved in the decision as to whether to include folate-related biomarkers in NHANES, but did not make policy recommendations. When we present serum and RBC folate data in this article, we express values in nmol/L (1.0 ng/mL = 2.266 nmol/L).
MEASUREMENT OF FOLATE BIOMARKERS IN NHANES
Barry Shane (12) described serum and RBC folate concentration measurement. The measurement of folate is complicated because biological (vitamin) activity is associated with many folate forms that vary by oxidation state of the pterin ring, one-carbon substitutes, and glutamate chain length. These multiple forms can also produce many folate breakdown products that lack biological activity but can complicate measurement.
Historically, nutrition scientists have favored the microbiological assay (MA) because, with appropriate sample processing, it measures all biologically active folate species but not degradation products that lack biological activity (12). In the 1970s and 1980s, commercial kits that used protein-binding procedures became popular because of their ease of use and high throughput. Recently, liquid chromatography–tandem mass spectrometry (LC-MS/MS) measurement procedures have gained interest because of their potential to quantify folate vitamers. Because NHANES has used the MA and competitive radio protein-binding measurement procedures and is considering the use of LC-MS/MS in the future, the roundtable reviewed the use of these 3 procedures in NHANES.
NHANES began the measurement of folate biomarkers in 1974–1975 with the use of an MA (Table 1); however, the roundtable focused primarily on NHANES measures after the 1991 introduction of the Bio-Rad Quantaphase II competitive protein-binding measurement procedure (Bio-Rad Laboratories, Hercules, CA). Previous expert panels have reviewed the measurement quality and comparability issues associated with MA in 1974–1975 and 1976–1978, the conversion to the Bio-Rad I kit in 1978, and the conversion to the Bio-Rad II kit in 1991 (2, 3).
TABLE 1.
Measurement of serum and red blood cell folate in NHANES1
| Survey date | Measurement procedure | Matrix | Laboratory | Population age |
| 1974–1975 | Microbiological | Serum | NCEH | 25–74 y |
| 1976–1978 | Microbiological | Serum | NCEH | 6 mo–74 y, subset |
| 1978–1980 | Bio-Rad QP I | Serum, WB lysate | NCEH | 6 mo–74 y, subset |
| 1988–1991 | Bio-Rad QP I | Serum, WB lysate | NCEH | ≥4 y |
| 1991–1994 | Bio-Rad QP II | Serum, WB lysate | NCEH | ≥4 y |
| 1999–2000 | Bio-Rad QP II | Serum, WB lysate | NCEH | ≥3 y |
| 2001–2002 | Bio-Rad QP II | Serum, WB lysate | NCEH | ≥3 y |
| 2003–2004 | Bio-Rad QP II | Serum, WB lysate | NCEH | ≥1 y |
| 2005–2006 | Bio-Rad QP II | Serum, WB lysate | NCEH | ≥1 y |
| 2007–2008 | Microbiological | Serum, WB lysate | NCEH | ≥1 y |
| Microbiological | Serum, WB lysate | Trinity, UAB | One-third subset, ≥1 y | |
| LC-MS/MS | Serum, WB lysate | NCEH | One-third subset, ≥1 y | |
| 2009–2010 | Microbiological | Serum, WB lysate | NCEH | ≥1 y |
| LC-MS/MS | Serum, WB lysate | NCEH | One-third subset, ≥1 y |
LC-MS/MS, liquid chromatography–tandem mass spectrometry; NCEH, Division of Laboratory Science, National Center for Environmental Health, Centers for Disease Control and Prevention; Bio-Rad QP, Bio-Rad Quantaphase radio protein-binding assay (Bio-Rad Laboratories, Hercules, CA); Trinity, Trinity College, Dublin, Ireland; UAB, University of Alabama at Birmingham, AL; WB, whole blood.
The Bio-Rad measurement procedure (NHANES 1991ndash1994 and 1999ndash2006)
Christine Pfeiffer of the Centers for Disease Control and Prevention's National Center for Environmental Health (NCEH) described the use of the Bio-Rad measurement procedure in NHANES. Details on the laboratory procedures and quality control results are available elsewhere (23–27). The Bio-Rad procedure is precise. The Bio-Rad Quantaphase II (1991–1994 and 1999–2006) showed CVs of 4–8% for serum folate across a range of ≈2–30 nmol/L, and 4–6% for RBC folate across a range of ≈75–1100 nmol/L (5, 23–27). In surveys that have used the Bio-Rad kits, the quality control pools for serum and RBC folate have been stable and reproducible (23–27).
However, the Bio-Rad procedure has had accuracy problems. The NCEH compared its Bio-Rad results with the assigned values of the reference materials that became available in 2006. The National Institute of Standards and Technology (NIST) standard reference material (SRM) 1955 has information values for 3 levels of serum total folate concentrations based on the LC-MS/MS, MA, and Bio-Rad measurement procedures (28). Compared with the SRM 1955 LC-MS/MS–derived values for total folate, the NCEH Bio-Rad results were ≈25% lower than the low and medium SRM 1955 materials (level 1 at 6.0 nmol/L and level 2 at 13.1 nmol/L) and ≈40% lower than the high concentration material (level 3 at 41 nmol/L). NCEH observed similar differences between the MA-derived SRM 1955 information values and its NCEH Bio-Rad–based results. In the National Institute for Biological Standards and Control (NIBSC; http://www.nibsc.ac.uk/) reference materials, the NCEH Bio-Rad results were ≈10% lower for serum (NIBSC 03/178 with an assigned concentration of 12.1 nmol/L by LC-MS/MS) (29) and ≈45% lower for whole blood (NIBSC 95/528 with an assigned concentration of 29.4 nmol/L by consensus value that several laboratories measured with the use of MA and radioimmunoassay procedures) (30). In the College of American Pathologists (http://www.cap.org/apps/cap.portal) Ligand Survey, NCEH's Bio-Rad results were ≈40% lower than those of the All Laboratory Trimmed Mean for serum and ≈50% lower for RBC folate. The All Laboratory Trimmed Mean is composed of results from all participating measurement procedures and, as a result, can fluctuate as different measurement procedures become part of the proficiency testing scheme. Because the Bio-Rad measurement procedure did not have enough respondents for the summary report to include as a peer group, the NCEH results could not be compared with the method-specific peer group results.
A likely contributor to the lower serum total folate concentrations with the Bio-Rad procedure is underrecovery of 5-methyltetrahydrofolic acid (5MTHF), the predominant folate species in serum and RBC (31, 32). In fortification and recovery studies with the Bio-Rad procedure, NCEH observed 5MTHF recoveries of ≈60% for serum and ≈50% for whole blood (Table 2). Although FA recovery was almost complete, the measured recovery of other minor folate species was much lower or higher than desirable. The UK National External Quality Assessment Service program obtained similar results in an independent spiking recovery experiment for 5MTHF and FA (33).
TABLE 2.
Fortification and recovery results for the Bio-Rad Quantaphase II measurement procedure and the microbiological assay1
| Bio-Rad Quantaphase II |
Microbiological assay |
|||
| Folate species | Serum | WB | Serum | WB |
| 5MTHF | 61 ± 9 | 51 ± 4 | 88 ± 9 | 97 ± 11 |
| FA | 91 ± 10 | 99 ± 5 | 69 ± 3 | — |
| 5FTHF | 38 ± 14 | 18 ± 0.1 | 120 ± 9 | 124 ± 7 |
| 5,10MethenylTHF | 234 ± 32 | 115 ± 10 | 101 ± 7 | 107 ± 13 |
| THF | 106 ± 27 | 152 ± 19 | 36 ± 10 | 46 ± 8 |
All values are percentages ± SDs. Serum results are from reference 31; recoveries of the microbiological assay and Bio-Rad Quantaphase II (Bio-Rad Laboratories, Hercules, CA) methods were tested over 2 d by the addition of each of the 5 folate calibrators (Merck Eprova; Merck & Cie, Schaffhausen, Switzerland) at 10 nmol/L to a serum pool (21.8 nmol total folate/L by liquid chromatography–tandem mass spectrometry). Whole-blood (WB) results are from reference 32; recoveries were measured over 3 d. 5MTHF, 5-methyltetrahydrofolic acid; FA, folic acid; 5FTHF, 5-formyltetrahydrofolic acid; 5,10MethenylTHF, 5,10-methenyltetrahydrofolic acid; THF, tetrahydrofolic acid.
The roundtable noted that unlike the MA that detects all biologically active folate species equally, the competitive binding procedures bind different folate species with different affinities. This is problematic not only because it adversely affects accuracy but also because the relative one-carbon distribution of folate forms in RBCs can differ by genotype between individuals. The only way to correct these binding affinity differences is through the use of a separate standard for each folate species in the sample. Manufacturers sometimes change commercial kit standards in ways that users cannot control or know of, matrix effects can affect results, and kits’ limited analytic range may be inadequate for the high serum and RBC folate concentrations that are typical in the post-FA fortification era.
Microbiological measurement procedure (NHANES 2007ndash2010)
Characteristics of the microbiological measurement procedure
After the manufacturer discontinued production of the Bio-Rad kits in 2006, NHANES began to use the MA in 2007 (Table 1). Pfeiffer reported on a study in which 3 laboratories analyzed samples from a one-third NHANES subsample (n = 2645 for serum and 2613 for RBC) to ascertain the comparability of results. Serum folate geometric means (±SE) were 39.5 ± 0.42, 59.2 ± 0.68, and 47.7 ± 0.53 nmol/L; the values for RBC folate that corresponded were 1118 ± 8.7, 1384 ± 13.9, and 1377 ± 10.9 nmol/L. Pearson's correlation coefficients (r) when 2 laboratories were compared at a time were 0.801–0.951 for serum and 0.651–0.917 for RBC folate. Bland-Altman relative bias results when 2 laboratories were compared at a time were 19–39% for serum and 1–21% for RBC folate. The main procedural differences between laboratories were use of different folate calibrators and a wild-type, compared with a chloramphenicol-resistant, Lactobacillus rhamnosus microorganism.
Comparisons of MA with Bio-Rad results
As described in the background article by Yetley and Johnson (1), meaningful time-trend analyses with the use of the NHANES data require that method comparability be evaluated. When different measurement procedures produce different results, statistical or other types of adjustments are often needed so that trends over time are reflective of real changes in nutritional status and are not simply due to methodologic artifacts. Because of the differences described above between the MA and Bio-Rad results, the NCEH conducted crossover evaluations for serum (31) and whole-blood folate (32) to assess the need for adjustments between the NHANES-based Bio-Rad (1988–1994 and 1999–2006) and MA (2007–2010) results for time-trend evaluations. The Bio-Rad results were, on average, 29% lower than the MA results for serum (n = 325) and 45% lower for RBC folates (n = 171). The roundtable agreed that the pronounced differences between the Bio-Rad and the MA necessitate data adjustments for time-trend analyses to make the results from the 2 procedures comparable (ie, as if they were done with the same measurement procedure). Without such adjustments, it is unclear what portion of time-trend differences are due to real changes in folate status and what portion are due to differences in measurement procedures.
Pfeiffer and Sempos presented several statistical models for the adjustment of the transition between the Bio-Rad and MA serum folate results. Each model gave a similar R2 of ≈0.95, but the regression line to fit the data varied between models (34). The linear (least-squares) regression model is the simplest, but it tends to overpredict at the tails of the serum folate distribution. The piecewise linear regression model, as performed by Fazili et al (31), requires predefinition of the knot but provides better estimates at the tails, which are important for the estimation of at-risk groups’ prevalence. A more sophisticated approach for a piecewise linear regression through a grid search that identifies the knot yields a very similar fit, but has the advantage of no break in the lines at the knot. Finally, the fractional polynomial model best satisfies the statistical assumptions that underlie these regression models and gives a slightly better fit than the other regression approaches. The roundtable noted that users prefer the model that best fits the data across the entire distribution of serum folate concentrations, rather than the simplest model.
For RBC folate, the methyltetrahydrofolate reductase (MTHFR) C677T polymorphism affects folate vitamer distribution, and the Bio-Rad results depend on the genotype (32, 35). The MA completely recovered folates added to whole-blood hemolysates except for tetrahydrofolic acid (THF) (46%), whereas the Bio-Rad had variable recoveries for several folate vitamers (Table 2). NCEH's Bio-Rad results were 48% lower than its MA results for samples with the MTHFR 677 C/C and C/T genotypes and 31% lower for samples with the T/T genotype. The Bio-Rad procedure's genotype dependence complicates the derivation of a single adjustment equation for NHANES data, which have no MTHFR genotype information. In addition, MA measurements come from whole-blood hemolysates. Calculations to convert results to RBC folate concentrations require the addition and adjustment of a serum folate value for the Bio-Rad–MA measurement procedure differences. Information on the NCHS recommendation on how best to deal with time-trend evaluations for RBC folate can be found on the NHANES website (34).
The LC-MS/MS measurement procedure
The roundtable considered the use of an LC-MS/MS procedure in future NHANES to measure total and individual folate vitamer concentrations in serum and RBCs. The goal is to accurately reflect the presence of all intact biologically active folate forms in vivo. Some folate species are more sensitive than others to oxidation and decomposition during sample processing and storage, which results in interconversions and degradations that make accurate measurement of individual species more of a challenge than measurement of total folate concentrations. For total folate estimates, accurately measured interconversions do not alter total folate concentration results from summing across the individual species (12, 36). However, individual folate species results do not reflect in vivo patterns and quantities after interconversions and degradations during processing and analysis.
Characteristics of the LC-MS/MS measurement procedure
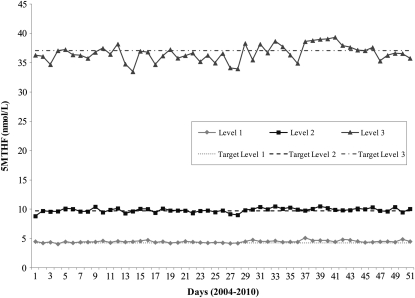
The NCEH LC-MS/MS procedure measures 5MTHF (the predominant folate species in serum and whole blood), FA, 5-formyltetrahydrofolic acid (5FTHF), THF, and 5,10-methenyl-tetrahydrofolic acid (31, 32, 37–39). The NCEH performance characteristics for the LC-MS/MS procedure show good precision (CVs) for total folate and 5MTHF (Table 3). CVs are higher for folate species, such as FA, present in lower concentrations. The NCEH procedure yields almost complete recovery of all folate species with the use of the respective stable-isotope–labeled internal standards. The LC-MS/MS procedure showed consistent performance over several years, and always matched the certified values for 5MTHF in the 3-level NIST SRM 1955 (Figure 1) and the assigned values for total folate, 5MTHF, FA, and 5FTHF in the one-level NIBSC 03/178 reference material (Figure 2).
TABLE 3.
Liquid chromatography–tandem mass spectrometry measurement performance characteristics of serum and red blood cell folates1
| Compound | Matrix | Imprecision2 | Recovery4 | LOD |
| % | % | nmol/L | ||
| Total folate | Serum | 6–7 | — | — |
| 5MTHF | Serum | 6–8 | 99 ± 6.9 | 0.5 |
| FA | Serum | 13–17 | 108 ± 2.8 | 0.3 |
| 5FTHF | Serum | 9–19 | 101 ± 1.9 | 0.1 |
| Total folate | WB lysate | 4–7 | — | — |
| 5MTHF | WB lysate | 5–6 | 92 ± 1.8 | 0.5 |
| FA | WB lysate | 13–19 | 90 ± 3.8 | 0.3 |
| THF | WB lysate | 17–29 | 101 ± 19.7 | 1.0 |
| 5FTHF | WB lysate | 12–17 | 93 ± 1.0 | 0.1 |
| 5,10MethenylTHF | WB lysate | 9–16 | 100 ± 12.3 | 0.3 |
Serum was analyzed over 47 d for imprecision assessment; whole blood (WB) was analyzed over 25 d for imprecision assessment. LOD, limits of detection; 5MTHF, 5-methyltetrahydrofolic acid; FA, folic acid; 5FTHF, 5-formyltetrahydrofolic acid; 5,10MethenylTHF, 5,10-methenyltetrahydrofolic acid; THF, tetrahydrofolic acid.
Source: C Pfeiffer, personal communication, 2011.
Values are mean percentages ± SDs.
FIGURE 1.
Performance of the National Center for Environmental Health, Centers for Disease Control and Prevention, liquid chromatography-tandem mass spectrometry measurement procedure over multiple years with the use of the National Institute of Standards and Technology standard reference material (SRM) 1955 (28). Dotted lines represent the target concentrations for SRM levels 1, 2, and 3. Solid lines track the performance of the measurement procedure for each SRM level between 2004 and 2010. 5MTHF, serum 5-methyltetrahydrofolate.
FIGURE 2.
Performance of the National Center for Environmental Health, Centers for Disease Control and Prevention, liquid chromatography–tandem mass spectrometry measurement procedure over multiple years with the use of the National Institute for Biological Standards and Control reference material 03/178 (29). Dotted lines represent the target concentrations for total folate, 5-methyltetrahydrofolate (5MTHF), folic acid (FA), and 5-formyltetrahydrofolic acid (5FTHF) in this reference material. Solid lines track the performance of the measurement procedure for each folate species and the total folate between 2005 and 2010 (CM Pfeiffer, unpublished observations, 2011).
For serum, Pfeiffer described the results of an NCEH evaluation of the reproducibility of folate species concentrations across multiple measurements with the use of a one-third subsample from NHANES 2007–2008. Pearson's correlation coefficients were high for serum 5MTHF (r = 0.97, n = 1340), FA (r = 0.95, n = 421), and 5FTHF (r = 0.90, n = 1338) between the first and repeat measurement. However, because of THF's poor stability, results showed no significant correlation between the first and repeat measurement (r = 0.04). An assessment of the agreement between the first and repeat measurement with the use of Deming regression analysis showed no proportional or constant bias for serum 5MTHF (repeat measurement = 1.00 × first measurement − 0.36 nmol/L) and 5FTHF (repeat measurement = 1.02 × first measurement + 0.03 nmol/L), whereas serum FA showed a small proportional and constant bias (log repeat measurement = 1.06 × log first measurement − 0.02 nmol/L; NCEH used log transformation because of the distribution's skewness).
The Joint Committee on Traceability in Laboratory Medicine database lists the NCEH isotope-dilution MS/MS procedure as an accepted reference measurement procedure for the measurement of serum 5MTHF, FA, and 5FTHF (40). Because the NCEH LC-MS/MS–based results for serum 5MTHF and FA were similar to results from 3 LC-MS/MS procedures that NIST developed (41), NIST included the NCEH results in the calculation of certified values for 5MTHF and reference values for FA for SRM 1955 (28). The NCEH LC-MS/MS measurement procedure also produced results for FA that were similar to those of an HPLC with electrochemical detection procedure that Steven Bailey of the University of Southern Alabama used [USAL = 0.99 × NCEH + 0.06 nmol/L, r = 0.00, n = 120 (CM Pfeiffer and SW Bailey, personal communication, 2011)]. However, for RBC folate, unresolved issues with the current LC-MS/MS measurement procedure preclude its consideration as a reference method at this time.
Comparison of LC-MS/MS and microbiological results for serum and RBC total folates
For serum total folate, Pfeiffer reported that the NCEH LC-MS/MS results were within ±10% of the MA results in a study (n = 325) of banked NHANES 1999–2004 samples (31) and studies of 120 plasma samples from the University of Southern Alabama Pharmacokinetics study and 48 proficiency testing samples from the National External Quality Assessment Service. The Pearson's correlation coefficients between the 2 measurement procedures were all >0.98 for these studies. With the use of a one-third subset of NHANES 2007–2008 data, the NCEH obtained identical means, medians, minimum and maximum values, and frequency distributions for the MA and LC-MS/MS procedures. Deming regression analysis showed no proportional or constant bias, with a correlation coefficient of 0.95.
Pfeiffer reported that for whole-blood folates, the NCEH MA results were 12% higher than the LC-MS/MS results for 171 samples from US and European blood banks (r = 0.94) (32) and 22% higher for 30 National External Quality Assessment Service samples (r = 0.93). The MA produced 25% higher values than the LC-MS/MS with NHANES 2007–2008 data (n = 295). NCEH continues to investigate its whole-blood extraction procedure because residual amounts of diglutamyl folates may be present in the incubated hemolysate. In addition, a slight loss of THF might occur during the hemolysate incubation that the presence of the internal standard does not compensate for.
The NCEH is also investigating the possibility that 4-α-hydroxy-5MTHF (4hmTHF), an oxidation product of 5MTHF, is generated during the preanalytic phase, hemolysate incubation, or both (42, 43). The current NCEH LC-MS/MS procedure does not separate 4hmTHF from 5FTHF. However, roundtable experts noted that 4hmTHF may be naturally present in serum. Whether 4hmTHF occurs naturally or is a methodologic artifact will determine whether to include it in the folate species summation to estimate total folate concentrations. The roundtable suggested that a study measure compounds individually to determine whether they generate other forms of folate.
The roundtable discussed other factors that may affect the differences that the NCEH observed between the MA and LC-MS/MS results for whole-blood folate. The oxygen in the hemoglobin and thawing of stored samples can alter folate species in whole-blood lysates. Because formylated folates are only found in RBCs from individuals with the TT MTHFR genotype, their presence in the RBCs of individuals with the CC genotype likely indicates the production of artifacts during sample processing or handling (44). Because of the pattern differences between folate species in the MTHFR C677T genotypes (32, 35), a need exists for information on the comparability of MA and LC-MS/MS results in other subgroups, such as alcohol or antifolate or anticonvulsant drug users and people with malabsorption problems.
Finally, the roundtable discussed ways to present the LC-MS/MS results. For total folate concentrations, interconversions between different forms are not a concern as long as they stay intact, retain their biological activity, and are measurable with equal efficiency (12). However, quantitative data on the individual species must accurately reflect what was in the original sample. For example, because of the pH that the NCEH procedure used, interconversions between 10FTHF, 5,10-methenyl-tetrahydrofolic acid, and 5FTHF occur. The SRM 1955 and NHANES 1999–2002 have produced quantitative values only for serum 5MTHF and FA. When LC-MS/MS is used, NCEH estimates total folate concentrations by the addition of the concentrations of methylated and nonmethylated folates.
Roundtable statisticians agreed that potential errors associated with summing across multiple species to obtain total folate values should not affect total means but could increase variances. For NHANES serum folate data, the 5MTHF that represents most folate in serum has a small CV, which results in a small CV (ie, imprecision) for total folate estimates (Table 3).
Measurement of folate vitamers in surplus serum from NHANES
Jacob Selhub discussed the measurement of serum 5MTHF and FA with the use of affinity/HPLC in surplus serum from NHANES 1999–2002 at Tufts University (Table 4) (10, 15, 45–47). With the use of the NHANES 2001–2002 data that Tufts University generated, Regan Bailey (8) provided folate vitamer results for an analytic sample of >1100 persons aged ≥60 y who had fasted; 38% had a detectable serum FA concentration. Although the trend for elevated serum FA concentrations with increased FA intakes was significant, this relation varied. Some people with low FA intakes had detectable serum FA concentrations. Morris et al (10), with the use of the same NHANES data, reported that in persons with low vitamin B-12 status (ie, serum vitamin B-12 < 148 pmol/L or plasma methylmalonic acid > 210 nmol/L), the presence of detectable circulating FA concentrations was related to lower cognitive test scores and lower mean cell volumes.
TABLE 4.
Measurement of serum 5-methyltetrahydrofolate and folic acid in NHANES1
| Survey | Assay | Matrix | Laboratory | Population age |
| 1974–1975 | — | — | — | — |
| 1976–1980 | — | — | — | — |
| 1988–1994 | — | — | — | — |
| 1999–2000 | Affinity/HPLC | Surplus sera | Tufts2 | ≥60 y |
| 2001–2002 | Affinity/HPLC | Surplus sera | Tufts2 | ≥60 y |
| 2003–2004 | — | — | — | — |
| 2005–2006 | — | — | — | — |
| 2007–2008 | LC-MS/MS | Serum, RBC | NCEH | One-third subset, ≥1 y |
| 2009–2010 | LC-MS/MS | Serum, RBC | NCEH | One-third subset, ≥1 y |
LC-MS/MS, liquid chromatography–tandem mass spectrometry; RBC, red blood cell; NCEH, National Center for Environmental Health, Centers for Disease Control and Prevention.
Jean Mayer USDA Human Nutrition Research Laboratory, Tufts University, Boston, MA. Measurement procedure: affinity/HPLC with electrochemical detection.
The roundtable agreed that the data on folate species are useful for the study of polymorphisms and their disease risks, but NHANES lacks MTHFR and other genotype information. Several experts suggested that, without genotype information, NHANES researchers use RBC patterns of folate species, if available, as a surrogate indicator of MTHFR C677T polymorphism status (38). However, inconsistencies in patterns from 2 laboratories (38, 44) underscore the need for accurate folate species measurement for this approach. Furthermore, the presence of circulating FA, associated with FA fortification and the increasing use of FA-fortified foods and supplements, might adversely affect cancer risk and vitamin B-12 status (18, 19, 48). The roundtable noted the current paucity of information on the metabolism and functional effects of serum FA, such as the degree to which free FA correlates with circulating folate-binding proteins; whether the body endogenously produces FA and, if so, by what mechanism; what effect polymorphisms have; and how long FA remains in circulation.
NIST REFERENCE MEASUREMENT PROCEDURES AND MATERIALS FOR SERUM FOLATES
Karen Phinney reported that the NIST SRM 1955 for folate in human serum contains 3 reference materials: level 2 is unaltered human serum, level 1 is diluted with phosphate-buffered saline because of the difficulty of obtaining low levels of some folate species, and level 3 is fortified with 5MTHF (28, 41). SRM 1955 has certified values for 5MTHF, reference values for FA, and information values for total folate and 5FTHF.
NIST developed several LC-MS/MS procedures to assign values for serum folate species in SRM 1955 for homocysteine and folate in human serum; these are described in detail elsewhere (28, 41, 49–51). The Joint Committee on Traceability in Laboratory Medicine database lists these as approved reference measurement procedures for serum 5MTHF and FA (40). NIST assigned values for the SRM 1955 folate species based on analyses from both NIST and NCEH (28).
The NIST is developing SRM 1950, its first plasma-based reference material for use by the metabolomics community to measure metabolites (52). The NIST is not developing this SRM specifically for folate but will provide a certified value for 5MTHF and a reference value for FA. SRM 1950 is a human plasma pool from ≥100 donors (equal numbers of men and women aged 40–50 y). The NIST and NCEH are characterizing the SRM 1950 values with the use of LC-MS/MS.
The NIST will revisit some of its methodologies for the quantification of folate vitamers, for example, to increase precision at low vitamer concentrations and agreement between measurement procedures (eg, FA), and to quantify additional vitamers. The NIST will evaluate potential interferences and will determine whether some measured vitamers are in vivo species or artifacts of sample handling. The NIST will also develop a certified value for total folate. The NIST does not have a measurement procedure for RBC folate, which precludes the development of reference materials for RBC folate at this time.
The roundtable noted the difficulties in the identification of the measurand or measurands and the need for a commutability study to determine whether the reference material behaves similarly to a patient sample with the use of SRM 1955 materials (11). The SRM 1955 should work reasonably well for the level 2 material because it is unaltered serum, but commutability may be an issue for levels 1 (derived from diluted materials) and 3 (a fortified material). Therefore, the value of the SRM 1955 materials as a reference material and trueness control for levels 1 and 3 is not currently clear.
ROUNDTABLE DIALOGUE
Measurement procedures for future NHANES
For serum folate, the roundtable generally agreed that NHANES should use the LC-MS/MS procedure in the future because of the identical results between the MA and the LC-MS/MS procedure and the increasing interest in the measurement of individual folate forms that the LC-MS/MS procedure, but not the MA, can detect. As a chemical measurement procedure, the LC-MS/MS is a metrologically higher-order procedure than the MA, which is a biological procedure. LC-MS/MS can also provide valuable information that other measurement procedures cannot (eg, individual folate species and the accumulation of byproducts). The NCEH is planning a round robin for serum folate with 2–3 laboratories, similar to the 3-laboratory comparison study for MA. The experts wondered whether, given the procedure's dynamic range, dilutions are necessary for good precision in the fortified US population. Pfeiffer noted that the calibration range for LC-MS/MS is 1–100 nmol/L for 5MTHF and 0.5–50 nmol/L for minor folate species, and linearity goes beyond even the calibration range. Only a few samples exceed the calibration range and require dilution.
For RBC folates, the roundtable experts agreed that the use of the LC-MS/MS procedure is premature because results with the LC-MS/MS and MA procedures in the NCEH laboratory differed by 20–25%. Research must identify the source of these differences. In addition, no reference materials are available for RBC folates. Therefore, for RBC folates, the roundtable agreed that MA is currently the preferred measurement procedure.
Which biomarkers?
The roundtable discussed whether future NHANES should continue to measure serum and RBC folate biomarkers given that public health concerns have shifted to the safety of high FA intakes and the potential for adverse effects from long-term exposures to FA in serum (also referred to as unmetabolized FA) after fortification.
The roundtable considered whether both serum and RBC folate measures are necessary to monitor folate concentrations in the US population. Experts had regarded RBC folate concentrations as better folate status measures because they are integrative measures of folate intakes over RBC's 90–120-d lifespan, whereas serum folate concentrations reflect recent intakes. The higher folate concentrations in RBC compared with serum also made RBC measurements easier. However, these factors may no longer be relevant to NHANES. The general population does not change daily intakes drastically, and current procedures can measure serum folate concentrations accurately. Therefore, RBC folate concentrations might not be more useful than serum folate concentrations for NHANES.
The roundtable agreed that serum or RBC folate would be useful in the assessment of folate status in NHANES, and noted that serum folate's advantages include its wide use in clinical settings. RBC folate falls with vitamin B-12 deficiency (and probably would not rise as high in folate-supplemented subjects with significant vitamin B-12 deficiency as it would in those without vitamin B-12 deficiency), which makes it useful in the evaluation of folate's effects on vitamin B-12–deficient subjects. However, the rationale for the selection of a folate-related biomarker for a large survey might not apply to clinical settings. RBC folate measures remain useful in clinical settings because recent intake changes with a disease or acute illness affect them less than they do serum folate concentrations. Therefore, RBC folate concentrations give a more accurate picture than do serum folate concentrations of patients’ underlying folate status.
Additional NHANES data analyses could provide information on the usefulness of single, compared with combination, folate status measures. Our incomplete knowledge of what serum and RBC folate concentrations actually reflect hampers the decision about which biomarker is preferable. For example, the magnitude of serum and RBC folate concentration responses to FA fortification in the United States showed marked differences (119–161% increase for serum, 44–64% increase for RBC) (5), and the RBC folate concentrations did not show the expected plateau effect if tissue saturation occurs at higher intakes (53). Monitoring serum and RBC folates could produce new information, particularly if NHANES uses LC-MS/MS procedures. Measurement of FA in serum could shed light on the prevalence of at-risk population groups and correlates of risk (8, 10, 54). Folate-related polymorphisms are more likely to relate to RBC than are serum folate concentrations, particularly to the pattern of folate species in RBC (32, 38).
SUMMARY
A roundtable evaluated the use of folate status biomarkers in past, current, and future NHANES. They evaluated the public health merits of the inclusion of these measures in NHANES, the quality of measurement procedures that past NHANES have used or are available for future NHANES, and the quality of the reference measurement procedures and materials available or under development. They noted a systematic bias in the Bio-Rad results that NHANES 1991–1994 and 1999–2006 used as compared with the MA and LC-MS/MS measurement procedures used in NHANES 2007–2010. Comparison of Bio-Rad results with the NHANES 2007–2010 MA-based results will require statistical adjustments if time trends are to be evaluated. The roundtable also supported the use of the LC-MS/MS procedure for the measurement of serum folate in future NHANES but agreed that this procedure is not yet fully validated for the measurement of RBC folates.
Acknowledgments
We thank Anne Thurn and Claudia Faigen of the Office of Dietary Supplements, National Institutes of Health; Kimberly Potter, Megan McNamee, and Therese Gibson of ICF International; and Mike Bykowski of Consolidated Solutions and Innovations for their outstanding logistical, organizational, and follow-up support. We thank Deborah Berlyne for her expert and timely technical editing services.
The authors’ responsibilities were as follows—MFP, CLJ, EAY, and PMC: conceived and sponsored the roundtable project; MFP, CLJ, EAY, PMC, RLB, DL, AMM, JLM, CMP, KWP, CS, BS, and TT: served on the planning committee; EAY, CLJ, JHE, JM, RA D-A, CS, BS, CMP, KWP, JS, RC, EB, RLB, and DL: presented the data and background information; CMP, ZF, KWP, DAL, RLB, RAD, AM, CS, and TT: developed the data under review; EAY: drafted the article; CMP and KWP: provided considerable input to the manuscript draft; and EAY, CLJ, and PMC: had primary responsibility for the final article content. All authors fully participated in the roundtable discussions and read, commented on, and approved the final manuscript. None of the authors had a conflict of interest.
REFERENCES
- 1.Yetley EA, Johnson CL. Folate and vitamin B-12 biomarkers in NHANES: history of their measurement and use. Am J Clin Nutr 2011;94(suppl):322S–31S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senti FR, Pilch SM. Analysis of folate data from the second National Health and Nutrition Examination Survey (NHANES II). J Nutr 1985;115:1398–402 [DOI] [PubMed] [Google Scholar]
- 3.Raiten DJ, Fisher KD. Assessment of folate methodology used in the third National Health and Nutrition Examination Survey (NHANES III, 1988-1994). J Nutr 1995;125:1371S–98S [DOI] [PubMed] [Google Scholar]
- 4.Yetley EA, Rader JI. Modeling the level of fortification and post-fortification assessments: U.S. experience. Nutr Rev 2004;62:S50–9 [DOI] [PubMed] [Google Scholar]
- 5.Pfeiffer CM, Johnson CL, Jain RB, et al. Trends in blood folate and vitamin B-12 concentrations in the United States, 1988-2004. Am J Clin Nutr 2007;86:718–27 [DOI] [PubMed] [Google Scholar]
- 6.McDowell MA, Lacher DA, Pfeiffer CM, et al. Blood folate levels: the latest NHANES results. NCHS Data Brief, No. 6. Hyattsville, MD: National Center for Health Statistics, 2008. Available from: http://www.cdc.gov/nchs/data/databriefs/db06.pdf (cited 14 January 2011) [PubMed] [Google Scholar]
- 7.Bailey RL, McDowell MA, Dodd KW, Gahche JJ, Dwyer JT, Picciano MF. Total folate and folic acid intakes from foods and dietary supplements of US children aged 1-13 y. Am J Clin Nutr 2010;92:353–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey RL, Mills JL, Yetley EA, et al. Unmetabolized serum folic acid and its relation to folic acid intake from diet and supplements in a nationally representative sample of adults aged ≥60 y in the United States. Am J Clin Nutr 2010;92:383–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brouwer I, Verhoef P. Folic acid fortification: is masking of vitamin B-12 deficiency what we should really worry about? Am J Clin Nutr 2007;86:897–8 [DOI] [PubMed] [Google Scholar]
- 10.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Circulating unmetabolized folic acid and 5-methyltetrahydrofolate in relate to anemia, macrocytosis, and cognitive test performance in American seniors. Am J Clin Nutr 2010;91:1733–44 [DOI] [PubMed] [Google Scholar]
- 11.Bock JL, Eckfeldt JH. Advances in standardization of laboratory measurement procedures: implications for measuring biomarkers of folate and vitamin B-12 status in NHANES. Am J Clin Nutr 2011;94(suppl):332S–6S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shane B. Folate status assessment history: implications for measurement of biomarkers in NHANES. Am J Clin Nutr 2011;94(suppl):337S–42S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmel R. Biomarkers of cobalamin (vitamin B-12) status in the epidemiologic setting: a critical overview of context, applications, and performance characteristics of cobalamin, methylmalonic acid, and holotranscobalamin II. Am J Clin Nutr 2011;94(suppl):348S–58S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nexo E, Hoffmann-Lücke E. Holotranscobalamin, a marker of vitamin B-12 status: analytical aspects and clinical utility. Am J Clin Nutr 2011;94(suppl):359S–65S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalmbach R, Paul L, Selhub J. Determination of unmetabolized folic acid in human plasma using affinity HPLC. Am J Clin Nutr 2011;94(suppl):343S–7S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yetley EA, Pfeiffer CM, Phinney KW, et al. Biomarkers of vitamin B-12 status in NHANES: a roundtable summary. Am J Clin Nutr 2011;94(suppl):313S–21S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yetley EA, Coates PM, Johnson CL. Overview of a roundtable on NHANES monitoring of biomarkers of folate and vitamin B-12 status: measurement procedure issues. Am J Clin Nutr 2011;94(suppl):297S–302S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sweeney MR, McPartlin J, Scott J. Folic acid fortification and public health: report on threshold doses above which unmetabolised folic acid appear in serum. BMC Public Health 2007;7:41–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sweeney MR, Staines A, Daly L, et al. Persistent circulating unmetabolised folic acid in a setting of liberal voluntary folic acid fortification. Implications for further mandatory fortification? BMC Public Health 2009;9:295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green R, Miller JW. Vitamin B12 deficiency is the dominant nutritional cause of hyperhomocysteinemia in a folic acid-fortified population. Clin Chem Lab Med 2005;43:1048–51 [DOI] [PubMed] [Google Scholar]
- 21.Carmel R, Green R, Jacobsen DW, Rasmussen K, Florea M, Azen C. Serum cobalamin, homocysteine, and methylmalonic acid concentrations in a multiethnic elderly population: ethnic and sex differences in cobalamin and metabolite abnormalities. Am J Clin Nutr 1999;70:904–10 [DOI] [PubMed] [Google Scholar]
- 22.Ganji V, Kafai MR. Demographic, lifestyle, and health characteristics and serum B vitamin status are determinants of plasma total homocysteine concentration in the post-folic acid fortification period, 1999-2004. J Nutr 2009;139:345–52 [DOI] [PubMed] [Google Scholar]
- 23.Gunter EW, Lewis BG, Koncikowski SM. Laboratory procedures used for the third National Health and Nutrition Examination Survey (NHANES III), 1988-1994. Atlanta, GA and NCHS. Hyattsville, MD: Centers for Disease Control and Prevention, 1996 [Google Scholar]
- 24.Centers for Disease Control and Prevention NHANES 1999-2000: folate/vitamin B12, serum and whole blood, Bio-Rad Laboratories’ “Quantaphase II folate/vitamin B12” radioassay kit. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_99_00/lab06_met_folate_b12.pdf (cited 14 January 2011)
- 25.Centers for Disease Control and Prevention NHANES 2001-2002: folate/vitamin B12, serum and whole blood, Bio-Rad Laboratories “Quantaphase II folate/vitamin B12” radioassay kit. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_01_02/l06_b_met_folate_b12.pdf (cited 14 January 2011)
- 26.Centers for Disease Control and Prevention NHANES 2003-2004: folate/vitamin B12, serum and whole blood, Bio-Rad Laboratories’ “Quantaphase II folate/vitamin B12” radioassay kit. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l06_c_met_folates%20B12.pdf (cited 14 January 2011)
- 27.Centers for Disease Control and Prevention NHANES 2005-2006: folate/vitamin B12, serum and whole blood, Bio-Rad Laboratories “Quantaphase II folate/vitamin B12” radioassay kit. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/folate_b12_d_met.pdf (cited 14 January 2011)
- 28.National Institute of Standards and Technology Certificate of analysis, standard reference material 1955, homocysteine and folate in frozen human serum. April 2008. Available from: https://www-s.nist.gov/srmors/certificates/1955.pdf?CFID=1126897&CFTOKEN=ad62a5219ffb884e-41496E79-A2D7-BED2-991CE742A98A0716&jsessionid=f030a960d9d22f92f6db3a34674d5a634a2a (cited 14 January 2011)
- 29.Thorpe SJ, Heath A, Blackmore S, et al. International standard for serum vitamin B12 and serum folate: international collaborative study to evaluate a batch of lyophilised serum for B12 and folate content. Clin Chem Lab Med 2007;45:380–6 [DOI] [PubMed] [Google Scholar]
- 30.Thorpe SJ, Sands D, Heath AB, Hamilton MS, Blackmore S, Barrowcliffe T. An international standard for whole blood folate: evaluation of a lyophilised haemolysate in an international collaborative study. Clin Chem Lab Med 2004;42:533–9 [DOI] [PubMed] [Google Scholar]
- 31.Fazili Z, Pfeiffer CM, Zhang M. Comparison of serum folate species analyzed by LC-MS/MS with total folate measured by microbiologic assay and Bio-Rad radioassay. Clin Chem 2007;53:781–4 [DOI] [PubMed] [Google Scholar]
- 32.Fazili Z, Pfeiffer CM, Zhang M, Jain RB, Koontz D. Influence of 5,10-methylenetetrahydrofolate reductase polymorphism on whole-blood folate concentrations measured by LC-MS/MS, microbiologic assay, and Bio-Rad radioassay. Clin Chem 2008;54:197–201 [DOI] [PubMed] [Google Scholar]
- 33.Blackmore S, Pfeiffer C, Hamilton MS, Lee A. Recoveries of folate species from serum pools sent to participants of the UK NEQAS Haematinics Scheme in February and March 2004. Clin Chim Acta 2005;335:S459 (abstr) [Google Scholar]
- 34.National Center for Health Statistics Analytic note for serum folate regression equations to compare 2007–2008 and 1999–2006 data and red blood cell folate comparison of 2007–2008 and 1999–2006 data. Hyattsville, MD: National Center for Health Statistics, 2011. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/FOLATE_E.htm (in press) [Google Scholar]
- 35.Molloy AM, Mills JL, Kirke PN, Whitehead AS, Weir DG, Scott JM. Whole-blood folate values in subjects with different methylenetetrahydrofolate reductase genotypes: differences between the radioassay and microbiological assays. Clin Chem 1998;44:186–8 [PubMed] [Google Scholar]
- 36.Pfeiffer CM, Fazili Z, Zhang M. Folate analytical methodology : Bailey LB. ed Folate in health and disease 2nd ed. Boca Raton, FL: CRC Press, 2010:517–74 [Google Scholar]
- 37.Pfeiffer CM, Fazili Z, McCoy L, Zhang M, Gunter EW. Determination of folate vitamers in human serum by stable-isotope-dilution tandem mass spectrometry and comparison with radioassay and microbiologic assay. Clin Chem 2004;50:423–32 [DOI] [PubMed] [Google Scholar]
- 38.Fazili Z, Pfeiffer CM. Measurement of folates in serum and conventionally prepared whole blood lysates: application of an automated 96-well plate isotope-dilution tandem mass spectrometry method. Clin Chem 2004;50:2378–81 [DOI] [PubMed] [Google Scholar]
- 39.Fazili Z, Pfeiffer CM, Zhang M, Jain R. Erythrocyte folate extraction and quantitative determination by liquid chromatography-tandem mass spectrometry: comparison of results with microbiologic assay. Clin Chem 2005;51:2318–25 [DOI] [PubMed] [Google Scholar]
- 40.Joint Committee on Traceability in Laboratory Medicine Bureau International des Poids et Mesures, Sèvres Cedex, Frances. Available from: http://www.bipm.org/jctlm/ (cited 4 December 2010)
- 41.Satterfield MB, Sniegoski LT, Sharpless KE, et al. Development of a new standard reference material: SRM 1955 (homocysteine and folate in human serum). Anal Bioanal Chem 2006;385:612–22 [DOI] [PubMed] [Google Scholar]
- 42.Hannisdal R, Ueland PM, Svardal A. Liquid chromatography–tandem mass spectrometry analysis of folate and folate catabolites in human serum. Clin Chem 2009;55:1147–54 [DOI] [PubMed] [Google Scholar]
- 43.Hannisdal R, Ueland PM, Eussen SJ, Svardal A, Hustad S. Analytical recovery of folate degradation products formed in human serum and plasma at room temperature. J Nutr 2009;139:1415–8 [DOI] [PubMed] [Google Scholar]
- 44.Bagley PJ, Selhub J. A common mutation in the methylenetetrahydrofolate reductase gene affects is associated with an accumulation of formylated tetrahydrofolates in red blood cells. Proc Natl Acad Sci USA 1998;95:13217–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bagley PJ, Selhub J. Analysis of folate form distribution by affinity followed by reversed-phase chromatography with electrochemical detection. Clin Chem 2000;46:404–11 [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention NHANES 1999-2000: unmetabolized folic acid (surplus sera) (SSFOL_A). Available from: http://www.cdc.gov/nchs/nhanes/nhanes1999-2000/ssfol_a.htm (cited 14 January 2011)
- 47.Centers for Disease Control and Prevention NHANES 2001-2002: unmetabolized folic acid (surplus sera) (SSFOL_B). Available from: http://www.cdc.gov/nchs/nhanes/nhanes2001-2002/ssfol_b.htm (cited 14 January 2011)
- 48.Mason JB. Folate, cancer risk, and the Greek god, Proteus: a tale of two chameleons. Nutr Rev 2009;67:206–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson BC, Pfeiffer CM, Margolis SA, Nelson CP. Affinity extraction combined with stable isotope dilution LC/MS for the determination of 5-methyltetrahydrofolate in human plasma. Anal Biochem 2003;313:117–27 [DOI] [PubMed] [Google Scholar]
- 50.Nelson BC, Pfeiffer CM, Margolis SA, Nelson CP. Solid-phase extraction-electrospray ionization mass spectrometry for the quantification of folate in human plasma or serum. Anal Biochem 2004;325:41–51 [DOI] [PubMed] [Google Scholar]
- 51.Nelson BC, Satterfield MB, Sniegoski LT, Welch MJ. Simultaneous quantification of homocysteine and folate in human serum or plasma using liquid chromatography/tandem mass spectrometry. Anal Chem 2005;77:3586–93 [DOI] [PubMed] [Google Scholar]
- 52.National Institute of Standards and Technology Development of a standard reference material for metabolites in plasma. Available from: http://www.nist.gov/mml/analytical/organic/metabolitesinserum.cfm (cited 4 December 2010)
- 53.Hoey L, McNulty H, Askin N, et al. Effect of a voluntary food fortification policy on folate, related B vitamin status, and homocysteine in healthy adults. Am J Clin Nutr 2007;86:1405–13 [DOI] [PubMed] [Google Scholar]
- 54.Kalmbach RD, Choumenkovitch SF, Troen AM, D'Agostino R, Jacques PF, Selhub J. Circulating folic acid in plasma: relation to folic acid fortification. Am J Clin Nutr 2008;88:763–8 [DOI] [PMC free article] [PubMed] [Google Scholar]