Abstract
Background: Altering dietary carbohydrate or fat content may have chronic effects on insulin secretion and sensitivity, which may vary with individual metabolic phenotype.
Objective: The objective was to evaluate the contribution of tightly controlled diets differing in carbohydrate and fat content for 8 wk to insulin sensitivity and β cell responsiveness and whether effects of diet would vary with race, free-living diet, or insulin response.
Design: Healthy overweight men and women (36 European Americans, 33 African Americans) were provided with food for 8 wk and received either a eucaloric standard diet (55% carbohydrate, 27% fat) or a eucaloric reduced-carbohydrate (RedCHO)/higher-fat diet (43% carbohydrate, 39% fat). Insulin sensitivity and β cell responsiveness were assessed at baseline and 8 wk by using a liquid meal tolerance test.
Results: Insulin sensitivity did not change with diet (P = 0.1601). Static β cell response to glucose (ФS) was 28.5% lower after the RedCHO/higher-fat diet. Subgroup analyses indicated that lower ФS with the RedCHO/higher-fat diet occurred primarily among African Americans. A significant inverse association was observed for change in glucose area under the curve compared with change in ФS.
Conclusions: Consumption of a eucaloric 43% carbohydrate/39% fat diet for 8 wk resulted in down-regulation of β cell responsiveness, which was influenced by baseline phenotypic characteristics. Further study is needed to probe the potential cause-and-effect relation between change in ФS and change in glucose tolerance. This trial is registered at clinicaltrials.gov as NCT00726908.
INTRODUCTION
Low insulin sensitivity is a well-established risk factor for both type 2 diabetes and cardiovascular disease (1). Accordingly, maintenance of adequate insulin sensitivity and secretion is important for ensuring appropriate metabolism of nutrients and for minimizing risk of chronic metabolic disease. Relatively low insulin secretion may portend the early stages of impaired glucose tolerance (2), whereas chronically high insulin secretion is a characteristic of populations at high risk of development of type 2 diabetes (3) and is associated with greater weight gain under certain circumstances (4).
Dietary macronutrient composition may play a role in determining insulin sensitivity and secretion. Diets with a relatively high carbohydrate content, glycemic index, or both increase insulin secretion on an acute basis (ie, while consuming a meal). Whether such diets have a residual effect on pancreatic β cell function is not clear. High-carbohydrate (or high–glycemic index) diets also may affect insulin sensitivity, given that experimental induction of hyperinsulinemia reduces insulin sensitivity (5). Results from experimental infusion of lipid suggest that chronic consumption of diets high in fat also may affect insulin secretion and sensitivity. Acute experimental exposure to elevated lipid enhances insulin secretion and impairs insulin sensitivity, whereas chronic exposure impairs insulin secretion (6–8). Limited data suggest that elevated dietary fat likewise may impair insulin sensitivity (9).
Metabolic responses to dietary factors may differ with baseline phenotype, such as race, insulin response, or free-living diet composition. Individuals with a relatively high 30-min insulin concentration after an oral glucose load (insulin-30) lost more weight with a lower carbohydrate diet than with a lower fat diet, whereas those with a lower insulin-30 showed no difference in weight loss with diet quality (10). Similarly, individuals with a high insulin-30 gained more weight over 6 y when consuming a diet higher in carbohydrate (4). Ethnic differences in metabolism have been extensively documented, particularly lower insulin sensitivity and greater acute insulin secretion among African Americans compared with European Americans (11, 12). Little research has been conducted regarding racial differences in the metabolic response to diets differing in macronutrient content. Habitual consumption of diets low or high in given macronutrients may augment or attenuate physiologic responses to test diets. Thus, it may be important to consider baseline (free-living) diet when assessing the response to a diet intervention.
The objective of this study was to evaluate the contribution of tightly controlled diets differing in carbohydrate and fat content for 8 wk to insulin sensitivity and β cell responsiveness and to determine whether effects of diet would vary with race, free-living diet, or insulin response.
SUBJECTS AND METHODS
Subjects
A total of 69 healthy African American and European American men and premenopausal women (52% European American, 45% men) aged 21–50 y participated in the study. Race was self-reported during a telephone screening. Inclusion criteria were body mass index (BMI; in kg/m2) in the overweight and obese range (>25 and <136 kg), age 21–50 y, relatively sedentary lifestyle, nondiabetic, and stable weight for 6 mo with no weight change >2.29 kg. Exclusion criteria included diagnosis of polycystic ovary disease, weight >136 kg, regular exercise >2 h/wk, pregnancy, currently breastfeeding, any disorders of glucose or lipid metabolism, use of medication that could affect body composition or glucose metabolism (including oral contraceptives, cholesterol medications, and blood pressure medications), current use of tobacco, reported use of illegal drugs in last 6 mo, history of hypoglycemic episodes, major food allergies or food dislikes, inconsistent or absence of regular menstrual cycles, or a medical history that contraindicated inclusion in the study. Subjects were evaluated for glucose tolerance (13) by using a 2-h oral-glucose-tolerance test. All but 2 subjects had normal glucose (2-h glucose <140 mg/dL). Two subjects had a 2-h glucose between 140 and 155 mg/dL but had normal fasting glucose (<110 mg/dL) (13). Subjects were informed of the experimental design, and oral and written consent were obtained. The study was approved by the Institutional Review Board for Human Use at the University of Alabama at Birmingham.
Procedures
Subjects completed a 4-d food record (3 weekdays and one weekend day) for assessment of typical, free-living, nutrient intake before starting an 8-wk diet intervention. After completing the food record, subjects were provided with a standard diet (STD; described below) for 3 d to eliminate intersubject variance in free-living dietary factors. Then, subjects reported to the General Clinical Research Center (GCRC) to complete a liquid meal tolerance test (LMTT). At approximately the midpoint of the diet intervention (week 4), a solid meal test was administered to assess whether the diets were having a noticeable effect on acute metabolic response to a representative meal. After 8 wk of the diet intervention, subjects reported to the GCRC to complete a follow-up LMTT. All tests are described below in detail.
For the first 30 subjects, testing was conducted on an inpatient basis, with subjects reporting to the GCRC the evening before the LMTT. Due to change in availability of inpatient services, for the remaining subjects testing was conducted on an outpatient basis, with subjects reporting to the GCRC on the morning of their test after an overnight fast. The number of subjects who consumed each diet [reduced-carbohydrate (RedCHO)/lower-fat and STD] was similar before and after this transition, and results did not differ if a variable for protocol was included. Therefore, it is unlikely that this change in protocol biased the results.
Diets
Subjects were assigned in groups to either a STD diet or RedCHO/lower-fat diet for 8 wk. Subjects were blinded to their assigned diet. The STD diet consisted of 55% carbohydrates, 18% protein, and 27% fat (with <10% saturated fat). The RedCHO/higher-fat diet consisted of 43% carbohydrates, 18% protein, and 39% fat (with <10% saturated fat). Both the percentage fat from omega-3 and oleic acid and the absolute amount of omega-3 and oleic acid were higher in the RedCHO/higher-fat diet. The average glycemic load (GL) for the STD diet was ≥75 points/1000 kcal, and that for the RedCHO/higher-fat diet was ≤45 points/1000 kcal. The GL was calculated per 1000 kcal to ensure that all subjects received a proportionate amount of GL per calorie level. A 1-d example of meals for both diets is shown in Table 1.
TABLE 1.
Example day of meals for the reduced-carbohydrate (RedCHO)/higher-fat diet and the standard (STD) diet
| RedCHO/higher-fat diet (2500 calories) | STD diet (2500 calories) |
| Breakfast | |
| 58 g White English muffin | 74 g Frozen waffles |
| 42 g 2% American cheese | 31 mL Sugar-free syrup |
| 21 g Canadian bacon | 76 g Meatless sausage patties |
| 236 mL Apple juice | 118 g Banana |
| 236 mL 2% Milk | 118 mL Cranberry juice |
| 236 mL Skim milk | |
| Snack | |
| 28.3 g Corn chips1 | 104 g Toaster pastries2 |
| 364 g Apple | |
| 21 g Peanut butter | |
| Lunch | |
| One prepackaged frozen meal (255 g): turkey with mushroom gravy and green beans3 | 126 g Ham-and-cheese microwaveable turnover4 |
| 200 g Iceberg lettuce | 50 g Roasted peanuts |
| 24.8 g Italian dressing | 226 g Fat-free, sugar-free yogurt |
| 28.3 g Multigrain chips5 | |
| Snack | |
| 325 mL Creamy milk chocolate shake6 | 236 mL Skim milk |
| 50 g Roasted, salted sunflower seeds | 36 g Sugar-free drink mix7 |
| Dinner | |
| Two prepackaged frozen meals (498 g): roasted garlic chicken with creamy Parmesan spinach8 | One prepackaged frozen meal (292 g): sesame chicken with rice, snow peas, and carrots9 |
| 155 g Frozen carrots | 75 g Frozen broccoli |
| 100 g Frozen broccoli | 19 g Fat-free American cheese |
| 10 g Margarine spread10 | 113 g Mandarin oranges |
| 32 g Rye bread | 28 g Dinner roll |
Fritos; Frito-Lay North America (Plano, TX).
Pop-Tarts; Kellogg's (Battle Creek, MI).
Weight Watchers Smart Ones; Weight Watchers Heinz North America (Pittsburgh, PA).
Hot Pocket; Nestle (Solon, OH).
Sun Chips; Frito-Lay North America.
Slim Fast shake; Slim Fast Unilever (Englewood Cliffs, NJ)
Carnation Instant Breakfast; Nestle (Minneapolis, MN).
Lean Cuisine; Stouffer's Nestle (Solon, OH).
Healthy Choice; ConAgra Foods (Omaha, NE).
Promise 60% margarine spread; Unilever (Lisle, IL).
For the duration of the intervention, subjects reported to the GCRC each weekday morning to be weighed, eat breakfast, and collect food for their remaining meals. On Fridays, subjects picked up food for Saturday and Sunday to consume at home. All food was provided by the GCRC Metabolic Kitchen. Body weight was recorded 5 times weekly to monitor weight maintenance. The total energy of all diets was calculated to be eucaloric by using the Harris-Benedict formula (14) with an activity factor of 1.35 for women and 1.5 for men. Energy intake was adjusted if necessary to maintain body weight within 2 kg of baseline weight. If a subject's weight trended below 2 kg of baseline weight, snacks were added to their menu that contained the appropriate macronutrient and GL content of the assigned diet. This addition added calories only to the assigned diet and did not affect the macronutrient compositions or GL.
Analyses of free-living dietary intake (based on the 4-d food records), and development of menus for the intervention diets, were conducted by using Nutrition Data System for Research (NDSR) software versions 2006 and 2007 (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN), which reflected the marketplace over the time frame during which menus were developed and intake was assessed. Glucose was used as the reference for determining GL. The NDSR time-related database updates analytic data while maintaining nutrient profiles true to the version used for data collection.
LMTT
Whole-body insulin sensitivity (SI) and β cell responsiveness were derived by obtaining frequent blood samples after ingestion of a standardized LMTT. Subjects were required to fast for 12 h before the test. Women were tested in the follicular stage of the menstrual cycle. To perform the test, a flexible intravenous catheter was placed in the antecubital space of one arm. At time “zero,” a liquid mixed-macronutrient meal was provided [Carnation Instant Breakfast (Nestle, Minneapolis, MN) and whole milk]. The liquid meal was calculated to provide 7 kcal/kg body weight as 24% fat, 58.6% carbohydrate, and 17.4% protein (mean glucose ingested was 57 g; range: 36–78 g). Subjects were required to consume the meal within 5 min. Blood was drawn at −15 and −5 min before meal consumption, which began at time zero. Subsequent samples were drawn every 5 min from time zero to 30 min, every 10 min from 30 to 180 min, and at 210 and 240 min. Sera were stored at −85°C until analyzed for glucose, insulin, and C-peptide.
Solid meal test
During one GCRC breakfast at approximately the midpoint of the diet intervention (week 4), subjects provided blood samples before, during, and after breakfast. Subjects were required to fast 12 h before the test. To perform the test, a flexible intravenous catheter was placed in the antecubital space of one arm. At time zero, subjects initiated consumption of breakfast, which was their normal, assigned breakfast meal for that particular day. Subjects were required to consume the meal within 20 min. Blood samples were collected at baseline (2 samples) and at 15, 60, 90, 120, 180, and 240 min. Samples were analyzed for insulin and glucose.
Analysis of glucose and hormones
Concentrations of glucose, insulin, and C-peptide were analyzed in the Core Laboratory of the GCRC, Nutrition Obesity Research Center, and Diabetes Research and Training Center. Glucose was measured in 3 μL sera by using the Glucose oxidase method on a Stanbio Sirrus analyzer (Stanbio Laboratory, Boerne, TX). This analysis had an intraassay CV of 1.21% and an interassay CV of 3.065%. Insulin was assayed in 50-μL aliquots by using immunofluorescence on a TOSOH AIA-II analyzer (TOSOH Corp, South San Francisco, CA). This analysis had an intraassay CV of 1.49% and an interassay CV of 4.42%. C-peptide was assayed in 20-μL aliquots by using the TOSOH analyzer (intraassay CV of 1.67% and interassay CV of 2.59%).
Mathematical modeling
Glucose, insulin, and C-peptide values from the LMTT were analyzed for measures of SI and β cell function following the procedure of Breda et al (15). Model output from this procedure includes SI and indexes of ФB, ФD, ФS, SRB, and X0). ФB is the β cell response to glucose during the basal condition. ФD (dynamic condition) is the response of the β cell to an increase in glucose. ФS (static condition) is the amount of insulin secreted for a given amount of glucose during nonbasal conditions. SRB is a measure of the basal insulin secretion rate. X0 is defined as a measure of the amount of insulin released immediately after the glucose stimulus. The glucose area under the curve (AUC) was calculated by using the trapezoidal method.
Statistical analyses
Descriptive statistics were computed for all study variables of interest. Distributions of these variables were examined for normality. SI, fasting insulin, 30-min insulin, fasting glucose, and β cell response measures (ФB, ФS, ФD, SRB, and X0) were log10-transformed before statistical analysis. Outliers were identified by plotting the residuals. Any data points that were outside the appropriate residual range (±2 SD) were omitted from analysis. The 30-min insulin value from the baseline (preintervention) LMTT (insulin-30) was used to dichotomize subjects by “low” compared with “high” insulin response, as shown by Chaput et al (4), by using the median value (124.8 μU/mL). Subjects’ free-living diet was dichotomized by using the median value for percentage carbohydrate consumed from total calories (45.6%). All statistical tests were performed by using a type I error rate of 0.05. All statistical analyses were performed by using SAS (version 9.2; SAS Institute Inc, Cary, NC).
Analysis of covariance (ANCOVA) was performed to examine the effect of diet on main outcomes. Main outcome measures at week 8 (postintervention) were used as the dependent variables, and baseline outcome measures were used as covariates. ANCOVA was also used to examine potential differences between subgroups based on race, insulin-30, and free-living diet quality. Race was chosen as a subgroup to examine potential racial differences in insulin sensitivity and β cell response. Insulin-30, a measure of insulin secretion, was used in our subgroup analysis on the basis of previous research by Chaput et al (4) who found strong associations with insulin-30, dietary macronutrient content, and body weight change. Free-living diet quality was used to determine whether those subjects who ate a higher-carbohydrate diet before the diet intervention showed greater metabolic improvements compared with those whose usual diet was relatively low in carbohydrate. For all subgroup analyses, interaction terms were examined but were not significant, likely due to small sample size (diet × race, P = 0.2256; diet × 30-min insulin, P = 0.5644; and diet × free living diet quality, P = 0.5699). As a result, we conducted analyses within each group.
The association between the change in ФS and the change in glucose AUC was examined by using simple Pearson correlation analysis.
RESULTS
Descriptive information on the subject population is shown in Table 2. Subjects were 45% men and 52% European American. All subjects were overweight at baseline (BMI of 25–46.9). Six subjects receiving the RedCHO/higher-fat diet dropped out before completion of the intervention. One LMTT (at 8 wk; STD diet) was not completed due to inability to obtain blood. One SI value (at baseline; STD diet) was considered an outlier and was omitted for analysis. Thus, complete data (baseline and 8-wk evaluations) were obtained on 63 subjects for fasting glucose and insulin, 62 subjects for β cell response measures, and 61 subjects for SI.
TABLE 2.
Baseline characteristics of study population by diet group1
| Variable | RedCHO/higher-fat diet | STD diet |
| n | 40 | 29 |
| Male sex [% (n)] | 43 (17) | 48 (14) |
| EA race [% (n)] | 55 (22) | 48 (14) |
| Age (y) | 35.6 ± 8.52 | 34.6 ± 8.1 |
| BMI (kg/m2)3 | 33.3 ± 4.0 | 31.3 ± 4.4 |
| Fasting glucose (mg/dL) | 100.3 ± 10.9 | 97.9 ± 9.2 |
| Fasting insulin (μU/mL) | 9.6 ± 5.5 | 11.1 ± 6.0 |
| SI [×10minus4 minminus1/(μIU/mL)]4 | 4.8 ± 3.9 | 3.7 ± 3.4 |
| ΦB (109 minminus1) | 10.2 ± 4.1 | 10.0 ± 4.4 |
| ΦS (109 minminus1) | 71.2 ± 39.6 | 67.8 ± 30.7 |
| ΦD (109)4 | 482.1 ± 268.5 | 598.9 ± 190.3 |
| X0 (ng/mL)4,5 | 4.6 ± 2.9 | 6.6 ± 3.4 |
| SRB (pg ⋅ mLminus1 ⋅ minminus1)5 | 143.8 ± 64.7 | 139.6 ± 49.5 |
| 30-min Insulin (μU/mL) | 139.6 ± 93.0 | 146.7 ± 89.6 |
RedCHO, reduced-carbohydrate; STD, standard; EA, European American; SI, insulin sensitivity; ФB, β cell response to glucose during the basal condition; ФS, amount of insulin secreted for a given amount of glucose during nonbasal conditions; ФD, response of the β cell to an increase in glucose; X0, measure of the amount of insulin released immediately after the glucose stimulus; SRB, measure of the basal insulin secretion rate.
Arithmetic mean ± SD, not log-transformed before analysis (all such values).
P ≤ 0.05 (2-sample t test for significant differences between diet groups at baseline).
n = 28 for the STD diet.
Conversion to International System of Units (pmol/L): multiply by 0.331.
Although each subject's daily energy intake was calculated on an individual basis to maintain body mass, fluctuations in body mass occurred over the 8-wk intervention period. On average, subjects showed a change of −1.86% (−1.68 kg) in body mass (range = −6.37% to +4.35%, −6.4 to +4.7 kg), which did not significantly differ with diet assignment. Inclusion of weight change in statistical analysis did not alter the results.
Changes in fasting glucose, insulin, and SI over the 8-wk intervention period are shown in Table 3. Fasting glucose was significantly higher after consumption of the 8-wk RedCHO/higher-fat diet than after the STD diet (P < 0.05). No effect of diet on 8-wk SI was observed after adjustment for baseline SI. Results did not differ if sex, race, and weight change were included as covariates. Subgroup analysis indicated no interaction with race, insulin-30, or baseline diet quality.
TABLE 3.
Fasting glucose, insulin, and insulin sensitivity (SI) at baseline and at 8 wk by diet group1
| Diet | Baseline | 8 wk | P for diet2 |
| No. of subjects3 | |||
| RedCHO/higher-fat | 40 | 34 | — |
| STD | 29 | 29 | — |
| Fasting glucose (mg/dL) | |||
| RedCHO/higher-fat | 99.7 ± 1.74 | 101.7 ± 1.7 | 0.01 |
| STD | 97.5 ± 1.7 | 95.8 ± 1.6 | — |
| Fasting insulin (μU/mL) | |||
| RedCHO/higher-fat | 8.0 ± 0.9 | 8.1 ± 1.1 | 0.312 |
| STD | 9.5 ± 1.1 | 8.1 ± 0.9 | — |
| SI [×10minus4 minminus1/(μIU/mL)] | |||
| RedCHO/higher-fat | 3.68 ± 0.47 | 3.71 ± 0.48 | 0.16 |
| STD | 2.96 ± 0.37 | 3.72 ± 0.48 | — |
RedCHO, reduced-carbohydrate; STD, standard. All variables were log10-transformed before analysis. Mean and SEM values reported were back-transformed from log10 values used for analysis. There were no significant differences between diet groups at baseline.
By ANCOVA; effect of diet on 8-wk fasting glucose, insulin, and SI after adjustment for baseline fasting glucose, insulin, and SI, respectively.
n = 28 for STD diet baseline and 8-wk SI.
Mean ± SEM (all such values).
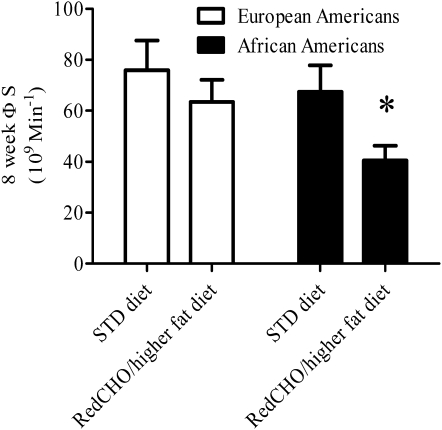
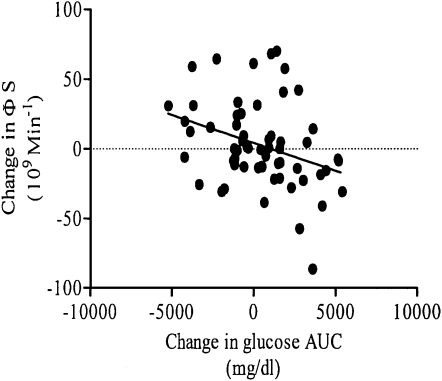
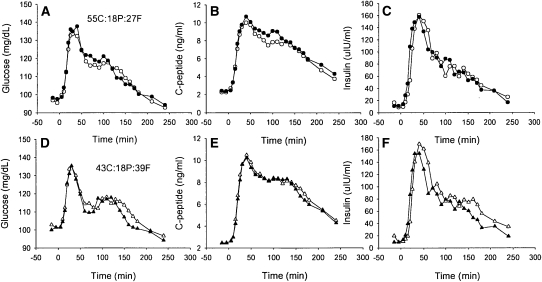
ФS was significantly lower at 8 wk in the RedCHO/higher-fat diet group than in the STD group (P < 0.05; Table 4). Subgroup analysis indicated that this difference in ФS with diet was specific to African Americans (40% lower in RedCHO/higher-fat compared with STD diet at 8 wk; P < 0.05) (Figure 1). No significant difference with diet was detected between European Americans (P = 0.200). Similarly, subgroup analysis based on insulin-30 indicated that, at week 8, individuals with relatively low baseline insulin-30 tended to have lower ФS on the RedCHO/higher-fat diet (P = 0.057). In contrast, diet did not contribute to ФS among individuals with a relatively high insulin-30 (P = 0.132). Subgroup analysis from the free-living diet indicated that those who consumed >45.6% carbohydrate from calories before the intervention (as calculated from the median) tended to show a greater decrease in ФS with the RedCHO/higher-fat diet (P = 0.0634). No significant difference was detected within subjects who consumed <45.6% carbohydrate from calories (P = 0.2379). Racial distribution was equal in the subgroups. No effects of diet were detected for ФB, ФD, X0, or SRB. A significant inverse association was observed for change in glucose AUC compared with change in ФS (r = −0.33, P < 0.05), after excluding 2 outliers (Figure 2). Serum concentrations of glucose, C-peptide, and insulin during the LMTT by diet group at baseline and 8 wk are shown in Figure 3.
TABLE 4.
β Cell response at baseline and at 8 wk by diet group1
| Diet | Baseline | 8 Weeks | P for diet2 |
| No. of subjects | |||
| RedCHO/higher-fat | 40 | 34 | — |
| STD | 29 | 28 | — |
| ΦB (109 minminus1) | |||
| RedCHO/higher-fat | 9.4 ± 0.63 | 9.5 ± 0.7 | 0.707 |
| STD | 9.3 ± 0.7 | 9.1 ± 0.5 | — |
| ΦS (109 minminus1) | |||
| RedCHO/higher-fat | 59.8 ± 6.5 | 51.7 ± 7.2 | 0.02 |
| STD | 60.0 ± 6.3 | 71.0 ± 6.6 | — |
| ΦD (109)4 | |||
| RedCHO/higher-fat | 405.9 ± 41.7 | 391.7 ± 48.5 | 0.2 |
| STD | 566.9 ± 38.3 | 568.9 ± 44.0 | — |
| X0 (ng/mL)45 | |||
| RedCHO/higher-fat | 3.8 ± 0.4 | 3.9 ± 0.6 | 0.502 |
| STD | 5.7 ± 0.7 | 5.5 ± 0.6 | — |
| SRB (pg ⋅ mLminus1 ⋅ minminus1)5 | |||
| RedCHO/higher-fat | 130.5 ± 9.7 | 133.8 ± 11.8 | 0.504 |
| STD | 131.1 ± 9.1 | 128.4 ± 8.2 | — |
RedCHO, reduced-carbohydrate; STD, standard; ФB, β cell response to glucose during the basal condition; ФS, amount of insulin secreted for a given amount of glucose during nonbasal conditions; ФD, response of the β cell to an increase in glucose; X0, measure of the amount of insulin released immediately after the glucose stimulus; SRB, measure of the basal insulin secretion rate. β Cell response measures were log10-transformed before analysis. Mean and SEM values were back-transformed from log10 values used for analysis.
By ANCOVA; effect of diet on 8-wk β cell response measures, after adjustment for baseline β cell response measures.
Mean ± SEM (all such values).
P ≤ 0.05 (2-sample t test for significant differences between diet groups at baseline).
Conversion to International System of Units (pmol/L): multiply by 0.331.
FIGURE 1.
Mean (±SEM) difference in 8-wk static β cell response to glucose (ФS) by diet in European Americans and African Americans, adjusted for baseline ФS by ANCOVA: *P < 0.05 for diet effect between African Americans. P = 0.200 for diet effect between European Americans. P = 0.226 for diet × race interaction. Reduced-carbohydrate (RedCHO)/higher-fat diet: European Americans, n = 18; African Americans, n = 16. Standard (STD) diet: European Americans, n = 14; African Americans, n = 14.
FIGURE 2.
Change in glucose area under the curve (AUC) compared with change in static β cell response to glucose (ФS) by correlation; n = 61 (r = −0.33, P < 0.05).
FIGURE 3.
Serum concentrations of glucose, C-peptide, and insulin during the liquid meal tolerance test (LMTT) by diet group at baseline and at 8 wk. Each symbol represents the group mean for that time point. Error bars were omitted for clarity. A–C: (O) standard (STD) diet [55% carbohydrate, 18% protein, 27% fat (55C:18P:27F)]; D–F: (Δ) Reduced-carbohydrate (RedCHO)/higher-fat diet [43% carbohydrate, 18% protein, 39% fat (43C:18P:39F)]. Filled circles and triangles = baseline; open circles and triangles = 8 wk. n = 28 for the STD diet, and n = 34 for the RedCHO/higher fat diet. Includes only subjects who completed both baseline and 8-wk LMTT.
Results from the solid meal test at 4 wk indicated that the insulin response to the 2 diets differed, with peak insulin concentration and incremental insulin AUC being significantly lower after the RedCHO/higher-fat breakfast meal (peak insulin: 88.40 ± 57.97 μU/mL during the RedCHO/higher-fat meal and 124.17 ± 63.32 μU/mL during the STD meal, P < 0.05; incremental AUC: 7055.07 ± 4960.74 μU/mL during the RedCHO/higher-fat meal and 10,294.67 ± 6273.53 μU/mL during the STD meal, P < 0.01).
DISCUSSION
We observed that consumption of a RedCHO/higher-fat diet compared with an STD diet resulted in a lower insulin response to a fixed liquid meal in the absence of weight change, suggesting a down-regulation of β cell responsiveness. This response was particularly apparent among African Americans, a racial group with a disproportionately high risk of obesity (16) and type 2 diabetes (17). Our results indicated that this down-regulation of β cell responsiveness was associated with greater postchallenge glucose, suggesting an association with glycemic control.
Our study offers novel information regarding β cell sensitivity to glucose after adaptation to eucaloric diets differing in carbohydrate and fat content. We observed that the RedCHO/higher-fat diet compared with the STD diet resulted in 28.5% lower ФS after 8 wk in response to a fixed liquid meal. This reduction in ФS occurred in the absence of significant changes in either body weight or insulin sensitivity, suggesting a direct effect of the RedCHO/higher-fat diet on pancreatic physiology. ФS is thought to reflect the β cell response to glucose concentrations above basal. It is possible that the lower carbohydrate content of the RedCHO/higher-fat diet, and subsequent reduced demand for insulin, which resulted in a down-regulation of β cell responsiveness. Similarly in rats, consumption of a very-low-carbohydrate diet for 8 wk resulted in reduced insulin response to a glucose challenge (18). However, an alternative explanation is the higher fat content of the diet had an inhibitory or lipotoxic effect on β cell function, as has been shown with administration of exogenous lipid (7, 8). Studies have suggested that greater intake of some fats is associated with a lower postprandial insulin response (19, 20). Therefore, in our study, greater intake of omega-3 and oleic fatty acids in the RedCHO/higher-fat diet could be associated with lower postprandial insulin response. Interventions with fixed dietary carbohydrate and varied fat (or vice versa) are needed to determine whether carbohydrate or fat is responsible for change in β cell function.
Whether reduced ФS after a RedCHO/higher-fat diet is considered to be a positive or negative outcome for risk of metabolic disease cannot be determined from this study. Among overweight, hyperinsulinemic individuals, lower circulating insulin may be beneficial, with the assumption that glucose tolerance is maintained. However, we observed that change in ФS was inversely associated with change in glucose AUC. This association may indicate that lower insulin secretion resulted in reduced glycemic control. Alternatively, reduced glycemic control could lead to compromised β cell function. Elevations in glycemia have been associated with reductions in glucose-stimulated insulin secretion (2, 21–23). We also found that fasting glucose was significantly higher after consumption of the 8-wk RedCHO/higher-fat diet, suggesting poorer glycemic control. Further research is needed to determine the cause-and-effect relation between ФS and glucose AUC.
Subgroup analysis indicated that lower ФS after the RedCHO/higher-fat intervention was apparent mainly among African American subjects. African Americans, relative to European Americans, are characterized by having a relatively robust insulin response to intravenous glucose (11, 12) but lower basal and phase 2 β cell response to intravenous glucose (Ф2) (24). Whether specific dietary factors are involved in racial differences in insulin dynamics has not been extensively studied. The limited data available are from observational studies in children. In African American and European American children, the dietary fat:carbohydrate ratio was positively associated with first-phase insulin response (P = 0.08) and inversely associated with insulin clearance (P < 0.01) (25); vegetable intake was inversely associated with the acute insulin response (26). Thus, the present observation may be the first to examine the insulin response to a carefully controlled diet intervention. In this study, African Americans had a lower ФS at baseline than did European Americans (P < 0.05), which is an observation that agrees with lower Ф2 to intravenous glucose (24). The possibility that lower ФS among African Americans plays a role in greater risk of type 2 diabetes deserves further attention.
Relatively high postchallenge insulin concentrations may increase risk for weight gain (27). Previous studies have shown that humans or experimental animals with relatively high postchallenge insulin concentrations were uniquely responsive to dietary macronutrient content with regard to body weight change (4, 10, 28). These observations provoke speculation that a subgroup of individuals exists who show a relatively greater insulin secretory response to dietary carbohydrate, which in turn facilitates weight gain and impedes weight loss. Thus, we examined whether baseline postprandial insulin status (high or low) affected the insulin secretory response to diets differing in macronutrient content. Results indicated no outcome measure differed by baseline insulin secretory status—ie, differences between diets at 8 wk, or lack thereof, were similar across groups. Although the difference between diets in ФS at week 8 tended to be slightly stronger among subjects with low instead of high insulin-30 (P = 0.057 compared with P = 0.132), our data do not support a differential response based on insulin secretion phenotype.
The response to the intervention differed somewhat based on habitual diet. Subjects who reported greater consumption of carbohydrate under free-living conditions subsequently tended to have greater reduction in ФS with the RedCHO/higher-fat diet compared with the STD diet (P = 0.063). Out of 34 of these subjects, 28 reported less consumption of fat, on the basis of the median percentage fat from total calories (37.7%). Conversely, ФS response to the intervention diets did not differ for subjects who reported consuming relatively less carbohydrate in their usual diet before the intervention (P = 0.238). These results could mean that the RedCHO/higher-fat diet did not provide a significant reduction in carbohydrate or elevation in fat for those whose usual diet was relatively low in carbohydrate or high in fat. This suggests that individuals who usually consume a relatively high carbohydrate/low-fat diet may reduce their β cell responsiveness and glycemic control by consuming a diet lower in carbohydrate and higher in fat.
We found no effect of the diet intervention on insulin sensitivity. This was true in the entire group and in the subgroups. Few previous intervention studies have examined the effect of diet quality on insulin sensitivity under eucaloric conditions, particularly using robust techniques. Results of existing studies have been equivocal; some have indicated a beneficial effect of a lower carbohydrate diet (29, 30), whereas others have indicated no effect (31), and one indicated a less beneficial effect than with a lower-fat diet (9). Our study extends these observations by providing all food for 8 wk and by considering the potential role of the baseline, habitual diet. Differences between studies may reflect differences between subject populations, the insulin sensitivity test used, or the nature of the diet intervention.
Strengths of this study included tight control of the diet interventions with all food supplied; provision of a eucaloric diet, which avoided confounding by large changes in body composition or energy balance; use of diets consisting of foods and macronutrient profiles that may be practically consumed; use of a robust measure of insulin sensitivity; and use of C-peptide–based measures of β cell responsiveness. Limitations included a relatively small sample size and inability to dissociate changes in dietary carbohydrate from those in dietary fat. Also, intention-to-treat analysis was not performed to minimize the uncertainty of dropouts.
In conclusion, 8 wk of a RedCHO/higher-fat diet relative to an STD diet resulted in a lower insulin secretory response to a fixed meal challenge that was associated with higher glucose AUC. This response was particularly apparent among African Americans, a racial group more prone to both type 2 diabetes and obesity. Neither the precise mechanism whereby the diet intervention altered β cell responsiveness, nor the cause and effect nature of the relation between β cell responsiveness and glucose AUC, can be determined from our study. Nonetheless, these results are important in that they clearly indicate that diet can affect aspects of β cell responsiveness. Future research is needed to determine whether carbohydrate or fat is responsible for the change in β cell function and to probe the potential cause-and-effect relation between β cell function and glycemic control.
Acknowledgments
We gratefully acknowledge the help of Maryellen Williams and Cindy Zeng with laboratory analyses and of Betty Darnell with experimental design and diet development.
The authors’ responsibilities were as follows—LLG, PC-L, ACE, and KC: conducted the research; LLG, PC-L, WMG, and BAG: analyzed the data; and LLG and BAG: wrote the manuscript and had primary responsibility for final content. All authors participated in the design of the research project and read and approved the final manuscript. The authors had no conflicts of interest to disclose.
REFERENCES
- 1.Reaven GM. Role of insulin resistance in human disease. Diabetes 1988;37:1595–607 [DOI] [PubMed] [Google Scholar]
- 2.Del Prato S, Tiengo A. The importance of first-phase insulin secretion: implications for the therapy of type 2 diabetes mellitus. Diabetes Metab Res Rev 2001;17:164–74 [DOI] [PubMed] [Google Scholar]
- 3.Lillioja S, Nyomba BL, Saad MF, et al. Exaggerated early insulin release and insulin resistance in a diabetes-prone population: A metabolic comparison of Pima Indians and Caucasians. J Clin Endocrinol Metab 1991;73:866–76 [DOI] [PubMed] [Google Scholar]
- 4.Chaput J-P, Tremblay A, Rimm EB, Bouchard C, Ludwig DS. A novel interaction between dietary composition and insulin secretion: effects on weight gain in the Quebec Family Study. Am J Clin Nutr 2008;87:303–9 [DOI] [PubMed] [Google Scholar]
- 5.Del Prato S, Leonetti F, Simonson DC, Sheehan P, Matsuda M, DeFronzo R. Effect of sustained physiologic hyperinsulinaemia and hyperglycaemia on insulin secretion and insulin sensitivity in man. Diabetologia 1994;37:1025–35 [DOI] [PubMed] [Google Scholar]
- 6.Carpentier A, Mittelman SD, Lamarche B, Bergman RN, Giacca A, Lewis GF. Acute enhancement of insulin secretion by FFA in humans is lost with prolonged FFA elevation. Am J Physiol Endocrinol Metab 1999;276:E1055–66 [DOI] [PubMed] [Google Scholar]
- 7.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 1997;46:536. [PubMed] [Google Scholar]
- 8.McGarry JD, Dobbins RL. Fatty acids, lipotoxicity and insulin secretion. Diabetologia 1999;42:128–38 [DOI] [PubMed] [Google Scholar]
- 9.Lovejoy JC, Windhauser MM, Rood JC, de la Bretonne JA. Effect of a controlled high-fat versus low-fat diet on insulin sensitivity and leptin levels in African-American and Caucasian women. Metabolism 1998;47:1520–4 [DOI] [PubMed] [Google Scholar]
- 10.Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA 2007;297:2092–102 [DOI] [PubMed] [Google Scholar]
- 11.Haffner SM, D'Agostino R, Jr, Saad MF, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes 1996;45:742–8 [DOI] [PubMed] [Google Scholar]
- 12.Gower BA, Fernandez JR, Beasley TM, Shriver MD, Goran MI. Using genetic admixture to explain racial differences in insulin-related phenotypes. Diabetes 2003;52:1047–51 [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association Screening for type 2 diabetes. Diabetes Care 2003;26(suppl 1):S21–4 [DOI] [PubMed] [Google Scholar]
- 14.Harris JA, Benedict FG. A biometric study of basal metabolism in man. Washington, DC: Carnegie Institution, 1919 [Google Scholar]
- 15.Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes 2001;50:150–8 [DOI] [PubMed] [Google Scholar]
- 16.Ogden CL, Carroll MD. Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1976–1980 through 2007–2008. Atlanta, GA: Centers for Disease Control and Prevention, 2010. Available from: http://www.cdc.gov/NCHS/data/hestat/obesity_adult_07_08/obesity_adult_07_08.pdf [Google Scholar]
- 17.National Center for Health Statistics, Centers for Disease Control and Prevention Age-adjusted prevalence of diagnosed diabetes by race/ethnicity and sex, United States, 1980-2005. Available from: http://www.cdc.gov/diabetes/statistics/prev/national/figraceethsex.htm (cited 4 May 2010)
- 18.Axen KV, Axen K. Longitudinal adaptations to very low-carbohydrate weight-reduction diet in obese rats: body composition and glucose tolerance. Obesity. 2010;18(8):1538–44 [DOI] [PubMed] [Google Scholar]
- 19.Ryan M, McInerney D, Owens D, et al. Diabetes and the Mediterranean diet: a beneficial effect of oleic acid on insulin sensitivity, adipocyte glucose transport and endothelium-dependent vasoreactivity. QJM 2000;93:85–91 [DOI] [PubMed] [Google Scholar]
- 20.Madigan C, Ryan M, Owens D, et al. Dietary unsaturated fatty acids in type 2 diabetes: higher levels of postprandial lipoprotein on a linoleic acid-rich sunflower oil diet compared with an oleic acid-rich olive oil diet. Diabetes Care 2000;23:1472–711023139 [Google Scholar]
- 21.Rossetti L, Shulman GI, Zawalich W, DeFronzo R. Effect of chronic hyperglycemia on in vivo insulin secretion in partially pancreatectomized rats. J Clin Invest 1987;80:1037–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdul-Ghani MA, Tripathy D, DeFronzo R. Contribution of B-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 2006;29:1130–9 [DOI] [PubMed] [Google Scholar]
- 23.Lillioja S, Mott DM, Howard BV, et al. Impaired glucose tolerance as a disorder of insulin action. Longitudinal and cross-sectional studies in Pima Indians. N Engl J Med 1988;318:1217–25 [DOI] [PubMed] [Google Scholar]
- 24.Chandler-Laney PC, Phadke RP, Granger WM, et al. Age-related changes in insulin sensitivity and β-cell function among European-American and African-American women. Obesity (Silver Spring) 2011;19(3):528–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arslanian SA, Saad R, Lewy V, Danadian K, Janosky JE. Hyperinsulinemia in African-American children. Decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes 2002;51:3014–9 [DOI] [PubMed] [Google Scholar]
- 26.Lindquist CH, Gower BA, Goran MI. Role of dietary factors in ethnic differences in early risk of cardiovascular disease and type 2 diabetes. Am J Clin Nutr 2000;71:725–32 [DOI] [PubMed] [Google Scholar]
- 27.Ludwig DS, Majzoub JA, Al-Zahrani A, Dallal GE, Blanco I, Roberts SB. High glycemic index foods, overeating, and obesity. Pediatrics 1999;103:e26–31 [DOI] [PubMed] [Google Scholar]
- 28.Pawlak DB, Kushner JA, Ludwig DS. Effects of dietary glycaemic index on adiposity, glucose homeostasis, and plasma lipids in animals. Lancet 2004;364:778–85 [DOI] [PubMed] [Google Scholar]
- 29.Rizkalla SW, Taghrid L, Laromiguiere M, et al. Improved plasma glucose control, whole-body glucose utilization, and lipid profile on a low-glycemic index diet in type 2 diabetic men. Diabetes Care 2004;27:1866–72 [DOI] [PubMed] [Google Scholar]
- 30.Clapp JFI, Lopez B. Low- versus high-glycemic index diets in women: effects on caloric requirements, substrate utilization, and insulin sensitivity. Metab Syndr Relat Disord 2007;5:231–42 [DOI] [PubMed] [Google Scholar]
- 31.Shikany JM, Phadke RP, Redden DT, Gower BA. Effects of low- and high-glycemic index/glycemic load diets on coronary heart disease risk factors in overweight/obese men. Metabolism 2009;58:1793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]