Abstract
Background: Food is a powerful reinforcer that motivates people to eat. The relative reinforcing value of food (RRVfood) is associated with obesity and energy intake and interacts with impulsivity to predict energy intake.
Objective: How RRVfood is related to macronutrient choice in ad libitum eating tasks in humans has not been studied; however, animal research suggests that sugar or simple carbohydrates may be a determinant of reward value in food. This study assessed which macronutrients are associated with food reinforcement.
Design: Two hundred seventy-three adults with various body mass indexes were assessed for RRVfood, the relative reinforcing value of reading, food hedonics, energy intake in an ad libitum taste test, and usual energy intake derived from repeated 24-h dietary recalls. Multiple regression was used to assess the relation between predictors of total energy and energy associated with macronutrient intake after control for age, sex, income, education, minority status, and other macronutrient intakes.
Results: The results showed that the relative proportion of responding for food compared with reading (RRVprop) was positively related to body mass index, laboratory-measured energy intake, and usual energy intake. In addition, RRVprop was a predictor of sugar intake but not of total carbohydrate, fat, or protein intake.
Conclusion: These results are consistent with basic animal research showing that sugar is related to food reward and with the hypothesis that food reward processes are more strongly related to eating than are food hedonics. This trial was registered at clinicaltrials.gov as NCT00962117.
INTRODUCTION
Food is a powerful reinforcer that motivates people to eat (1). Food reinforcement is associated with energy intake in laboratory ad libitum eating tasks (2, 3), because those who find food more reinforcing have greater energy intakes. Obese people consume more food than their leaner peers, and food is more reinforcing for obese than for lean people (2, 4, 5). The identification of food as a powerful natural reinforcer for behavior has led to speculation that food may produce addictive behaviors similar to those associated with drug addiction (6–9). The most studied component of food potentially related to the strong self-administration characteristic of drug addiction is sugar. Animals will respond to self-administer sugar and increase responding after deprivation, similar to responding for drugs of abuse (10). Responding for sugar meets many of the general characteristics of addictions, including bingeing and escalation of sugar intake (11), withdrawal (12, 13), craving (10, 14), and sensitization to other drugs (15–17).
Food reinforcement has been studied by using a wide variety of snack and entrée foods (2–5) and sweetened caffeinated beverages (18). However, to our knowledge, no research has been conducted in humans to attempt to relate reinforcing properties of food to particular characteristics of food, such as the macronutrient or sugar content of the food. Our approach to relating food reinforcement to eating is to assess the breakpoint for subjects working for their favorite snack food or an alternative noneating activity and in a separate session to measure ad libitum energy intake in a snack “buffet” taste test that includes the subject's favorite snack food and other snack foods that vary in their macronutrient composition (2, 19). This provides the opportunity to relate the relative reinforcing value of food (RRVfood) to the macronutrient composition of the snack foods that were studied.
A secondary aim was to compare the reinforcing value of snack food compared with the hedonic value of snack foods as predictors of energy intake. A central prediction of incentive salience theory (20, 21), as extended to natural rewards such as food (22–24), is that the incentive value of food is a more powerful influence on energy intake than is liking of food. Food reinforcement is not identical to the incentive value of a food; however, these 2 constructs are related (25), which provides an opportunity to evaluate this prediction in a large sample extending previous observations of this relation in a smaller sample (3).
SUBJECTS AND METHODS
Participants
The participants were 273 adults (79 nonobese women, 72 nonobese men, 60 obese women, and 62 obese men) who participated in a study of genetic factors associated with food reinforcement. Participants were recruited from an existing family database, newspaper ads, flyers posted around the University at Buffalo campuses and in community settings, Web-based sources (eg, ads on Craig's list and on the department's website), and direct mailings targeted to community residents between the ages of 18–50 y. Participants were excluded from the study if they were taking medications associated with loss of appetite, were smokers, had diabetes, had a previous diagnosis of an eating disorder or psychiatric disorder (eg, anxiety, depression, and attention-deficit hyperactivity disorder), were allergic to the ingredients in the study foods, were currently dieting, were pregnant, and did not rate at least a moderate liking (≥4 on a 9-point Likert-type scale) for 5 of the 6 study foods. Participants received a $50 gift certificate to local stores for completing the study. Recruitment began in January 2008 and continued throughout June 2010. The study was approved by the University at Buffalo Health Sciences Institutional Review Board. The participants’ characteristics are shown in Table 1.
TABLE 1.
Participant characteristics1
| Overall (n = 273) | |
| Age (y) | 34.4 ± 10.7 |
| BMI (kg/m2) | 29.9 ± 7.4 |
| Restraint score | 7.7 ± 4.7 |
| Disinhibition score | 6.3 ± 3.4 |
| Hunger score | 5.2 ± 3.2 |
| Sex (M/F) | 134/139 |
| Education (n) | |
| Attended high school | 6 |
| Completed high school | 36 |
| Some college/vocational training | 81 |
| Completed 2 y of college | 45 |
| Completed 4 y of college | 73 |
| Completed graduate/professional school | 31 |
| Minority status (n) | |
| Minority | 75 |
| Nonminority | 198 |
| Laboratory intake (kcal) | |
| Total energy | 589.9 ± 312.1 |
| Fat energy | 270.4 ± 143.9 |
| Carbohydrate energy | 306.5 ± 161.0 |
| Protein energy | 131.6 ± 66.9 |
| Sugar | 163.3 ± 89.2 |
| Intake from 24-h recalls (kcal) | |
| Total energy | 2070.8 ± 716.8 |
| Fat energy | 732.4 ± 347.4 |
| Carbohydrate energy | 1005.4 ± 355.9 |
| Protein energy | 336.6 ± 148.7 |
| RRVprop | 0.43 ± 0.31 |
| Liking favorite food (Likert scale 1–9) | 8.22 ± 1.00 |
RRVprop, relative proportion of responding for food compared with reading: RRVprop = Pmax food/(Pmax food + Pmax reading). Pmax represents the breakpoint.
Procedures
The participants visited the laboratory for 2 sessions: an ad libitum snack-eating task and a food-reinforcement task. Both experimental sessions were scheduled between the hours of 1400 and 1700, during a time when the individuals would normally consume additional calories outside of meal time (26, 27). The participants were asked to refrain from consuming food or drinking beverages, other than water, for ≥3 h before the test session and to refrain from consuming the experimental foods in the 24 h before the test session. On initial arrival to the laboratory, participants read and signed consent forms and completed a same-day and 24-h food recall and hunger questionnaires. The participants were provided a choice of 2 isocaloric energy bar preloads (Clif Bar & Company; Berkeley, CA; 42 g, 150 kcal, 4 g fat, 23 g carbohydrates, 7 g protein) after collection of the baseline hunger questionnaire measure to minimize the effects of hunger on energy intake and food reinforcement. The inclusion of a standard preload increases the ability to show individual differences in food reinforcement (28). Demographic information, height and weight measurements, and 3 dietary-habits questionnaires were administered at the end of the ad libitum eating session.
Ad libitum eating task
The ad libitum food-consumption task was presented as a taste test. The participants were presented multiple servings of 6 palatable, high-energy-density snack foods that were of similar energy density (4.5–5.4 kcal/g) but that varied in fat, carbohydrate, and total sugar content (Table 2). Water was provided ad libitum. The participants were told that they could consume as much or as little of the food that they wanted as long as they tasted each food so that they could accurately rate the food's characteristics. The participants rated each food on many different characteristics, including pleasurability, sweetness, blandness, flavorfulness, and bitterness using 9-point Likert-type scales. Participants were then given the Three-Factor Eating Questionnaire (TFEQ) (29), the Questionnaire of Eating and Weight Patterns (QEWP) (30), and the Binge Eating Scale (BES) (31) to complete. Food from the taste test was left in the room, and the participants were told that the food would be discarded after the session and that they could continue eating if they chose to do so. When the participants indicated that they were finished eating, they were asked to identify their favorite food from among the 6 available foods and were told that this was the food that would be used in the next test session. The participants’ height and weight measurements were taken, and they were reminded of the next visit (food reinforcement session), which was scheduled 2–3 wk after the ad libitum eating session.
TABLE 2.
Foods used in the ad libitum taste test1
| Amount | Weight | Energy | Energy density | Protein | Carbohydrate | Sugar | Fat | |
| g | kcal | kcal/g | g (% of energy) | g (% of energy) | g (% of energy) | g (% of energy) | ||
| Food | ||||||||
| Potato chips2 | ≈30 Chips | 57 | 305 | 5.4 | 4.0 (5.3) | 30.5 (40.0) | 0 (0) | 20.4 (60.2) |
| Tortilla chips3 | ≈36 Chips | 56 | 300 | 5.4 | 3.9 (5.2) | 36.0 (48.0) | 2 (2.7) | 16.0 (48.0) |
| Shell-covered chocolate candy4 | ≈69 Candies | 60 | 300 | 5.0 | 3.0 (4.0) | 42.9 (57.2) | 38.6 (51.5) | 12.9 (38.7) |
| Chocolate and caramel-covered biscuit5 | 3 Fun-size bars | 48 | 240 | 5.0 | 3.0 (5.0) | 30.0 (50.0) | 24 (40.0) | 12.0 (45.0) |
| Milk-chocolate biscuit6 | 3 Fun-size bars | 42 | 210 | 5.0 | 3.0 (5.7) | 27.0 (51.4) | 21 (40.0) | 11.0 (47.1) |
| Chocolate-covered caramel crisp7 | 3 Fun-size bars | 57 | 255 | 4.5 | 3.0 (4.7) | 40.5 (63.5) | 25.5 (40.0) | 10.5 (37.1) |
Values are from the package labels as of January 2010 and may represent some rounding error.
Wavy Lays Potato Chips; Frito-Lay, Dallas, TX.
Cool Ranch Doritos; Frito-Lay.
M&M candies; Mars, Hackettstown, NJ.
Twix bars; Mars.
Kit Kat Bars; Hershey Co, Hershey, PA.
Butterfingers; Nestlé, Glendale, CA.
Food reinforcement task
RRVfood was measured by determining the number of responses (mouse button presses) participants made for food or food alternatives on progressive ratio schedules of reinforcement. The experimental environment included 2 computer stations that participants could go back and forth between. At one station, participants could earn points toward food and at the other station they could earn points for time to spend reading Time and Newsweek magazines. This alternative activity was provided to reduce the likelihood that participants would engage in responding out of boredom. Participants were instructed on how to use the computer task and were allowed a practice session. After the instructions for the task were provided, the experimenter left the room. An intercom and closed-circuit video system were present in the room so that the experimenter could observe the participant and the participant could communicate with the experimenter.
The program used for the reinforcement task is similar to a slot machine, having shapes that rotate on a screen. A point is earned each time that the 3 shapes that appear match in shape and color. For every 5 points earned, the subject was able to receive a 70–101-kcal (14–20-g) portion of his or her preferred snack food, which had been selected during the ad libitum eating session, or 2 min of time to spend reading, depending on which reward they were working for. The programmed reinforcement schedules for food and reading were progressive fixed-ratio schedules with response requirements of 4, 8, 16, 32, 64, 128, 256, 512, 1024, 2048 and so forth for each point. The participants were instructed to perform one activity at a time (ie, play the computer game, eat, or read) and that the session would end when they no longer wished to earn points for access to food or time to spend reading. Water was provided ad libitum.
The breakpoint, or Pmax (32), which was the last schedule (4, 8, 16, 32, etc) at which subjects met response requirements for access to the food or nonfood alternative, was calculated for each alternative. In addition, the proportion of breakpoint for food compared with the alternative (RRVprop) was calculated (Pmax food)/(Pmax food + Pmax reading) to have a metric for the relative value of food to a noneating alternative. The analyses showed that that proportional measure was a better predictor of intake than were the reinforcement measures considered separately, and the proportional measure was used for all analyses.
Measurement
Dietary recalls
At the beginning of each session, the participants were interviewed by the experimenter, using a multipass same-day and 24-h food recall, to verify that the participants complied with the study protocol, that they had not consumed food or drink (except water) 3 h before the appointment, that they had not eaten the preferred snack food in the 24 h before the appointment, and to quantify usual energy intake. Briefly, the first pass included making a quick, uninterrupted list of all the foods and beverages consumed. For the second pass, the experimenter reviewed the quick list and probed for any large gaps of time between eating bouts. For the third pass, the experimenter returned to the beginning of the list and asked for portion sizes and any condiments, fats, and sugars that may have been added to the food item. The final pass was to prompt the participants to recall any other foods that they may have forgotten to report, such as foods that were eaten in small amounts. Measuring cups and spoons, rulers, and pictures of food portions were provided to help the participants estimate portion sizes. The total number of calories consumed was calculated for the recall based on manufacturers’ labels and the Food Works nutrition database (33). Usual energy intake was calculated based on a 2-d average of the 24-h dietary recalls collected on the day before the testing days.
Height and weight
The participant's weight and height were measured by using a digital scale (TANITA Corporation of America Inc, Arlington Heights, IL) and a digital stadiometer (Measurement Concepts & Quick Medical, North Bend, WA). On the basis of height and weight data, body mass index (BMI) was calculated as weight (kg)/height squared (m2). Individuals were considered obese if their BMI (in kg/m2) was ≥30 and nonobese if their BMI was <30 (34).
Eating questionnaires
The participants were excluded if they currently had an eating disorder or if they had a history of an eating disorder. This was determined in 2 stages. First, the participants completed the TFEQ (29), the QEWP (30), and the BES (31). The TFEQ is a validated instrument used to detect dietary restraint (35) and contains 3 subscales that assess dietary restraint, hunger, and disinhibition. The QEWP and BES are used to assess binge-eating disorder. The participants were identified as potentially having a binge-eating disorder if they scored >27 on the BES or were indicated as having the disorder by the QEWP. Because diagnoses cannot be made on the basis of questionnaire scores, in a second stage, any participant identified as potentially having an eating disorder completed the Eating Disorders Examination (36), which was administered by trained personnel in an additional session. One participant was excluded for having an eating disorder.
Food liking and hunger
Subjective ratings of hunger were collected before and after intake of the preload and after both test sessions by using a 10-point Likert-type scale. Food hedonics were also measured before and after intake of the preload and after the session for the food-reinforcement session. For hunger, 1 indicated “not hungry at all/not full at all” and 10 indicated “extremely hungry/extremely full,” whereas for hedonics 1 indicated “do not like at all” and 9 indicated “like very much.”
Analytic plan
The goal of the analyses was to establish the independent effects of food hedonics and the proportion of responding for food on total energy intake or energy intake for fats, carbohydrates, sugar, and protein by using hierarchical linear regression analyses. Age, sex, minority status, education, and BMI were included as covariates in the initial set of regression models. However, because all of the foods that were studied were complex foods, consisting of a mixture of fat, carbohydrates, and protein, it is necessary to control for the other macronutrients to assess the independent relation between relative reinforcing value of food to fat, carbohydrates, protein, or sugar. Thus, in the second set of regression models, the other macronutrients were included as control variables in the model. The data were entered and analyzed by using SYSTAT 11 (37).
RESULTS
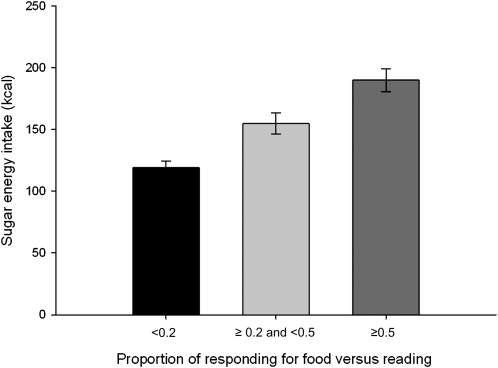
The regression model to predict laboratory energy intake (Table 3) showed that RRVprop was a strong predictor of energy intake (P = 0.00002), but liking of the favorite food used in the reinforcing value task was not a significant predictor (P = 0.83). Average liking for all foods used in the ad libitum task also was not significant in place of liking of favorite food (P = 0.51). With the use of similar covariates, RRVprop was a significant independent predictor for dietary fat (β = 109.91, P < 0.00001), carbohydrate (β = 123.05, P < 0.00001), protein (β = 41.25, P = 0.00047), and sugar (β = 70.78, P < 0.00001). However, when the other macronutrients were controlled for, RRVprop was only related to sugar intake (β = 13.24, P < 0.04) and not to fat, total carbohydrate, or protein intake (Table 4). The relation between RRVprop and sugar energy intake is shown in Figure 1, with RRVprop divided into those with low allocation of responding for food (RRVprop < 0.2), moderate allocation of responding for food (RRVprop ≥ 0.2 and <0.5), and greater allocation of responding for food than the alternative (RRVprop ≥ 0.5).
TABLE 3.
Predictors of energy intake1
| β | SE | t | P | |
| Constant | 824.74 | 178.01 | 4.63 | 0.00001 |
| RRVprop | 237.36 | 54.44 | 4.36 | 0.00002 |
| Age | −5.84 | 1.63 | 3.58 | 0.00042 |
| Sex | −197.97 | 33.76 | 5.86 | <0.0001 |
| Education | −27.55 | 13.19 | 2.08 | 0.038 |
| Minority status | −123.44 | 38.60 | 3.20 | 0.0016 |
| BMI | 4.364 | 2.25 | 1.94 | 0.053 |
| Liking favorite food | 3.58 | 16.77 | 0.21 | 0.83 |
RRVprop, relative proportion of responding for food compared with reading. For the multiple regression analysis to predict laboratory energy intake, RRVprop = Pmax food/(Pmax food + Pmax reading); minority status is 0 = nonminority, 1 = minority; and liking of favorite food is scored from 1 (do not like at all) to 9 (like very much). Pmax represents the breakpoint.
TABLE 4.
Predictors of macronutrient intake and sugar adjusted for other macronutrients1
| Fat |
Carbohydrate |
Protein |
Sugar |
|||||||||
| β | SE | P | β | SE | P | β | SE | P | β | SE | P | |
| Constant | −11.71 | 8.49 | 0.17 | 18.811 | 8.03 | < 0.02 | −12.05 | 11.38 | 0.29 | 2.01 | 14.09 | 0.89 |
| RRVprop | −1.51 | 3.76 | 0.69 | 4.10 | 3.57 | 0.25 | 5.04 | 5.03 | 0.32 | 13.24 | 6.27 | <0.04 |
| Age | 0.37 | 0.11 | 0.00096 | −0.42 | 0.11 | 0.00012 | 0.32 | 0.15 | 0.039 | −0.08 | 0.19 | 0.69 |
| Sex | −0.43 | 2.39 | 0.86 | −0.96 | 2.28 | 0.67 | −1.51 | 3.21 | 0.64 | −1.61 | 4.00 | 0.69 |
| Education | −0.34 | 0.89 | 0.70 | 0.05 | 0.85 | 0.95 | 0.01 | 1.19 | 0.99 | −0.37 | 1.49 | 0.80 |
| Minority status | 5.61 | 2.66 | 0.036 | −4.89 | 2.54 | 0.056 | −3.86 | 3.60 | 0.28 | −6.70 | 4.46 | 0.13 |
| BMI | 0.06 | 0.16 | 0.69 | -0.14 | 0.15 | 0.35 | 0.65 | 0.20 | < 0.002 | −0.01 | 0.26 | 0.98 |
| Carbohydrate/fat/fat/fat | 1.01 | 0.02 | <0.0001 | 0.44 | 0.03 | <0.0001 | −0.56 | 0.07 | <0.0001 | 0.25 | 0.03 | <0.0001 |
| Protein/protein/carbohydrate/protein | −0.31 | 0.04 | <0.0001 | 0.92 | 0.02 | <0.0001 | 0.87 | 0.07 | <0.0001 | 0.72 | 0.06 | <0.0001 |
RRVprop, relative proportion of responding for food compared with reading. For the multiple regression analysis to predict whether food reinforcement predicted fat, carbohydrate, protein, or sugar consumption, RRVprop = Pmax food/(Pmax food + Pmax reading); minority status is 0 = nonminority, 1 = minority; and liking of favorite food is scored from 1 (do not like at all) to 10 (like very much). Carbohydrate/fat/fat/fat and Protein/protein/carbohydrate/protein represent the macronutrients controlled for in the order presented in the table. For example, for the model to predict fat consumption, carbohydrate and protein were controlled for; to predict carbohydrate consumption, fat and protein were controlled for; and so forth. Pmax represents the breakpoint.
FIGURE 1.
Mean (±SEM) sugar energy intake by participants who were stratified by their responses to receive food or reading time (Pmax food/(Pmax food + Pmax reading): low preference for food (<0.2; n = 55), moderate preference for food (≥0.2 and <0.5; n = 95), or high preference for food (≥0.5; n = 123) relative to preference for reading. The relation between the relative reinforcing value of food compared with that of reading and sugar energy intake was P = 0.036 after control for age, sex, education, minority status, BMI, and energy intake from fat and protein. Pmax represents the breakpoint.
The regression models were also computed by using both the absolute reinforcing values for food and reading considered separately, rather than as a proportion of responding. The models showed that food reinforcement, but not reading reinforcement, was a predictor of laboratory energy, fat, carbohydrate, protein, or sugar intake (P < 0.0005). However, when the other macronutrients were controlled for, the individual reinforcing value of food measure was not related to fat (P = 0.38), carbohydrate (P = 0.86), protein (P = 0.22), or sugar (P = 0.17) intake.
With the use of Pearson product-moment correlations, RRVfood was related to energy intake in the laboratory (r = 0.30, P < 0.001) and to energy intake from repeated 24-h recalls (r = 0.28, P < 0.001). Energy intake in the laboratory was related to energy intake assessed by dietary recall (r = 0.33, P < 0.001), and macronutrient intake in the laboratory was related to usual macronutrient intake for fat (r = 0.30, P < 0.001), carbohydrate (r = 0.25, P < 0.001), and protein (r = 0.24, P < 0.001) assessed by repeated 24-h recalls.
DISCUSSION
This study replicated previous observations that RRVfood is related to laboratory energy intake (2, 3) and is a stronger predictor of intake than is the hedonics of that food (3). The new finding is that, after control for the presence of other macronutrients, the relative reinforcing value of food is only related to sugar intake. This finding is consistent with a large body of basic science on sugar as a characteristic of food that is related to RRVfood or a food reward (6). To our knowledge, this is the initial demonstration of sugar as a component of food as a reinforcer in humans.
This research replicates previous research showing that RRVfood is greater for obese than for leaner participants (2, 4, 38). The positive association between food reinforcement and energy intake provides a mechanistic link between food reinforcement and obesity. The relation between the reward value of food and obesity has led to considerable interest in research on whether food can be considered an addictive substance, and, if so, which component or components of food may be addictive. Previous research has shown that RRVfood may sensitize in obese persons, and the amount or dose of food is related to the degree of sensitization (39, 40). In addition, considerable evidence indicates that people crave the foods that they usually eat (6, 41–43), and people may develop tolerance to food (44) in a similar manner to how people develop tolerance to drugs, which may drive increased eating or binges. Good evidence from animal studies indicates that food can be considered an addictive substance (6), and this study supports the hypothesis that sugar is a component of food that drives reinforcement of reward processes.
This study had some limitations. The variety of foods that the participants could choose to eat was limited and these foods were all complex, containing a mixture of macronutrients. The relation between food reward and a specific macronutrient or sugar can be studied by using foods that vary only in that component. For example, to assess the reinforcing value of sugar, it would be ideal to manipulate access to foods that vary only in the amount of sugar they contain, with other macronutrients being equal. This could be done by using sweetened beverages, such as soda. This is an excellent vehicle for administering doses of sugar, because it is commonly consumed and its reinforcing value has been researched; however, previous research focused on its caffeine content and not on its sugar content (18). Of course, the reinforcing value of beverages may be different from that of solid foods (45, 46); however, there are solid foods that can be used as a vehicle for providing different amounts of sugar, such as gelatin (47). People also consume a lot of food that contains fat, and because of its higher energy density, fat may contribute more to positive energy balance than does sugar. While rats will respond to self-administer pure dietary fat (48, 49), humans do not consume fat on its own, but rather in combination with other components of food (50, 51), such as combinations of fat and sugar in candy and baked goods or fat and salt in chips and fried foods. This makes it more challenging to study fat, but it is possible to use a vehicle such as milk or a dairy product, for which the fat content can be varied while keeping the contents of protein and carbohydrates the same. Whereas the current research suggests that sugar can drive food reinforcement, while the other macronutrients in the snack foods studied are statistically controlled for, it is premature to argue that fat and protein do not also drive food reinforcement without the properly designed experiments to evaluate the role of these individual components of food while keeping the other components of the food constant.
One implication of this research involves the role of different components of food on food reinforcement, with the implication that changes in the contents of macronutrients in foods would reduce the RRVfood, and people would eat less. It is not surprising that more palatable foods are more reinforcing (52), and many people may strive to improve the quality of their diets by eating healthier, although in many instances, less-palatable foods. However, it is well known that many people who deprive themselves of foods they are motivated to eat do not maintain these healthier eating habits over time (53). This may be due in part to the fact that these healthier foods do not derive the same degree of pleasure or reinforcing value, and people return to consuming these less healthy, but more reinforcing foods. One place to focus research attention is on behavioral substitutes for less healthy foods, such that people could derive equivalent pleasure from alternative activities, and they would not have to return to consuming less-healthy, high-energy-dense foods. As observed in this study, RRVfood versus alternatives was a stronger predictor of energy and macronutrient intakes than was food reinforcement alone. It is possible that people consume too much food because they do not have good alternative reinforcers to eating (54–56). Obese people may find alternatives to eating less reinforcing than food (5). Understanding how to increase the choice of substitutes for food may be very important in facilitating long-term changes in eating behavior (57).
In addition, the results replicate previous findings with the use of repeated 24-h food recalls that food reinforcement is related to laboratory energy intake (2, 19) and to energy intake assessed outside of the laboratory. Previous research has shown that the reinforcing value of physical activity is related to physical activity in the natural environment, but this is the first demonstration that food reinforcement is related to energy intake in the natural environment. This provides increased confidence in the laboratory food-reinforcement measure as relevant to usual eating as well as laboratory eating. The ad libitum taste test was related to usual energy intake, and macronutrient intake in the laboratory was related to usual macronutrient intake. This is surprising given the very limited range of foods studied, but for the average person in the study the proportion of macronutrients in their usual diet may have been similar to the proportion in the snack foods provided in the taste test. The significant relation between the laboratory taste test eating and energy and macronutrient distribution in usual eating provides strong validation for the use of the taste test to estimate usual energy and macronutrient intakes.
In conclusion, this study replicates basic animal research showing that sugar can drive food reward (6), and opens up a new area of human research to identifying what characteristics of food most drive food reinforcement. This study also provides evidence that laboratory measures of reinforcing value and a taste test ad libitum eating task are related to usual eating, providing additional support for using laboratory measures to study human eating. The observation that RRVfood provides a better index of reinforcing value than does the absolute reinforcing values of food and alternatives to food also supports the need to consider assessment of eating behavior as a function of choice. Increasing the value of substitutes for eating may be a powerful method for combating the overconsumption of high-energy-density and less healthy but highly reinforcing foods.
Acknowledgments
Appreciation is expressed to Lora G Roba, Vida Rostami, Lauren Angelucci, Nicole Gens, Caitlin Hart, and Kirstie Clune for data collection and data entry and for assistance with the implementation of the protocol.
The authors’ responsibilities were as follows—LHE: design of the experiment; KAC and KDF: supervision and collection of data; LHE and HL: statistical analysis and interpretation of data; LHE: draft of the initial manuscript; and LHE, HL, KAC, and KDF: critical revision of the manuscript. The study sponsors had no role in the study design; collection, analysis, or interpretation of the data; writing of the report; or decision to submit the manuscript for publication. LHE is a consultant for NuVal. None of the other authors declared a conflict of interest.
REFERENCES
- 1.Epstein LH, Leddy JJ, Temple JL, Faith MS. Food reinforcement and eating: a multilevel analysis. Psychol Bull 2007;133:884–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epstein LH, Temple JL, Neaderhiser BJ, Salis RJ, Erbe RW, Leddy JJ. Food reinforcement, the dopamine D2 receptor genotype and energy intake in obese and non-obese humans. Behav Neurosci 2007;121:877–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epstein LH, Wright SM, Paluch RA, et al. Food hedonics and reinforcement as determinants of laboratory food intake in smokers. Physiol Behav 2004;81:511–7 [DOI] [PubMed] [Google Scholar]
- 4.Saelens BE, Epstein LH. The reinforcing value of food in obese and non-obese women. Appetite 1996;27:41–50 [DOI] [PubMed] [Google Scholar]
- 5.Temple JL, Legierski CM, Giacomelli AM, Salvy SJ, Epstein LH. Overweight children find food more reinforcing and consume more energy than do nonoverweight children. Am J Clin Nutr 2008;87:1121–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev 2008;32:20–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang GJ, Volkow ND, Fowler JS. The role of dopamine in motivation for food in humans: implications for obesity. Expert Opin Ther Targets 2002;6:601–9 [DOI] [PubMed] [Google Scholar]
- 8.Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis 2004;23:39–53 [DOI] [PubMed] [Google Scholar]
- 9.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci 2005;8:555–60 [DOI] [PubMed] [Google Scholar]
- 10.Avena NM, Long KA, Hoebel BG. Sugar-dependent rats show enhanced responding for sugar after abstinence: evidence of a sugar deprivation effect. Physiol Behav 2005;84:359–62 [DOI] [PubMed] [Google Scholar]
- 11.Colantuoni C, Schwenker J, McCarthy J, et al. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport 2001;12:3549–52 [DOI] [PubMed] [Google Scholar]
- 12.Colantuoni C, Rada P, McCarthy J, et al. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res 2002;10:478–88 [DOI] [PubMed] [Google Scholar]
- 13.Wideman CH, Nadzam GR, Murphy HM. Implications of an animal model of sugar addiction, withdrawal and relapse for human health. Nutr Neurosci 2005;8:269–76 [DOI] [PubMed] [Google Scholar]
- 14.Grimm JW, Fyall AM, Osincup DP. Incubation of sucrose craving: effects of reduced training and sucrose pre-loading. Physiol Behav 2005;84:73–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neuroscience 2003;122:17–20 [DOI] [PubMed] [Google Scholar]
- 16.Avena NM, Hoebel BG. Amphetamine-sensitized rats show sugar-induced hyperactivity (cross-sensitization) and sugar hyperphagia. Pharmacol Biochem Behav 2003;74:635–9 [DOI] [PubMed] [Google Scholar]
- 17.Gosnell BA. Sucrose intake enhances behavioral sensitization produced by cocaine. Brain Res 2005;1031:194–201 [DOI] [PubMed] [Google Scholar]
- 18.Temple JL, Bulkley AM, Briatico L, Dewey AM. Sex differences in reinforcing value of caffeinated beverages in adolescents. Behav Pharmacol 2009;20:731–41 [DOI] [PubMed] [Google Scholar]
- 19.Epstein LH, Wright SM, Paluch RA, et al. Relation between food reinforcement and dopamine genotypes and its effect on food intake in smokers. Am J Clin Nutr 2004;80:82–8 [DOI] [PubMed] [Google Scholar]
- 20.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 1993;18:247–91 [DOI] [PubMed] [Google Scholar]
- 21.Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive- sensitization view. Addiction 2000;95:S91–117 [DOI] [PubMed] [Google Scholar]
- 22.Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev 1996;20:1–25 [DOI] [PubMed] [Google Scholar]
- 23.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 1998;28:309–69 [DOI] [PubMed] [Google Scholar]
- 24.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci 2002;22:3306–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci 2003;26:507–13 [DOI] [PubMed] [Google Scholar]
- 26.Hampl JS, Heaton CL, Taylor CA. Snacking patterns influence energy and nutrient intakes but not body mass index. J Hum Nutr Diet 2003;16:3–11 [DOI] [PubMed] [Google Scholar]
- 27.Popkin BM, Duffey KJ. Does hunger and satiety drive eating anymore? Increasing eating occasions and decreasing time between eating occasions in the United States. Am J Clin Nutr 2010;91:1342–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiss S, Havercamp S. The sensitivity theory of motivation: implications for psychopathology. Behav Res Ther 1996;34:621–32 [DOI] [PubMed] [Google Scholar]
- 29.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985;29:71–83 [DOI] [PubMed] [Google Scholar]
- 30.Spitzer RL, Devlin MJ, Walsh BT, et al. Binge eating disorder: a multisite field trial of the diagnostic criteria. Int J Eat Disord 1992;11:191–203 [Google Scholar]
- 31.Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addict Behav 1982;7:47–55 [DOI] [PubMed] [Google Scholar]
- 32.Bickel WK, Marsch LA, Carroll ME. Deconstructing relative reinforcing efficacy and situating the measures of pharmacological reinforcement with behavioral economics: a theoretical proposal. Psychopharmacology (Berl) 2000;153:44–56 [DOI] [PubMed] [Google Scholar]
- 33.Nutrition Company FoodWorks nutrient analysis software: the professional's choice. Version 9 ed Long Valley, NJ: Nutrition Company, 2007 [Google Scholar]
- 34.NHLBI Obesity Education Initiative Expert Panel Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults–the evidence report. Obes Res 1998;6(suppl 2):51S–209S [PubMed] [Google Scholar]
- 35.Allison DB, Kalinsky LB, Gorman BS. A comparison of the psychometric properties of three measures of dietary restraint. Psychol Assess 1992;4:391–8 [Google Scholar]
- 36.Bryant-Waugh RJ, Cooper PJ, Taylor CL, Lask BD. The use of the eating disorder examination with children: a pilot study. Int J Eat Disord 1996;19:391–7 [DOI] [PubMed] [Google Scholar]
- 37.Systat Software Systat 11.0. Richmond, CA: SYSTAT Software Inc, 2004 [Google Scholar]
- 38.Giesen JC, Havermans RC, Douven A, Tekelenburg M, Jansen A. Will work for snack food: the association of BMI and snack reinforcement. Obesity (Silver Spring) 2010;18:966–70 [DOI] [PubMed] [Google Scholar]
- 39.Temple JL, Chappel A, Shalik J, Volcy S, Epstein LH. Daily consumption of individual snack foods decreases their reinforcing value. Eat Behav 2008;9:267–76 [DOI] [PubMed] [Google Scholar]
- 40.Clark EN, Dewey AM, Temple JL. Effects of daily snack food intake on food reinforcement depend on body mass index and energy density. Am J Clin Nutr 2010;91:300–8 [DOI] [PubMed] [Google Scholar]
- 41.Lappalainen R, Sjoden PO, Hursti T, Vesa V. Hunger/craving responses and reactivity to food stimuli during fasting and dieting. 1990;14:679–88 [PubMed] [Google Scholar]
- 42.Kassel JD, Shiffman S. What can hunger teach us about drug craving? A comparative analysis of the two constructs. Adv Behav Res Ther 1992;14:141–67 [Google Scholar]
- 43.Lowe MR, Butryn ML. Hedonic hunger: a new dimension of appetite? Physiol Behav 2007;91:432–9 [DOI] [PubMed] [Google Scholar]
- 44.Woods SC. The eating paradox: how we tolerate food. Psychol Rev 1991;98:488–505 [DOI] [PubMed] [Google Scholar]
- 45.Mattes R. Fluid calories and energy balance: the good, the bad, and the uncertain. Physiol Behav 2006;89:66–70 [DOI] [PubMed] [Google Scholar]
- 46.Mattes RD, Campbell WW. Effects of food form and timing of ingestion on appetite and energy intake in lean young adults and in young adults with obesity. J Am Diet Assoc 2009;109:430–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Epstein LH, Caggiula AR, Rodefer JS, Wisniewski L, Mitchell SL. The effects of calories and taste on habituation of the human salivary response. Addict Behav 1993;18:179–85 [DOI] [PubMed] [Google Scholar]
- 48.Freed DE, Green L. A behavioral economic analysis of fat appetite in rats. Appetite 1998;31:333–49 [DOI] [PubMed] [Google Scholar]
- 49.Wojnicki FH, Babbs RK, Corwin RL. Reinforcing efficacy of fat, as assessed by progressive ratio responding, depends upon availability not amount consumed. Physiol Behav 2010;100:316–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drewnowski A, Greenwood MRC. Cream and sugar: human preferences for high-fat foods. Physiol Behav 1983;30:629–33 [DOI] [PubMed] [Google Scholar]
- 51.Drewnowski A. Sensory preferences for fat and sugar in adolescence and adult life. Ann N Y Acad Sci 1989;561:243–50 [DOI] [PubMed] [Google Scholar]
- 52.Lappalainen R, Epstein LH. A behavioral economics analysis of food choice in humans. Appetite 1990;14:81–93 [DOI] [PubMed] [Google Scholar]
- 53.Jeffery RW, Drewnowski A, Epstein LH, et al. Long-term maintenance of weight loss: current status. Health Psychol 2000;19(suppl):5–16 [DOI] [PubMed] [Google Scholar]
- 54.Doell SR, Hawkins RC. Pleasures and pounds: an exploratory study. Addict Behav 1982;7:65–9 [DOI] [PubMed] [Google Scholar]
- 55.Jacobs SB, Wagner MK. Obese and nonobese individuals: behavioral and personality characteristics. Addict Behav 1984;9:223–6 [DOI] [PubMed] [Google Scholar]
- 56.Jacobs SB, Wagner MK. Obese and nonobese individuals: behavioral and personality characteristics. 1984;9:223–6 [DOI] [PubMed] [Google Scholar]
- 57.Epstein LH, Salvy SJ, Carr KA, Dearing KK, Bickel WK. Food reinforcement, delay discounting and obesity. Physiol Behav 2010;100:438–45 [DOI] [PMC free article] [PubMed] [Google Scholar]