Abstract
Background: Low concentrations of serum 25-hydroxyvitamin D [25(OH)D] may be associated with cardiometabolic disorders; however, little is known about their relation to intermediate metabolic and lipid markers.
Objective: We investigated the relation of serum 25(OH)D concentrations to fasting insulin, glucose, dyslipidemia, adiposity, and prevalent metabolic syndrome.
Design: We conducted this cross-sectional analysis in 292 postmenopausal women aged 50–79 y in the Women's Health Initiative Calcium–Vitamin D (WHI-CaD) trial. Data were collected from 3 nested case-control studies that measured baseline serum 25(OH)D concentrations. Inverse probability weighting was used to approximate parameter estimates for the WHI-CaD population.
Results: In weighted linear regression models adjusted for age, race-ethnicity, month of blood draw, region, case-control status, smoking, alcohol, physical activity, and history of cardiometabolic risk factors, there was an inverse association of serum 25(OH)D with adiposity [body mass index (BMI): β = −1.12 ± 0.30, P = 0.0002; waist circumference: β = −3.57 ± 0.49, P < 0.0001; waist-hip ratio: β = −0.01 ± 0.002, P < 0.0001], triglycerides (β = −0.10 ± 0.02, P < 0.0001), and triglyceride:HDL-cholesterol ratio (β = −0.11 ± 0.03, P = 0.0003). The multivariable-adjusted odds ratio for metabolic syndrome for the highest (≥52 nmol/L) compared with the lowest (<35 nmol/L) tertile of serum 25(OH)D concentrations was 0.28 (95% CI: 0.14, 0.56). Significant associations remained after adjustment for BMI. We observed no significant associations with LDL cholesterol, HDL cholesterol, insulin, glucose, homeostatic model assessment of insulin resistance (HOMA-IR), or homeostatic model assessment of β cell function (HOMA-β).
Conclusion: Higher serum 25(OH)D concentrations may be inversely associated with adiposity, triglycerides, triglyceride:HDL-cholesterol ratio, and metabolic syndrome but are not associated with LDL and HDL cholesterol, insulin, glucose, HOMA-IR, or HOMA-β in postmenopausal women. This trial was registered at clinicaltrials.gov as NCT00000611.
INTRODUCTION
Vitamin D deficiency is an increasingly recognized health concern related to skeletal and nonskeletal outcomes. Although accumulating evidence suggests that low concentrations of serum 25-hydroxyvitamin D [25(OH)D] may be associated with increased risk of cardiometabolic disorders including type 2 diabetes (1) and cardiovascular disease (2–4), biological mechanisms that underlie these relations remain poorly understood.
Vitamin D receptors are present on pancreatic β cells and insulin-sensitive tissues including skeletal muscle tissue (5), and vitamin D repletion improves insulin and glucose homeostasis in animal models of vitamin D deficiency (6, 7). However, findings from cross-sectional and prospective cohort studies that examined the relation of serum 25(OH)D to fasting insulin (8, 9), fasting glucose (8, 10), insulin resistance (8–13), and β cell dysfunction (10, 11, 13) in observational settings have been inconsistent. Low serum 25(OH)D concentrations may also be associated with dyslipidemia (14–16), but data to support this relation are sparse. Furthermore, the role of adiposity remains unclear. There have been few studies of the relation of serum 25(OH)D concentrations to these intermediate metabolic and lipid markers. Additional research in this area may provide insight into possible intermediate pathways for complex cardiometabolic diseases.
To investigate the hypothesis that higher serum 25(OH)D concentrations may be protective for cardiometabolic disease through beneficial effects on intermediate metabolic biomarkers and adiposity, we conducted a cross-sectional analysis in postmenopausal women enrolled in the Women's Health Initiative Calcium–Vitamin D (WHI-CaD) trial. In particular, we examined concentrations of serum 25(OH)D in relation to metabolic biomarkers including total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, the triglyceride:HDL cholesterol ratio, insulin, glucose, and insulin resistance and β cell dysfunction as measured by homeostatic model assessment, measures of adiposity, including body mass index (BMI; in kg/m2), waist circumference, and waist-hip ratio, and prevalent metabolic syndrome.
SUBJECTS AND METHODS
Study population
The WHI-CaD trial was designed to test the effect of calcium and vitamin D supplementation on bone fracture and colorectal cancer in postmenopausal women. A total of 36,282 participants were randomly assigned in a double-blind fashion to consume either 1000 mg elemental calcium (as calcium carbonate) and 400 IU of vitamin D3 or a placebo. Details on the design and recruitment have been published elsewhere (17, 18). Eligibility criteria for the WHI-CaD trial included no medical condition associated with a predicted survival of <3 y, no prior history of renal calculi, hypercalcemia, corticosteroid use, and calcitriol use, and no safety, adherence, or retention issues (18). All Women's Health Initiative (WHI) study procedures were approved by the institutional review board at each clinical center, and all women provided written informed consent before participating in the study.
The current study took advantage of data collected from 3 nested case-control studies examining fractures (19), breast cancer (20), and colorectal cancer (21) that measured baseline serum 25(OH)D concentrations in women enrolled in the WHI-CaD trial. Controls were free of disease for the duration of the study and were individually matched to case participants according to age, latitude of the clinical center, race-ethnic group, and date of venipuncture. The sample for the current study included women with available measurements of serum 25(OH)D from these case-control studies as well as overlapping measurements of fasting insulin, glucose, triglycerides, total cholesterol, LDL cholesterol, and HDL cholesterol collected previously in a 6% subsample of clinical trial participants. The sample included incident cases of fracture, breast cancer, and colorectal cancer (n = 166 cases total) ascertained over a mean follow-up period of 7.0 y.
To account for sampling on the basis of case-control status and prior matching, we used inverse probability weighting to provide approximate parameter estimates for the entire WHI-CaD population. In addition, the case-control status and all matching variables including age, ethnicity, geographic region (proxy for latitude of clinical center), and month of blood draw were adjusted for in weighted multivariable models.
Baseline measurements
Certified WHI trained staff measured the height, weight, waist and hip circumference, and blood pressure of each subject at the baseline visit. Height (in cm) was measured with a wall-mounted stadiometer, and weight (in kg) was measured with a balance-beam scale. BMI was calculated as weight (in kg) divided by height (in m2). Waist and hip circumferences (in cm) were determined with a standardized measuring tape. Standardized questionnaires including information on age, ethnicity, education, income, occupation, medical and family histories, smoking status, alcohol use, recreational physical activity, and medication and supplement use were administered at the baseline visit. Metabolic syndrome was defined on the basis of updated guidelines proposed by the International Diabetes Federation, American Heart Association, and National Heart, Lung, and Blood Institute (22) as a presentation with ≥3 of the following criteria: 1) waist circumference ≥88 cm for women, 2) triglyceride concentration ≥150 mg/dL, 3) HDL concentration <50 mg/dL for women, 4) systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg, and 5) fasting glucose concentration ≥100 mg/dL.
Blood collection and assessment of biomarkers
Fasting blood specimens were collected from all participants at baseline according to a standardized protocol. Participants were instructed to fast for 12 h before collection, take all regular medications except for diabetes medication, take no aspirin or nonsteroidal antiinflammatory drugs for 48 h before the visit except for those medications taken regularly, refrain from smoking for 1 h before the visit, and perform no vigorous physical activity for 12 h before the visit. Aliquots of serum, plasma, and buffy coat were frozen and shipped on dry ice to a central repository and stored at −70°C for future assays.
Serum 25(OH)D was measured with the DiaSorin Liaison 25(OH)D chemiluminescent immunoassay system at Diasorin headquarters (Stillwater, MN). Serum insulin was measured by using the stepwise sandwich enzyme-linked immunosorbent assay procedure with an ES 300 (Boehringer Mannheim Diagnostics, Indianapolis, IN). Glucose was measured in serum by using the hexokinase method with a Hitachi 747 analyzer (Boehringer Mannheim Diagnostics). Total cholesterol and triglycerides were measured by enzymatic methods with a Hitachi 747 analyzer (Boehringer Mannheim Diagnostics) as previously described (23). HDL cholesterol was isolated by using heparin-manganese chloride and measured enzymatically with a Hitachi 747 analyzer (Boehringer Mannheim Diagnostics). LDL cholesterol concentrations were calculated by using Friedewald's formula as follows (23, 24):
The CVs for each analyte were 11.8% for serum 25(OH)D, 5.3–8.8% for insulin, 2.0–2.3% for glucose, 0.8–1.3% for total cholesterol, 1.5–2.2% for LDL cholesterol, 1.8–2.0% for triglycerides, and 2.4–2.6% for HDL cholesterol.
Statistical analysis
Differences in baseline characteristics of the study population across tertiles of serum 25(OH)D were compared by using analysis of variance and Pearson's chi-square test. Continuous outcomes with skewed distributions were logarithmically transformed before analysis to achieve normal distributions. The homeostatic model assessment of insulin resistance (HOMA-IR) was computed by using the formula
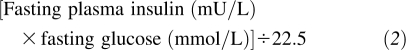 |
The homeostatic model assessment of β cell function (HOMA-β) was calculated as follows (25, 26):
 |
We divided participants into clinically relevant categories of serum 25(OH)D concentrations (<50, 50–75, and >75 nmol/L) (27–29) as well as tertiles for categorical analysis [to convert 25(OH)D concentrations in nmol/L to ng/mL, divide by 2.496). Tertiles are presented because of small numbers within certain clinically relevant categories (>75 nmol/L).
We used inverse probability weighting to account for prior matching in the 3 nested case-control studies to allow findings in our sampled study population to be representative of the entire WHI-CaD population (n = 36,282). Inverse probability weights equal to the inverse of the conditional probability of being included in the sample were estimated by fitting a logistic regression model that included outcomes from the 3 case-control studies (hip, spine, lower arm, and wrist fractures; invasive breast cancer; and colorectal cancer) and matching variables (age, race-ethnicity, month of blood draw, and geographic region) as predictor variables. All analyses were performed as weighted analyses (with the Proc GenMod procedure in SAS software; version 9.2; SAS Institute, Cary, NC).
We performed weighted multiple linear regression models to compute geometric means of biomarker concentrations across categories of serum 25(OH)D after adjusting for potential confounding variables. Geometric means were calculated by regressing the natural logarithmic values of plasma concentrations of biomarkers on serum 25(OH)D concentrations and taking the antilog of the resulting mean logarithmic value. To test for the linear trend across increasing categories of serum 25(OH)D, we used the median value of each category as a continuous variable in the model. We also calculated the corresponding changes in biomarker concentrations and measures of adiposity associated with an increase of 25 nmol/L (10 ng/mL) of serum 25(OH)D concentration. To model the shape of the dose-response relation between serum 25(OH)D and biomarker outcomes while allowing for variation within and across categories (30), we fit restricted, weighted, quadratic spline models with knots at medians of tertiles of serum 25(OH)D concentrations (26, 43, and 70 nmol/L). The resulting curves from the adjusted spline models were plotted to provide a visual representation of the dose-response trend. In addition, we performed weighted logistic regression models to assess the odds ratios (ORs) and 95% CIs of prevalent metabolic syndrome across tertiles of serum 25(OH)D concentrations. A comparison of the highest (≥52 nmol) to the lowest (<35 nmol/L) tertile of serum 25(OH)D concentrations was of particular interest because tertile boundaries approximately coincided with clinically relevant cutoffs (<30 and ≥50 nmol/L) recently proposed in the 2011 Institute of Medicine report (28, 31).
In multivariable analyses, we first adjusted for case-control status (yes or no) and matching factors including age (continuous), race-ethnicity (white, black, Hispanic, and Asian), geographic region (Northeast, South, Midwest, West), and month of blood draw (model 1). In addition, we adjusted for smoking status (never, past, and current smokers), alcohol intake (never, past, and current drinkers), physical activity (continuous), and a composite risk-factor profile that incorporated the history of metabolic risk factors including hypertension, high cholesterol that required medication, myocardial infarction, stroke, or prior treatment of diabetes (“yes” if participant had at least one history of a risk factor and “no” if otherwise) (model 2). The composite risk-factor variable was excluded when we modeled odds of metabolic syndrome because of an overlap between conditions included in the historical profile and metabolic syndrome, the outcome of interest. Further adjustments were made for the use of supplemental vitamin D, calcium, or magnesium or multivitamins with minerals (yes or no) (model 3), and BMI (continuous) (model 4). We also adjusted for waist circumference, although because of the high correlation between BMI and waist circumference (R = 0.84) we did not adjust for both variables in the same model. To further examine whether the association between serum 25(OH)D and cardiometabolic biomarker concentrations was modified by measures of adiposity including BMI and waist circumference prior history of disease, season, and vitamin D supplementation, we conducted stratified analyses by BMI (<30 and ≥30), waist circumference (<88 cm and ≥88 cm), prior history of metabolic risk factors including hypertension, high cholesterol requiring medication, myocardial infarction, stroke, or prior treatment of diabetes (yes or no), season (winter, spring, summer, an fall), and use of vitamin D supplementation (yes or no). We also entered multiplicative interaction terms into the model for other lifestyle and demographic confounders and tested their significance by using likelihood ratio tests.
All P values were 2-tailed, and P < 0.05 was considered to indicate statistical significance unless otherwise specified. All statistical analyses were conducted with SAS software (version 9.2; SAS Institute).
RESULTS
As shown in Table 1, women in the highest tertile of serum 25(OH)D concentrations were generally healthier than women with lower concentrations; these women had a lower BMI and waist circumference, were more likely to be physically active, were less likely to have prevalent metabolic syndrome, and reported higher intakes of vitamin D, calcium, and magnesium. Metabolic profiles also varied across tertiles of serum 25(OH)D concentrations; insulin, HOMA-IR, and LDL cholesterol were lower in women with higher serum 25(OH)D concentrations, and HDL cholesterol concentrations were higher in this group.
TABLE 1.
Baseline characteristics according to tertiles of serum 25-hydroxyvitamin D [25(OH)D] in postmenopausal women (n = 292)1
| Serum 25(OH)D |
|||||
| Total (median: 47 nmol/L) | Tertile (<35 nmol/L) | Tertile 2 (35–51 nmol/L) | Tertile 3 (≥52 nmol/L) | P | |
| n | 292 | 96 | 94 | 102 | |
| Age (y) | 63.3 ± 7.52 | 62.4 ± 7.6 | 64.3 ± 7.5 | 63.4 ± 7.3 | 0.48 |
| BMI (kg/m2) | 28.7 ± 5.6 | 30.9 ± 6.3 | 28.3 ± 5.1 | 27.0 ± 4.6 | <0.0001 |
| Waist circumference (cm) | 87.7 ± 12.5 | 91.9 ± 13.1 | 87.5 ± 12.6 | 84.0 ± 10.7 | <0.0001 |
| Waist-hip ratio | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.13 |
| Current smoker (%) | 9 | 13 | 11 | 4 | 0.21 |
| Current drinker (%) | 65 | 62 | 63 | 68 | 0.70 |
| Physical activity (METs/wk) | 9.8 ± 12.0 | 7.9 ± 10.1 | 9.3 ± 9.1 | 12.0 ± 15.3 | 0.02 |
| Metabolic syndrome (%) | 33 | 42 | 31 | 26 | <0.0001 |
| Diabetes ever (% yes) | 8 | 8 | 9 | 6 | 0.73 |
| Hypertension ever (% yes) | 35 | 40 | 34 | 30 | 0.36 |
| Systolic blood pressure (mm Hg) | 127.8 ± 16.2 | 127.4 ± 15.9 | 128.3 ± 16.2 | 127.5 ± 16.7 | 0.98 |
| Diastolic blood pressure (mm Hg) | 74.6 ± 8.8 | 75.5 ± 8.4 | 74.6 ± 9.4 | 73.7 ± 8.6 | 0.14 |
| Race-ethnicity (%) | |||||
| White | 64 | 51 | 71 | 70 | 0.005 |
| Black | 14 | 24 | 14 | 6 | 0.001 |
| Hispanic | 11 | 16 | 7 | 9 | 0.14 |
| Asian | 7 | 4 | 3 | 12 | 0.03 |
| Geographic region (%) | |||||
| Northeast | 24 | 22 | 30 | 22 | 0.32 |
| South | 27 | 29 | 26 | 26 | 0.84 |
| Midwest | 24 | 22 | 22 | 28 | 0.48 |
| West | 24 | 27 | 22 | 24 | 0.73 |
| Supplemental nutrient intake | |||||
| Vitamin D (μg/d) | 4.6 ± 6.4 | 1.8 ± 3.9 | 5.8 ± 5.5 | 6.2 ± 7.9 | <0.0001 |
| Calcium (mg/d) | 284.6 ± 407.1 | 100.9 ± 245.1 | 301.8 ± 368.0 | 439.7 ± 488.7 | <0.0001 |
| Magnesium (mg/d) | 49.7 ± 89.3 | 21.5 ± 56.7 | 53.8 ± 73.8 | 72.1 ± 116.6 | 0.0001 |
| Metabolic biomarkers | |||||
| Insulin (μIU/mL) | 11.0 ± 7.0 | 12.9 ± 8.5 | 10.5 ± 6.9 | 9.8 ± 5.0 | 0.003 |
| Glucose (mg/dL) | 100.2 ± 28.3 | 103.2 ± 28.0 | 98.7 ± 26.8 | 98.8 ± 30.1 | 0.33 |
| HOMA-IR | 2.9 ± 2.7 | 3.6 ± 3.1 | 2.7 ± 2.5 | 2.6 ± 2.5 | 0.02 |
| HOMA-β | 126.7 ± 97.5 | 131.9 ± 87.1 | 129.9 ± 124.8 | 118.7 ± 75.0 | 0.33 |
| Total cholesterol (mg/dL) | 218.2 ± 37.4 | 220.9 ± 38.7 | 219.9 ± 36.6 | 214.2 ± 36.8 | 0.19 |
| LDL cholesterol (mg/dL) | 128.0 ± 34.3 | 133.1 ± 35.0 | 128.6 ± 32.5 | 122.6 ± 34.7 | 0.03 |
| HDL cholesterol (mg/dL) | 60.0 ± 15.7 | 56.7 ± 15.2 | 60.6 ± 14.2 | 62.6 ± 17.1 | 0.01 |
| Triglycerides (mg/dL) | 151.2 ± 70.3 | 155.5 ± 63.2 | 153.4 ± 81.6 | 145.1 ± 65.4 | 0.28 |
| Triglyceride:HDL-cholesterol ratio | 2.8 ± 1.8 | 3.0 ± 1.7 | 2.8 ± 2.1 | 2.6 ± 1.6 | 0.08 |
METs, metabolic equivalent tasks; HOMA-IR, homeostatic model assessment of insulin resistance; HOMA-β, homeostatic model assessment of β cell function.
Mean ± SD (all such values).
The multivariable-adjusted geometric means of cardiometabolic biomarkers across tertiles of serum 25(OH)D concentrations as well as the linear regression coefficients for a corresponding increase in 25 nmol/L of the serum 25(OH)D concentration is shown in Table 2. Overall, we observed that higher serum 25(OH)D concentrations were inversely associated with insulin, HOMA-IR, HOMA-β, triglycerides, and the triglyceride:HDL-cholesterol ratio but not fasting glucose, total cholesterol, LDL cholesterol, or HDL cholesterol after controlling for age, race-ethnicity, month of blood draw, geographic region, and case-control status in categorical analyses. Multivariable-adjusted geometric means across increasing tertiles of serum 25(OH)D were 11.3, 10.1, 9.9 μIU/mL for insulin concentrations (P for linear trend < 0.0001); 2.82, 2.48, and 2.45 for HOMA-IR (P = 0.002); 117.9, 111.9, and 103.0 for HOMA-β (P = 0.02); 152.9, 142.4, and 117.0 mg/dL for triglyceride concentrations (P < 0.0001); and 2.9, 2.5, and 2.1 for the triglyceride:HDL cholesterol ratio (P < 0.0001) (model 1). After further adjustment for smoking status, alcohol intake, physical activity, history of cardiometabolic risk factors, use of supplements, and BMI, inverse associations with insulin, HOMA-IR, and HOMA-β were attenuated, whereas inverse associations with triglycerides and the triglyceride:HDL- cholesterol ratio remained statistically significant. With the assumption of a linear relation, an increase of 25 nmol/L of the serum 25(OH)D concentration was inversely associated with triglycerides (β = −0.08 ± 0.02, P < 0.0001) and the triglyceride:HDL cholesterol ratio (β = −0.08 ± 0.04, P = 0.04) (model 4). There appeared to be a suggestion of an inverse trend with total cholesterol with a small but significant decrease observed per 25 nmol/L increase in the 25(OH)D concentration (β = −0.03 ± 0.01; P = 0.04), although the trend was not significant in categorical analyses (model 4). After further adjustment for waist circumference (in place of BMI), the inverse associations remained significant for triglycerides (β = −0.06 ± 0.02; P = 0.0001) and borderline significant for the triglyceride:HDL ratio (β = −0.04 ± 0.02; P = 0.10) (data not shown).
TABLE 2.
Adjusted geometric means of insulin, glucose, homeostatic model assessment of insulin resistance (HOMA-IR), homeostatic model assessment of β cell function (HOMA-β), triglycerides, total cholesterol, LDL cholesterol, HDL cholesterol, and the triglyceride:HDL ratio across tertiles of serum 25-hydroxyvitamin D [25(OH)D] and linear regression coefficients for a corresponding 25-nmol/L increase in serum 25(OH)D in a sample of postmenopausal women in the Women's Health Initiative Calcium–Vitamin D (WHI-CaD) trial (n = 292)1
| Serum 25(OH)D |
Change in biomarker concentrations for each 25-nmol/L increase in the serum 25(OH)D concentration | ||||
| Tertile 1 (<35 nmol/L) | Tertile 2 (35–51 nmol/L) | Tertile 3 (≥52 nmol/L) | P for linear trend | ||
| Median (nmol/L) | 25.7 | 43.0 | 69.6 | ||
| Insulin (μIU/mL) | |||||
| Model 1 | 11.3 (10.4, 12.3)2 | 10.1 (9.0, 11.3) | 9.9 (9.5, 10.3) | <0.0001 | −0.07 ± 0.03 (0.01)3 |
| Model 2 | 10.0 (8.8, 11.3) | 9.4 (8.2, 10.7) | 9.3 (8.1, 10.6) | 0.07 | −0.02 ± 0.02 (0.47) |
| Model 3 | 9.8 (8.6, 11.3) | 9.5 (8.3, 10.9) | 9.4 (8.3, 10.7) | 0.40 | −0.003 ± 0.03 (0.92) |
| Model 4 | 9.2 (8.1, 10.5) | 10.3 (9.3, 11.4) | 10.3 (9.4, 11.3) | 0.11 | 0.06 ± 0.03 (0.05) |
| Glucose (mg/dL) | |||||
| Model 1 | 100.5 (94.0, 107.4) | 99.7 (96.6, 102.9) | 100.5 (93.8, 107.6) | 0.95 | −0.02 ± 0.01 (0.008) |
| Model 2 | 101.3 (98.6, 104.1) | 98.1 (94.7, 101.5) | 99.3 (92.7, 106.4) | 0.59 | −0.006 ± 0.01 (0.54) |
| Model 3 | 99.8 (96.2, 103.6) | 99.6 (96.5, 102.8) | 101.3 (93.8, 109.4) | 0.69 | 0.01 ± 0.01 (0.52) |
| Model 4 | 98.7 (93.7, 103.9) | 101.5 (98.0, 105.2) | 103.2 (96.0, 110.8) | 0.37 | 0.02 ± 0.01 (0.13) |
| HOMA-IR | |||||
| Model 1 | 2.82 (2.69, 2.95) | 2.48 (2.18, 2.83) | 2.45 (2.23, 2.69) | 0.002 | −0.09 ± 0.03 (0.002) |
| Model 2 | 2.53 (2.31, 2.77) | 2.29 (1.99, 2.64) | 2.31 (2.01, 2.65) | 0.22 | −0.02 ± 0.04 (0.54) |
| Model 3 | 2.47 (2.21, 2.75) | 2.36 (2.07, 2.69) | 2.40 (2.08, 2.76) | 0.83 | 0.01 ± 0.04 (0.86) |
| Model 4 | 2.28 (2.02, 2.58) | 2.61 (2.35, 2.89) | 2.64 (2.39, 2.91) | 0.25 | 0.08 ± 0.04 (0.09) |
| HOMA-β | |||||
| Model 1 | 117.9 (96.4, 144.1) | 111.9 (99.7, 125.6) | 103.0 (87.4, 121.3) | 0.02 | −0.03 ± 0.02 (0.25) |
| Model 2 | 96.5 (79.4, 117.2) | 104.8 (89.3, 123.0) | 95.4 (75.7, 120.3) | 0.39 | −0.001 ± 0.01 (0.87) |
| Model 3 | 99.1 (82.1, 119.6) | 101.5 (83.4, 123.4) | 91.7 (70.5, 119.2) | 0.18 | −0.03 ± 0.03 (0.28) |
| Model 4 | 96.3 (79.9, 116.2) | 105.0 (88.6, 124.5) | 94.8 (74.0, 121.4) | 0.36 | −0.01 ± 0.02 (0.80) |
| Triglycerides (mg/dL) | |||||
| Model 1 | 152.9 (149.3, 156.6) | 142.4 (118.4, 171.3) | 117.0 (111.1, 123.3) | <0.0001 | −0.13 ± 0.01 (<0.0001) |
| Model 2 | 148.6 (142.1, 155.3) | 136.6 (118.9, 156.9) | 117.3 (108.4, 127.0) | <0.0001 | −0.10 ± 0.02 (<0.0001) |
| Model 3 | 151.0 (141.5, 161.0) | 134.3 (113.4, 159.0) | 114.8 (102.7, 128.4) | <0.0001 | −0.11 ± 0.02 (<0.0001) |
| Model 4 | 145.8 (135.4, 157.1) | 139.6 (118.1, 165.0) | 119.7 (108.7, 131.9) | <0.0001 | −0.08 ± 0.02 (<0.0001) |
| Total cholesterol (mg/dL) | |||||
| Model 1 | 231.9 (206.0, 222.0) | 207.1 (195.0, 220.0) | 202.6 (192.0, 231.9) | 0.23 | −0.04 ± 0.02 (0.03) |
| Model 2 | 225.3 (213.6, 237.6) | 213.3 (202.2, 224.9) | 208.6 (197.2, 220.7) | 0.05 | −0.04 ± 0.01 (0.004) |
| Model 3 | 221.6 (213.2, 230.3) | 217.0 (203.5, 231.5) | 213.4 (201.7, 225.7) | 0.26 | −0.02 ± 0.01 (0.05) |
| Model 4 | 221.9 (214.0, 230.1) | 216.5 (202.9, 231.1) | 212.8 (201.5, 224.8) | 0.22 | −0.03 ± 0.01 (0.04) |
| LDL cholesterol (mg/dL) | |||||
| Model 1 | 126.3 (119.8, 133.1) | 115.7 (108.0, 124.0) | 116.9 (114.8, 119.1) | 0.08 | −0.05 ± 0.01 (<0.0001) |
| Model 2 | 136.1 (127.9, 144.8) | 121.7 (113.6, 130.3) | 123.1 (111.7, 135.7) | 0.04 | −0.05 ± 0.02 (0.0006) |
| Model 3 | 133.5 (127.4, 140.0) | 124.2 (115.3, 133.8) | 126.4 (114.1, 140.1) | 0.43 | −0.03 ± 0.02 (0.09) |
| Model 4 | 133.4 (127.1, 140.0) | 124.6 (115.7, 134.3) | 126.8 (114.9, 139.9) | 0.47 | −0.03 ± 0.02 (0.07) |
| HDL cholesterol (mg/dL) | |||||
| Model 1 | 52.0 (50.4, 53.7) | 57.2 (54.5, 60.1) | 56.8 (48.6, 66.3) | 0.32 | 0.03 ± 0.03 (0.41) |
| Model 2 | 54.2 (51.3, 57.2) | 58.5 (56.9, 60.1) | 57.5 (52.2, 63.3) | 0.47 | 0.01 ± 0.03 (0.73) |
| Model 3 | 53.2 (51.3, 55.3) | 59.6 (58.1, 61.2) | 58.9 (54.9, 63.3) | 0.13 | 0.02 ± 0.02 (0.42) |
| Model 4 | 54.4 (52.0, 56.8) | 57.8 (56.3, 59.4) | 57.1 (53.6, 60.8) | 0.54 | −0.005 ± 0.03 (0.85) |
| Triglyceride:HDL cholesterol | |||||
| Model 1 | 2.9 (2.8, 3.1) | 2.5 (2.0, 3.1) | 2.1 (1.8, 2.4) | <0.0001 | −0.15 ± 0.03 (<0.0001) |
| Model 2 | 2.7 (2.6, 2.8) | 2.3 (2.0, 2.7) | 2.0 (1.8, 2.3) | <0.0001 | −0.11 ± 0.03 (0.0003) |
| Model 3 | 2.8 (2.7, 3.0) | 2.3 (1.9, 2.7) | 1.9 (1.7, 2.3) | <0.0001 | −0.13 ± 0.04 (0.0002) |
| Model 4 | 2.7 (2.5, 2.9) | 2.4 (2.0, 2.8) | 2.1 (1.9, 2.3) | <0.0001 | −0.08 ± 0.04 (0.04) |
Model 1 was adjusted for matching factors (age, race-ethnicity, month of blood draw, and geographic region) and case-control status (yes or no). Model 2 was adjusted for variables in model 1 plus smoking status, alcohol intake, physical activity, and history of cardiometabolic risk factors [including hypertension, high cholesterol that required medication, myocardial infarction, stroke, or prior treatment of diabetes (yes or no)]. Model 3 was adjusted for variables in model 2 plus the use of supplemental vitamins including vitamin D, calcium, or magnesium or multivitamins with minerals (yes or no). Model 4 was adjusted for variables in model 3 plus BMI. To convert 25(OH)D concentrations in nmol/L to ng/mL, divide by 2.496.
Adjusted geometric mean; 95% CI in parentheses (all such values).
Linear regression coefficient ± SD; P values in parentheses (all such values).
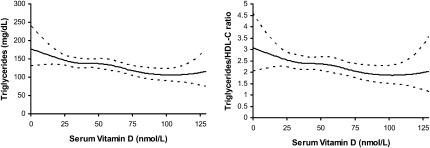
Smoothed dose-response curves generated from restricted quadratic spline models (model 4) were consistent with these findings and showed a decrease in triglycerides and the triglyceride:HDL cholesterol ratio as serum 25(OH)D concentrations rose (Figure 1). In subgroup analyses stratified by BMI (<30 and ≥30) and waist circumference (<88 and ≥88 cm), we observed a significant positive association with HDL cholesterol in women with higher adiposity that was not present in normal-weight women (HDL cholesterol: P for trend = 0.0002 in women with BMI ≥30; P = 0.001 in women with a waist circumference ≥88 cm). Although multiplicative interaction terms entered into the model were not significant (BMI: P for interaction = 0.16; waist circumference: P for interaction = 0.31), the notable difference in significant trends across groups suggested that adiposity may have modified the association of serum 25(OH)D with HDL cholesterol concentrations. Similarly, inverse associations with triglycerides and the triglyceride:HDL ratio appeared more pronounced during the winter and spring (compared with during the summer and fall) (data not shown). We observed no significant interactions with a prior history of disease, use of supplements, and other lifestyle factors including alcohol, smoking, and physical activity for all metabolic outcomes.
FIGURE 1.
Restricted quadratic spline plots showing the fully adjusted geometric means (solid lines) and pointwise 95% CIs (dashed lines) of triglycerides (mg/dL) (left panel) and the triglyceride:HDL-cholesterol (HDL-C) ratio (right panel) by serum vitamin D concentrations (nmol/L); n = 292. Three knots at medians of serum 25-hydroxyvitamin D (Serum Vitamin D) concentration tertiles (26, 43, and 70 nmol/L). All models were adjusted for matching factors (age, race-ethnicity, month of blood draw, and geographic region), case-control status (yes or no), smoking status, alcohol intake, physical activity, history of cardiometabolic risk factors [including hypertension, high cholesterol that required medication, myocardial infarction, stroke, or prior treatment of diabetes (yes or no)], use of supplemental vitamins including vitamin D, calcium, or magnesium or multivitamins with minerals (yes or no), and BMI.
The multivariable-adjusted associations of serum 25(OH)D concentrations with BMI, waist circumference, and the waist-hip ratio (model 2) are shown in Table 3. Serum 25(OH)D concentrations were consistently and inversely associated with all 3 measures of adiposity (P < 0.001 for all) even after controlling for demographic and lifestyle risk factors including physical activity and geographic region, suggesting that serum 25(OH)D concentrations may contribute to or be affected by adiposity apart from these factors (the magnitude and statistical significance of the regression coefficients did not change materially in models 1 and 3).
TABLE 3.
Multivariable-adjusted relations of serum 25-hydroxyvitamin D [25(OH)D] with measures of adiposity in a sample of postmenopausal women in the Women's Health Initiative Calcium–Vitamin D (WHI-CaD) trial (n = 292)1
| Change in metabolic risk factor for each 25-nmol/L increase in serum 25(OH)D | P | |
| BMI (kg/m2) | −1.12 ± 0.30 | 0.0002 |
| Waist circumference (cm) | −3.57 ± 0.49 | <0.0001 |
| Waist-hip ratio | −0.01 ± 0.002 | <0.0001 |
All values are linear regression coefficients ± SDs. Models were adjusted for matching factors (age, race-ethnicity, month of blood draw, and geographic region), case-control status (yes or no), smoking status, alcohol intake, physical activity, and history of cardiometabolic risk factors [including hypertension, high cholesterol that required medication, myocardial infarction, stroke, or prior treatment of diabetes (yes or no)].
As shown in Table 4, women in the highest tertile of serum vitamin D concentrations (≥52 nmol/L) were less likely to have prevalent metabolic syndrome compared with those in the lowest tertile (<35 nmol/L) (26% compared with 42% of women, respectively; P < 0.0001). After adjustment for matching factors (age, race-ethnicity, month of blood draw, and region), case-control status, smoking, alcohol, physical activity, and use of supplements, the multivariable-adjusted OR for metabolic syndrome for the highest (≥52 nmol/L) compared with the lowest (<35 nmol/L) tertile of serum 25(OH)D concentrations was 0.28 (95% CI: 0.14, 0.56) (model 3). After the final adjustment for BMI, the association was slightly attenuated but remained significant.
TABLE 4.
Multivariable-adjusted odds ratios (95% CIs) of metabolic syndrome across tertiles of serum 25-hydroxyvitamin D [25(OH)D] concentrations in a sample of postmenopausal women in the Women's Health Initiative Calcium–Vitamin D (WHI-CaD) trial (n = 292)1
| Serum 25(OH)D |
||||
| Tertile 1 (<35 nmol/L) | Tertile 2 (35–51 nmol/L) | Tertile 3 (≥52 nmol/L) | P for linear trend | |
| Median (nmol/L) | 25.7 | 43.0 | 69.6 | |
| Metabolic syndrome | ||||
| Unadjusted prevalence (%) | 42 | 31 | 26 | <0.0001 |
| Model 1 | 1.00 | 0.37 (0.21, 0.67) | 0.35 (0.25, 0.48) | <0.0001 |
| Model 2 | 1.00 | 0.33 (0.20, 0.52) | 0.31 (0.21, 0.47) | <0.0001 |
| Model 3 | 1.00 | 0.30 (0.15, 0.61) | 0.28 (0.14, 0.56) | 0.0002 |
| Model 4 | 1.00 | 0.43 (0.20, 0.93) | 0.38 (0.16, 0.91) | 0.03 |
Odds ratios and 95% CIs were derived from weighted logistic regression models. Model 1 was adjusted for matching factors (age, race-ethnicity, month of blood draw, and geographic region) and case-control status (yes or no). Model 2 was adjusted for variables in model 1 plus smoking status, alcohol intake, and physical activity. Model 3 was adjusted for variables in model 2 plus the use of supplemental vitamins including vitamin D, calcium, or magnesium or multivitamins with minerals (yes or no). Model 4 was adjusted for variables in model 3 plus BMI.
DISCUSSION
In apparently healthy postmenopausal women enrolled in the WHI-CaD trial, serum 25(OH)D concentrations were inversely associated with triglycerides and the triglyceride:HDL cholesterol ratio, measures of adiposity, and prevalent metabolic syndrome. These associations appeared to be independent of demographic characteristics and traditional risk factors for cardiometabolic disorders. We observed no significant associations between serum 25(OH)D concentrations and LDL cholesterol, HDL cholesterol, fasting insulin and glucose, or insulin resistance and β cell dysfunction as reflected by HOMA measures.
Abnormalities in concentrations of triglycerides, which are a primary source of fat storage in the blood, and HDL cholesterol, and the lipoprotein responsible for the transport of cholesterol back to the liver for excretion are 2 major criteria for metabolic syndrome. The ratio of triglycerides to HDL cholesterol is also a marker for the atherogenic effect of circulating lipids (32). Although substantial variability has been observed in the association between serum 25(OH)D and dyslipidemia (11, 15, 33–37), the inverse association with triglycerides has been reported fairly consistently in both cross-sectional and prospective cohort studies in diverse populations (8, 11, 14–16, 38–42). In line with these findings, we observed an inverse association between serum 25(OH)D and triglycerides as well as the triglyceride:HDL ratio. The potential biological mechanism underlying this relation is still not completely understood but may be mediated, in part, by the effects of dietary calcium. Higher serum 25(OH)D concentrations increase the absorption of intestinal calcium (43), which may bind to fatty and bile acids and form insoluble lipid-calcium complexes, thereby inhibiting the absorption of cholesterol and increasing fecal excretion (44, 45). Alternatively, the association may be mediated by reductions in the hepatic triglyceride formation or secretion in response to increased hepatocellular calcium amounts (46). Excess concentrations of parathyroid hormone associated with low serum 25(OH)D concentrations (47) may also drive this association; the decreased peripheral removal of triglycerides and hypertriglyceridemia has been observed in states of hyperparathyroidism (48). Some (49) but not all (50, 51) randomized trials that tested the effects of varying doses of vitamin D supplementation on metabolic outcomes have reported decreases in triglycerides in response to vitamin D supplementation, although the dosage in the null studies (50) may have been too low to achieve beneficial serum 25(OH)D concentrations. Thus, additional investigation of the relation of serum 25(OH)D to triglycerides seems warranted in large randomized settings of vitamin D supplementation.
Vitamin D receptors have been identified on pancreatic β cells (52), and the active metabolite of vitamin D, 1,25-dihydroxyvitamin D, is thought to be required for normal glucose-stimulated insulin release from β cells (53). Although cross-sectional and prospective inverse associations with insulin resistance (8, 10–13, 33), β cell function (10, 11, 13), and glycemia (8, 10) have been reported, we observed no consistent significant associations between serum 25(OH)D and insulin, glucose, HOMA-IR, and HOMA-β. This is an interesting finding because of the biological evidence and prior cross-sectional and prospective cohort findings that reported an association. Our findings may differ, in part, because we controlled for the history of cardiometabolic risk factors in all multivariable-adjusted models, unlike in some previous studies (10, 11), which more adequately controlled for the confounding effects of adiposity. Our findings are consistent with evidence from randomized settings (49, 54–61) in which confounding by adiposity would generally not have been an issue because of randomization; a recent meta-analysis reported that 5 of 8 randomized trials of vitamin D supplementation observed no effect on fasting plasma glucose or incident diabetes (62).
Increased adiposity has been consistently associated with reduced serum 25(OH)D concentrations and adverse cardiometabolic outcomes, although the mechanism underlying the relation to serum 25(OH)D is not clear. It may be that overweight individuals at increased risk of cardiometabolic disorders are more likely to have low serum 25(OH)D concentrations because of the high lipid-solubility of serum 25(OH)D and sequestration in excess adipose tissue that result in reduced bioavailability (63). An alternate explanation is that adiposity confounds the relation because overweight individuals have less exposure to ultraviolet light because of lower levels of outdoor physical activity, which results in lower serum 25(OH)D concentrations. In the current analysis, we accounted for BMI and waist circumference as potential confounders and effect modifiers of the relation. Our primary findings were unchanged after controlling for BMI and waist circumference, which suggested that serum 25(OH)D may be related to triglycerides and the triglyceride:HDL ratio independently of adiposity. We also directly examined the relation of serum 25(OH)D to BMI, waist-circumference, and the waist-hip ratio to determine whether serum 25(OH)D was associated with adiposity independent of physical activity. Although we cannot confirm causality in this study, our findings lend support to the hypothesis that lower serum 25(OH)D concentrations may be physiologically associated with increased adiposity apart from the effects of physical activity and sunlight exposure.
In line with our findings regarding triglycerides and measures of adiposity, we observed a significant inverse association between serum 25(OH)D concentrations and metabolic syndrome. Although 3 cross-sectional studies reported no association with metabolic syndrome (64–66), the observed OR of 0.38 in the current study was approximately consistent in magnitude with 6 cross-sectional studies that reported ORs that ranged from 0.26 to 0.59 (16, 35, 42, 67–69). Inconsistencies in the literature regarding this association may be due to differences in baseline concentrations of serum 25(OH)D, with higher mean concentrations potentially making it difficult to detect an association (64). Varying control for measures of adiposity may also contribute; 2 (65, 66) of 3 null studies controlled for adiposity, whereas other studies that reported an association (16, 35, 41, 42, 69) did not control for adiposity. Our findings are of note because we observed a significant inverse association with metabolic syndrome even after controlling for BMI.
Principal limitations to this study included the relatively small sample size that limited our statistical power and the cross-sectional design. To account for sample-size limitations, we compared results including serum 25(OH)D in the model continuously and categorically and plotted the adjusted results by using a restricted quadratic spline model to more realistically model the relation and allow for variation within categories. Our findings were generally consistent across methods. The cross-sectional design precluded us from making causal conclusions about the effect of serum 25(OH)D concentrations on cardiometabolic outcomes; we could not rule out the possibility that metabolic disturbances that were already present led to lower serum 25(OH)D concentrations because of excess adipose tissue or other unknown mechanisms.
In conclusion, we observed no relation between serum 25(OH)D concentrations and LDL and HDL cholesterol, insulin, glucose, HOMA-IR, and HOMA-β after adjustment for demographic and lifestyle factors. In contrast, we observed a consistent inverse association between serum 25(OH)D concentrations and triglycerides and the triglyceride:HDL cholesterol ratio as well as measures of adiposity and prevalent metabolic syndrome in this population of postmenopausal women. These findings support the need for future, large-scale longitudinal studies to quantify the potential beneficial effects of increasing serum 25(OH)D on the components of metabolic syndrome, especially in obese populations. Randomized clinical trials may ultimately be necessary to confirm these findings.
Acknowledgments
We acknowledge all WHI centers, principal investigators, and committed participants in this research.
The authors' responsibilities were as follows—SAC: analyzed data and wrote the manuscript; SL: supervised the analysis and edited the manuscript; and all authors: read and approved the final manuscript. CE has received funding from Pfizer, Merck, Amylin, and Roche and has served as a consultant to Pfizer. SAC, YS, JEM, LVH, LM, AM, JDC, JW-R, LSP, RAP, and SL had no conflicts of interest.
REFERENCES
- 1.Knekt P, Laaksonen M, Mattila C, et al. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology 2008;19:666–71 [DOI] [PubMed] [Google Scholar]
- 2.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med 2008;168:1340–9 [DOI] [PubMed] [Google Scholar]
- 3.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med 2008;168:1174–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008;117:503–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zittermann A. Vitamin D in preventive medicine: are we ignoring the evidence? Br J Nutr 2003;89:552–72 [DOI] [PubMed] [Google Scholar]
- 6.Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science 1980;209:823–5 [DOI] [PubMed] [Google Scholar]
- 7.Kadowaki S, Norman AW. Dietary vitamin D is essential for normal insulin secretion from the perfused rat pancreas. J Clin Invest 1984;73:759–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng S, Massaro JM, Fox CS, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes 2010;59:242–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulseth HL, Gjelstad IM, Tierney AC, et al. Serum vitamin D concentration does not predict insulin action or secretion in European subjects with the metabolic syndrome. Diabetes Care 2010;33:923–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 2004;27:2813–8 [DOI] [PubMed] [Google Scholar]
- 11.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr 2004;79:820–5 [DOI] [PubMed] [Google Scholar]
- 12.Forouhi NG, Luan J, Cooper A, Boucher BJ, Wareham NJ. Baseline serum 25-hydroxy vitamin d is predictive of future glycemic status and insulin resistance: the Medical Research Council Ely Prospective Study 1990-2000. Diabetes 2008;57:2619–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kayaniyil S, Vieth R, Retnakaran R, et al. Association of vitamin D with insulin resistance and beta-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care 2010;33:1379–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karhapää P, Pihlajamäki J, Pörsti I, et al. Diverse associations of 25-hydroxyvitamin D and 1,25-dihydroxy-vitamin D with dyslipidaemias. J Intern Med 2010;268:604–10 [DOI] [PubMed] [Google Scholar]
- 15.Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 2007;167:1159–65 [DOI] [PubMed] [Google Scholar]
- 16.Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care 2005;28:1228–30 [DOI] [PubMed] [Google Scholar]
- 17.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol 2003;13:S5–17 [DOI] [PubMed] [Google Scholar]
- 18.Hays J, Hunt JR, Hubbell FA, et al. The Women's Health Initiative recruitment methods and results. Ann Epidemiol 2003;13:S18–77 [DOI] [PubMed] [Google Scholar]
- 19.Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 2006;354:669–83 [DOI] [PubMed] [Google Scholar]
- 20.Chlebowski RT, Johnson KC, Kooperberg C, et al. Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst 2008;100:1581–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 2006;354:684–96 [DOI] [PubMed] [Google Scholar]
- 22.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. 2009;120:1640–5 [DOI] [PubMed] [Google Scholar]
- 23.Steiner P, Freidel J, Bremner W, Stein E. Standardization of micromethods for plasma cholesterol, triglyceride and HDL-cholesterol with the Lipid Clinics methodology. J Clin Chem 1981;19:850 [Google Scholar]
- 24.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502 [PubMed] [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9 [DOI] [PubMed] [Google Scholar]
- 26.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–95 [DOI] [PubMed] [Google Scholar]
- 27.Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol 2006;92:39–48 [DOI] [PubMed] [Google Scholar]
- 28.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin d from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011;96:53–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81 [DOI] [PubMed] [Google Scholar]
- 30.Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology 1995;6:356–65 [DOI] [PubMed] [Google Scholar]
- 31.IOM Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press, 2011 [PubMed] [Google Scholar]
- 32.Gaziano JM, Hennekens CH, O'Donnell CJ, Breslow JL, Buring JE. Fasting triglycerides, high-density lipoprotein, and risk of myocardial infarction. Circulation 1997;96:2520–5 [DOI] [PubMed] [Google Scholar]
- 33.Liu E, Meigs JB, Pittas AG, et al. Plasma 25-hydroxyvitamin d is associated with markers of the insulin resistant phenotype in nondiabetic adults. J Nutr 2009;139:329–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gannage-Yared MH, Chedid R, Khalife S, Azzi E, Zoghbi F, Halaby G. Vitamin D in relation to metabolic risk factors, insulin sensitivity and adiponectin in a young Middle-Eastern population. Eur J Endocrinol 2009;160:965–71 [DOI] [PubMed] [Google Scholar]
- 35.Lu L, Yu Z, Pan A, et al. Plasma 25-hydroxyvitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes Care 2009;32:1278–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinelli NR, Jaber LA, Brown MB, Herman WH. Serum 25-hydroxy vitamin d and insulin resistance, metabolic syndrome, and glucose intolerance among Arab Americans. Diabetes Care 2010;33:1373–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson MD, Nader NS, Weaver AL, Singh R, Kumar S. Relationships between 25-hydroxyvitamin D levels and plasma glucose and lipid levels in pediatric outpatients. J Pediatr 2010;156:444–9 [DOI] [PubMed] [Google Scholar]
- 38.Hypponen E, Power C. Vitamin D status and glucose homeostasis in the 1958 British birth cohort: the role of obesity. Diabetes Care 2006;29:2244–6 [DOI] [PubMed] [Google Scholar]
- 39.Rodríguez-Rodríguez E, Ortega RM, González-Rodríguez LG, López-Sobaler AM; UCM Research Group VALORNUT (920030) Vitamin D deficiency is an independent predictor of elevated triglycerides in Spanish school children. Eur J Nutr (Epub ahead of print 20 November 2010) [DOI] [PubMed] [Google Scholar]
- 40.Reis JP, von Muhlen D, Miller ER, 3rd, Michos ED, Appel LJ. Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Pediatrics 2009;124:e371–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Botella-Carretero JI, Alvarez-Blasco F, Villafruela JJ, Balsa JA, Vázquez C, Escobar-Morreale HF. Vitamin D deficiency is associated with the metabolic syndrome in morbid obesity. Clin Nutr 2007;26:573–80 [DOI] [PubMed] [Google Scholar]
- 42.Hypponen E, Boucher BJ, Berry DJ, Power C. 25-hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: a cross-sectional study in the 1958 British Birth Cohort. Diabetes 2008;57:298–305 [DOI] [PubMed] [Google Scholar]
- 43.Barger-Lux MJ, Heaney RP, Lanspa SJ, Healy JC, DeLuca HF. An investigation of sources of variation in calcium absorption efficiency. J Clin Endocrinol Metab 1995;80:406–11 [DOI] [PubMed] [Google Scholar]
- 44.Welberg JW, Monkelbaan JF, de Vries EG, et al. Effects of supplemental dietary calcium on quantitative and qualitative fecal fat excretion in man. Ann Nutr Metab 1994;38:185–91 [DOI] [PubMed] [Google Scholar]
- 45.Vaskonen T, Mervaala E, Sumuvuori V, Seppanen-Laakso T, Karppanen H. Effects of calcium and plant sterols on serum lipids in obese Zucker rats on a low-fat diet. Br J Nutr 2002;87:239–45 [DOI] [PubMed] [Google Scholar]
- 46.Cho HJ, Kang HC, Choi SA, Ju YC, Lee HS, Park HJ. The possible role of Ca2+ on the activation of microsomal triglyceride transfer protein in rat hepatocytes. Biol Pharm Bull 2005;28:1418–23 [DOI] [PubMed] [Google Scholar]
- 47.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 2001;22:477–501 [DOI] [PubMed] [Google Scholar]
- 48.Lacour B, Basile C, Drueke T, Funck-Brentano JL. Parathyroid function and lipid metabolism in the rat. Miner Electrolyte Metab 1982;7:157–65 [PubMed] [Google Scholar]
- 49.Zittermann A, Frisch S, Berthold HK, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr 2009;89:1321–7 [DOI] [PubMed] [Google Scholar]
- 50.Rajpathak SN, Xue X, Wassertheil-Smoller S, et al. Effect of 5 y of calcium plus vitamin D supplementation on change in circulating lipids: results from the Women's Health Initiative. Am J Clin Nutr 2010;91:894–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andersen R, Brot C, Mejborn H, et al. Vitamin D supplementation does not affect serum lipids and lipoproteins in Pakistani immigrants. Eur J Clin Nutr 2009;63:1150–3 [DOI] [PubMed] [Google Scholar]
- 52.Johnson JA, Grande JP, Roche PC, Kumar R. Immunohistochemical localization of the 1,25(OH)2D3 receptor and calbindin D28k in human and rat pancreas. Am J Physiol 1994;267:E356–60 [DOI] [PubMed] [Google Scholar]
- 53.Maestro B, Campion J, Davila N, Calle C. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J 2000;47:383–91 [DOI] [PubMed] [Google Scholar]
- 54.Lind L, Pollare T, Hvarfner A, Lithell H, Sorensen OH, Ljunghall S. Long-term treatment with active vitamin D (alphacalcidol) in middle-aged men with impaired glucose tolerance. Effects on insulin secretion and sensitivity, glucose tolerance and blood pressure. Diabetes Res 1989;11:141–7 [PubMed] [Google Scholar]
- 55.Orwoll E, Riddle M, Prince M. Effects of vitamin D on insulin and glucagon secretion in non-insulin-dependent diabetes mellitus. Am J Clin Nutr 1994;59:1083–7 [DOI] [PubMed] [Google Scholar]
- 56.Fliser D, Stefanski A, Franek E, Fode P, Gudarzi A, Ritz E. No effect of calcitriol on insulin-mediated glucose uptake in healthy subjects. Eur J Clin Invest 1997;27:629–33 [DOI] [PubMed] [Google Scholar]
- 57.Borissova AM, Tankova T, Kirilov G, Dakovska L, Kovacheva R. The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int J Clin Pract 2003;57:258–61 [PubMed] [Google Scholar]
- 58.Nilas L, Christiansen C. Treatment with vitamin D or its analogues does not change body weight or blood glucose level in postmenopausal women. Int J Obes 1984;8:407–11 [PubMed] [Google Scholar]
- 59.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care 2007;30:980–6 [DOI] [PubMed] [Google Scholar]
- 60.de Boer IH, Tinker LF, Connelly S, et al. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women's Health Initiative. Diabetes Care 2008;31:701–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hsia J, Heiss G, Ren H, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation 2007;115:846–54 [DOI] [PubMed] [Google Scholar]
- 62.Pittas AG, Chung M, Trikalinos T, et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med 2010;152:307–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000;72:690–3 [DOI] [PubMed] [Google Scholar]
- 64.Reis JP, von Muhlen D, Kritz-Silverstein D, Wingard DL, Barrett-Connor E. Vitamin d, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care 2007;30:1549–55 [DOI] [PubMed] [Google Scholar]
- 65.Rueda S, Fernandez-Fernandez C, Romero F, Martinez de Osaba J, Vidal J, Vitamin D. PTH, and the metabolic syndrome in severely obese subjects. Obes Surg 2008;18:151–4 [DOI] [PubMed] [Google Scholar]
- 66.Hjelmesaeth J, Hofso D, Aasheim ET, et al. Parathyroid hormone, but not vitamin D, is associated with the metabolic syndrome in morbidly obese women and men: a cross-sectional study. Cardiovasc Diabetol 2009;8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ford ES, Zhao G, Li C, Pearson WS. Serum concentrations of vitamin D and parathyroid hormone and prevalent metabolic syndrome among adults in the United States. J Diabetes 2009;1:296–303 [DOI] [PubMed] [Google Scholar]
- 68.Kim MK, Il Kang M, Won Oh K, et al. The association of serum vitamin D level with presence of metabolic syndrome and hypertension in middle-aged Korean subjects. Clin Endocrinol (Oxf) 2010;73:330–8 [DOI] [PubMed] [Google Scholar]
- 69.Reis JP, von Muhlen D, Miller ER., 3rd Relation of 25-hydroxyvitamin D and parathyroid hormone levels with metabolic syndrome among US adults. Eur J Endocrinol 2008;159:41–8 [DOI] [PubMed] [Google Scholar]