Abstract
Background: Acute deviations in protein intake before the quantification of protein kinetics in older humans may explain the controversy over the effects of older age on muscle protein synthesis and proteolysis rates.
Objective: We hypothesized that an acute decrease in protein intake from the habitual intake is associated with lower muscle protein synthesis and higher proteolysis rates, whereas an acute increase in protein intake from the habitual intake is associated with higher muscle protein synthesis and lower proteolysis rates.
Design: In 112 community-dwelling healthy men aged 65–90 y, we quantified resting whole-body [1,2-13C2]leucine kinetics, muscle mixed protein fractional synthesis rates (FSRs), and muscle proteasome proteolytic enzyme activities after participants consumed for 3 d controlled research meals (0.9–1.1 g protein · kg−1 · d−1) that contained more or less protein than that habitually consumed and that induced alterations in nitrogen balance.
Results: Protein kinetic parameters were not significantly different between the groups, despite controlled research protein intakes that were lower (−0.2 to −0.3 g · kg−1 · d−1) or higher (+0.2 g · kg−1 · d−1) than habitual intakes and that induced negative (−22 to −25 mg · kg−1 · d−1) or positive (22–25 mg · kg−1 · d−1) nitrogen balance. Within these acutely altered protein intake and nitrogen balance boundaries, a reduction in protein intake from habitual intake and induction of negative nitrogen balance were not associated with higher proteolysis or lower muscle FSR, and an acute increase in protein intake from habitual intake and induction of positive nitrogen balance were not associated with lower proteolysis or higher muscle FSR. A higher quantitative insulin sensitivity check index was associated with lower whole-body proteolysis rates.
Conclusions: The practice of acutely controlling protein intake, even at intakes lower than habitual intakes that induce negative nitrogen balance, before quantifying human protein kinetics does not significantly reduce muscle protein synthesis or increase proteolysis. Factors other than protein intake explain lower muscle protein synthesis rates with advanced age. This trial is registered at clinicaltrials.gov as NCT00183040.
INTRODUCTION
Sarcopenia is the loss of muscle protein mass and function that accompanies advanced age. Sarcopenia is associated with physical inactivity (1, 2); endocrine changes (3–5); neuronal/denervation (6, 7); anorexia or insufficient macronutrient intake, digestion, and absorption (8–11); proinflammatory processes (12); impaired kidney function (13); and reduced muscle blood flow (14). These factors contribute to a loss of muscle protein that ultimately results from changes in the rates of muscle proteolysis and muscle protein synthesis.
However, the effects of older age on muscle proteolysis and protein synthesis rates are controversial. Several reports indicate that resting muscle protein synthesis is lower in older persons than in young control subjects (2, 15–18), whereas other reports indicate that aging has no effect on resting muscle protein synthesis rate (19, 20). One potential explanation for this discrepancy is methodologic. Perhaps the research meals used by some investigators to control energy and protein intakes for 2 to 3 d before the quantification of muscle protein synthesis rate lower the rate of muscle protein synthesis or increase the rate of muscle proteolysis in comparison with measurements made when participants consume their habitual protein and energy intakes. The underlying premise is that habitual protein and energy intakes are greater than those provided in research meals and that a relative protein and energy deficiency introduced by controlled research meals artifactually lowers the rate of muscle protein synthesis or increases the rate of muscle proteolysis measured after several days of controlled intake. If true, this finding clarifies the relative contribution of changes in muscle protein synthesis and proteolysis to sarcopenia.
We tested this hypothesis in the Hormonal Regulators of Muscle and Metabolism in Aging (HORMA) study, in which resting muscle protein synthesis and proteolysis rates were quantified in a relatively large number (n = 112) of healthy men aged 65–90 y (4, 5). We hypothesized that resting muscle protein synthesis rates would be lower and muscle proteolysis higher when habitual protein consumption (g · kg−1 · d−1) was higher than that consumed over 3 d of controlled research meals and that the resting muscle protein synthesis rate would be higher and muscle proteolysis lower when habitual protein consumption was lower than that consumed over 3 d of controlled research meals. This study tested the importance of controlling dietary protein intake on the days before the measurement of in vivo protein/amino acid metabolism and directly addresses the controversy over the influence of acute changes in protein and energy intakes (3 d) preceding the measures of muscle protein synthesis and proteolysis. We also collected information on several potential regulators of muscle protein synthesis and proteolysis and determined their relative contribution to the resting measures of muscle protein synthesis and proteolysis.
SUBJECTS AND METHODS
The HORMA was a randomized, controlled, double-masked multicenter investigation of physiologic supplementation with testosterone and recombinant human growth hormone given to 65–90-y-old community-dwelling men with testosterone and insulin-like growth factor I (IGF-I) concentrations typical of older men (4, 5). The current report focuses only on baseline (before hormonal interventions) nutritional, metabolic, physiologic, and quality-of-life measures.
Study participants
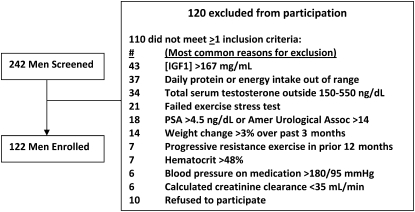
Male participants provided local institutional review board–approved informed consent and were screened at the University of Southern California (USC), Tufts University, and Washington University. Inclusion criteria were as follows: 65–90 y of age, fasting serum IGF-I concentration in the lower tertile for adults (<167 ng/mL, or 21.9 nmol/L) and morning total serum testosterone in the lower half (150–550 ng/dL, or 5.21–19.1 nmol/L) of the adult range for males, prostate-specific antigen ≤ 4.0 ng/mL, hematocrit ≤50%, and fasting blood glucose <126 mg/dL (6.99 mmol/L). Serum albumin and creatinine were quantified in local university hospital laboratories, and creatinine clearance was estimated by using the following equation: [(140 − age) × body weight]/72 × serum creatinine (4). None of the men were current tobacco users. We screened 242 men and enrolled 122 men; exclusion events are outlined in Figure 1.
FIGURE 1.
Screening, exclusion, and enrollment of the older men studied in the Hormonal Regulators of Muscle and Metabolism in Aging (HORMA) study. IGF1, insulin-like growth factor I; PSA, prostate-specific antigen; Amer, American; Assoc, Association.
Dietary intake
Habitual dietary intake
Habitual nutrient intake was assessed from 3-d dietary diaries collected from participants before the study and reviewed by the research nutritionists. Total energy and macronutrient intakes were quantified by using Nutritionist Pro (Axxya Systems, Stafford, TX). The Harris-Benedict equation (1.3 physical activity factor) predicted that these men required 2116 ± 260 kcal/d (median: 2096; minimum: 1405; maximum: 2726 kcal/d), and they reported their habitual energy consumption to be 2198 ± 484 kcal/d (median: 2166; minimum: 983; maximum: 3638; Table 1). These men did not underreport their habitual total daily energy intake.
TABLE 1.
Participant characteristics1
| Mean ± SD | Median (minimum, maximum) | |
| Age (y) | 70.2 ± 4.2 | 69 (64, 85) |
| Nutritional data | ||
| Albumin (mg/dL) | 4.1 ± 0.3 | 4.1 (3.5, 5.4) |
| Creatinine clearance (mL/min) | 82.5 ± 18.5 | 82.9 (40.2, 162.3) |
| Habitual energy intake (kcal/d) | 2198 ± 484 | 2166 (983, 3638) |
| Habitual protein intake (g/d) | 93 ± 24 | 92 (30, 181) |
| Habitual carbohydrate intake (g/d) | 261 ± 82 | 253 (98, 492) |
| Habitual fat intake (g/d) | 87 ± 42 | 82 (20, 425) |
| Energy intake, research meal plan (kcal/d) | 2327 ± 399 | 2326 (1359, 3197) |
| Protein intake, research meal plan (g/d) | 88 ± 14 | 86 (58, 141) |
| Carbohydrate intake, research meal plan (g/d) | 351 ± 72 | 346 (184, 556) |
| Fat intake, research meal plan (g/d) | 69 ± 13 | 70 (32, 100) |
| Resting energy expenditure (kcal/d) | 1696 ± 245 | 1652 (1206, 2664) |
| Estimated total daily energy expenditure (kcal/d) | 2205 ± 318 | 2148 (1567, 3463) |
| Body composition | ||
| Habitual weight (kg)2 | 85.2 ± 12.7 | 83.0 (53.7, 121.0) |
| Research meal plan weight (kg)3 | 84.7 ± 12.5 | 83.0 (51.5, 115.9) |
| BMI (kg/m2)4 | 27.8 ± 3.4 | 27.4 (21.1, 35.1) |
| Lean body mass (kg) | 58.2 ± 6.9 | 57.5 (41.6, 78.0) |
| Appendicular lean mass (kg) | 25.5 ± 3.3 | 25.5 (16.7, 34.0) |
| Appendicular fat mass (kg) | 8.7 ± 3.2 | 8.1 (2.4, 18.7) |
| Trunk fat mass (kg) | 12.7 ± 4.3 | 12.4 (3.5, 26.3) |
| Body fat (%) | 26.3 ± 5.4 | 26.2 (12.4, 39.7) |
| Endocrine and metabolic data | ||
| Total testosterone (ng/dL) | 363 ± 97 | 361 (155, 546) |
| IGF-I (ng/mL) | 125 ± 34 | 118 (68, 260) |
| HOMA-IR (n = 111) | 1.58 ± 1.03 | 1.22 (0.21, 6.97) |
| QUICKI (n = 111) | 0.161 ± 0.016 | 0.161 (0.126, 0.224) |
| Physiologic and behavioral data | ||
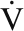 O2 peak (mL · kg−1 · min−1) (n = 93) O2 peak (mL · kg−1 · min−1) (n = 93) |
24.6 ± 4.9 | 24.3 (9.2, 36.8) |
| PASE (n = 100) | 147 ± 65 | 143 (29.6, 368.7) |
| PCS (n = 109) | 52 ± 6 | 54 (29, 59) |
n = 112 unless noted otherwise. PASE, Physical Activity Scale for the Elderly; PCS, SF-12 Physical Component Score;  O2, oxygen consumption; IGF-I, insulin-like growth factor I; HOMA-IR, homeostasis model assessment of insulin resistance; QUICKI, quantitative insulin sensitivity check index.
O2, oxygen consumption; IGF-I, insulin-like growth factor I; HOMA-IR, homeostasis model assessment of insulin resistance; QUICKI, quantitative insulin sensitivity check index.
Habitual body weight at the time of screening.
Weight on day 3 of the research meals, before testing began.
Calculated by using body weight from the controlled research meal period.
Controlled research dietary intake
The participants were admitted to a local Clinical Research Unit (CRU) for ≈20 h. For 2 d before and on the day of admission, the participants consumed a controlled energy and protein meal plan based on their self-reported habitual intake. The controlled research meals consisted of 0.9–1.1 g protein · kg−1 · d−1 with 50–60% of energy as carbohydrate, 25–35% of energy as total fat (≤7% saturated fat), and a total daily energy intake of 2327 ± 399 kcal/d (median: 2326; minimum: 1359; maximum: 3197 kcal/d). Mean total daily energy intake exceeded the Harris-Benedict prediction for total daily energy requirement by 211 ± 404 kcal/d (P < 0.0001).
The controlled meals were prepared in the local CRU research kitchens. The participants were instructed to arrive in the morning (3 d before testing) and to pick up a cooler that contained all food that was to be consumed for the 3 d before the isotope-dilution studies. An additional 500 kcal food was provided in the coolers in case the participants were still hungry after consuming the controlled research meals. The participants were instructed to eat only the food/drink provided and to return all uneaten food. Food/drink that was not consumed was weighed, and the daily intake record was corrected accordingly. Total daily nitrogen intake during the controlled meals was calculated (dietary protein is ≈16% nitrogen). The participants consumed 101–296 mg N · kg−1 · d−1; all but 5 men consumed adequate protein nitrogen (132 mg N · kg−1 · d−1; 0.83 g protein · kg−1 · d−1) (21). We compared muscle and whole-body protein kinetics between 4 groups of older men (Table 2): group 1: lower than habitual protein intake (g · kg−1 · d−1) and negative nitrogen balance (n = 27); group 2: lower than habitual protein intake and positive nitrogen balance (n = 40); group 3: higher than habitual protein intake and negative nitrogen balance (n = 23); and group 4: higher than habitual protein intake and positive nitrogen balance (n = 22).
TABLE 2.
Whole-body and muscle protein metabolism as a function of altered protein intake and nitrogen balance in elderly men1
| Group 1: lower than habitual protein intake and negative nitrogen balance | Group 2: lower than habitual protein intake and positive nitrogen balance | Group 3: higher than habitual protein intake and negative nitrogen balance | Group 4: higher than habitual protein intake and positive nitrogen balance | Overall P value2 | |
| Subjects [n (%)] | 27 (24) | 40 (36) | 23 (21) | 22 (19) | |
| Difference in protein intake: research meals minus habitual intake | |||||
| (g · kg−1 · d−1) | |||||
| Mean ± SD | −0.24 ± 0.18 | −0.28 ± 0.27 | 0.21 ± 0.1334 | 0.22 ± 0.1834 | <0.0001 |
| Median (minimum, maximum) | −0.20 (−0.71, −0.01) | −0.21 (−1.38, −0.02) | 0.21 (0.03, 0.54) | 0.20 (0.01, 0.66) | |
| (% of total energy) | |||||
| Mean ± SD | −3.2 ± 3.1 | −4.0 ± 3.5 | 1.4 ± 2.634 | 0.9 ± 2.734 | <0.0001 |
| Median (minimum, maximum) | −2.3 (−9.9, 2.2) | −3.8 (−16.1, 2.5) | 2.1 (−5.2, 4.5) | 0.8 (−4.9, 5.9) | |
| Estimated nitrogen balance (mg · kg−1 · d−1) | |||||
| Mean ± SD | −21.5 ± 17.1 | 24.9 ± 26.83 | −24.6 ± 15.94 | 21.9 ± 19.435 | <0.0001 |
| Median (minimum, maximum) | −17.8 (−65.9, −1.4) | 16.7 (0.1, 134.9) | −21.8 (−54.5, −1.2) | 16.6 (0.3, 84.1) | |
| Habitual weight (kg)6 | |||||
| Mean ± SD | 84.4 ± 11.4 | 82.2 ± 13.8 | 89.2 ± 12.5 | 87.7 ± 12.2 | 0.15 |
| Median (minimum, maximum) | 82.9 (64.2, 108.2) | 79.8 (53.7, 112.5) | 89.1 (69.0, 121.0) | 84.3 (73.3, 110.7) | |
| Research meal weight (kg)6 | |||||
| Mean ± SD | 83.6 ± 11.0 | 82.3 ± 13.8 | 88.1 ± 12.0 | 87.1 ± 12.1 | 0.25 |
| Median (minimum, maximum) | 81.5 (63.8, 107.6) | 80.2 (51.5, 110.6) | 88.7 (68.3, 115.9) | 84.7 (71.5, 112.7) | |
| Whole-body proteolysis rate (mg · kg LBM−1 · h−1)6 | |||||
| Mean ± SD | 16.4 ± 1.4 | 15.8 ± 2.5 | 16.0 ± 1.4 | 15.9 ± 2.3 | 0.25 |
| Median (minimum, maximum) | 16.4 (13.3, 19.4) | 15.8 (10.9, 23.8) | 15.8 (14.0, 19.4) | 16.3 (12.7, 22.0) | |
| Mixed muscle protein fractional synthesis rate (%/h) (n = 107)6 | |||||
| Mean ± SD | 0.097 ± 0.048 | 0.085 ± 0.025 | 0.097 ± 0.032 | 0.091 ± 0.039 | 0.49 |
| Median (minimum, maximum) | 0.075 (0.048, 0.222) | 0.084 (0.041, 0.157) | 0.088 (0.056, 0.170) | 0.072 (0.051, 0.202) | |
| Chymotrypsin-like enzyme activity (mU/g)6 | |||||
| Mean ± SD | 46.1 ± 20.2 | 39.2 ± 15.6 | 49.1 ± 14.1 | 42.2 ± 13.8 | 0.11 |
| Median (minimum, maximum) | 44.9 (21.0, 92.6) | 38.9 (9.5, 69.3) | 46.1 (24.2, 80.6) | 37.1 (19.7, 74.4) | |
| Trypsin-like enzyme activity (mU/g)6 | |||||
| Mean ± SD | 8.3 ± 3.8 | 7.0 ± 3.1 | 9.0 ± 3.2 | 7.8 ± 3.5 | 0.14 |
| Median (minimum, maximum) | 8.0 (3.4, 17.2) | 6.4 (1.8, 13.7) | 8.5 (3.9, 15.8) | 7.2 (3.0, 17.3) | |
| PGPD enzyme activity (mU/g)6 | |||||
| Mean ± SD | 8.7 ± 4.9 | 7.5 ± 3.9 | 10.1 ± 4.1 | 8.9 ± 4.1 | 0.13 |
| Median (minimum, maximum) | 6.5 (2.9, 26.0) | 6.4 (1.7, 18.4) | 8.9 (3.2, 18.1) | 7.7 (3.0, 16.3) | |
LBM, lean body mass; PGPD, peptidyl glutamyl peptide hydrolase.
Comparison between means (2-sample t test).
Significantly different from group 1 after adjustment for multiple comparisons, P ≤ 0.01 (post hoc).
Significantly different from group 2 after adjustment for multiple comparisons, P ≤ 0.01 (post hoc).
Significantly different from group 3 after adjustment for multiple comparisons, P ≤ 0.01 (post hoc).
Post hoc test not appropriate.
All urine excreted during the CRU admission was collected, and an aliquot was analyzed for urine urea nitrogen concentration in the local university hospital laboratory. Total (24 h) urine nitrogen excretion was calculated (urea nitrogen is ≈90% of total urine nitrogen), and total nitrogen losses were estimated from urine nitrogen plus 1.75 g N/d (from fecal, dermal, and miscellaneous losses). Nitrogen balance was estimated from total nitrogen intake minus total nitrogen excretion.
Primary outcome measures
Whole-body leucine rate of appearance, proteolysis rate, and fractional synthesis rate of mixed muscle proteins
The primary study outcomes were whole-body and muscle protein metabolism measured in each participant on day 3 of the controlled research meal plan. The rationale, approach, and technical procedures for these measures were described previously (2, 15–18, 22–24). Standardized protocols for tracer infusion studies and blood and muscle sampling procedures were applied across the 3 clinical testing centers, where research team members received training and adhered to a detailed standard operating procedure manual. All samples for protein kinetic analyses were analyzed in the Biomedical Mass Spectrometry Research Facility at Washington University.
Briefly, at 1800 h (≈2 h after the evening meal), a primed bolus of [1,2-13C2]leucine ([13C2]Leu; Cambridge Isotope Laboratories Inc, Waltham, MA; ≈98 atom%; 4.58 μmol/kg) was given, and a 14-h intravenous infusion (1 mg · kg−1 · d−1) was continued throughout the overnight fasted condition. Venous blood (7 mL) was collected before the start of the [13C2]Leu infusion (−15, 0 min) and at 30-min intervals during the last 2 h of the constant infusion (12, 12.5, 13, 13.5, and 14 h). [13C2]Leu was used (rather than [1-13C]Leu) because it provides more 13C incorporation into muscle proteins, which facilitates mass spectrometric quantitation of the expected slow rate of amino acid incorporation into muscle protein.
Plasma Leu and the intracellular product of Leu transamination, α-keto-isocaproic acid (αKIC), were chemically derivatized (23, 25–27) and [13C2]abundance of each was quantified by using gas chromatography–quadrupole mass spectrometry [Agilent 6890N Gas Chromatograph and Agilent 5973N Mass Selective Detector (GC-MS); Agilent, Palo Alto, CA] (23, 27). The average plasma [13C2]Leu enrichment during the last 2 h of infusion was used to calculate the whole-body Leu rate of appearance. The average plasma [13C2]αKIC enrichment was used to calculate the whole-body proteolysis rate (23, 27, 28).
Indirect calorimetry was used to quantify resting oxygen consumption and carbon dioxide production rates. Measures (5 min stabilization plus 15 min collection) were performed twice; before the infusion started (at 0600 and 0730) and during the last hour of the [13C2]Leu infusion (1530 and 1600). The Weir equation was used to convert oxygen and carbon dioxide kinetics to a measure of resting energy expenditure (29). A 1.3 physical activity factor was used to estimate total daily energy requirements for these men.
Vastus lateralis muscle samples (≈100 mg) were obtained 90 min after the infusion started (1930) and at the end of the infusion (13.5–14 h; 0730–0800). [13C2]Leu enrichment in the muscle free amino acid pool was quantified by using GC-MS (22, 24, 30). [13C2]Leu enrichment in mixed muscle proteins was quantified by using GC-combustion-isotope ratio mass spectrometry (17, 23, 30–32) (Delta+ XL-IRMS; Finnigan, Bremen, Germany).
The fractional synthesis rate for mixed muscle proteins (MMPs) was calculated by using the established equation (2, 3, 11, 14–16, 18, 20, 22–24, 26, 30–35):
 |
This approach uses estimates for 13C2-enrichment in the true precursor pool for muscle protein synthesis ([13C2]leucyl-tRNA); the average muscle free pool [13C2]Leu enrichment or the average plasma [13C2]αKIC enrichment during the last 2 h of the [13C2]Leu infusion (15, 17, 24, 30–32, 36).
Muscle proteasome catalytic activity
To assess functional activation of the ubiquitin-proteasome pathway in skeletal muscle extracts, we measured the 3 best characterized ATP-ubiquitin–independent peptidase activities in the 20S proteasome core: chymotrypsin-like, trypsin-like (T-L), and peptidyl glutamyl peptide hydrolase (37). As described previously (23), peptidase activities were measured ex vivo in crude muscle (10 mg) extracts by monitoring the release of 7-amido-4-methylcoumarin (AMC) from synthetic peptide substrates: N-succinyl-Leu-Leu-Val-Tyr-7-amido-4-methylcoumarin (N-suc-LLVY-AMC) for chymotrypsin-like activity, N-tert-butyloxycarbonyl-Leu-Ser-Thr-Arg-7-amido-4-methylcoumarin (N-t-BOC-LSTR-AMC) for trypsin-like activity and N-benzyloxycarbonyl-Leu-Leu-Glu-7-amido-4-methylcoumarin (N-CBZ-LLE-AMC) for peptidyl glutamyl peptide hydrolase activity. All reagents were obtained from Sigma Chemical (St Louis, MO). Tissue extract protein concentrations were quantified by using the Biuret method, and bovine serum albumin was used as the reference standard. AMC release was monitored fluorometrically at 37°C in 96-well plates. Slopes corresponding to maximum reaction rates (increase in fluorescence/min) were compared with AMC standards to quantify the amount of AMC released. Proteasome peptidase activities were determined in triplicate by the difference in rates of substrate hydrolysis in muscle extracts that had been pre-incubated in the absence or presence of lactacystin (BIOMOL Research Laboratories, Plymouth Meeting, PA)—a proteasome-specific inhibitor (38). Enzyme activity is expressed as mU/g; one unit of activity is equivalent to the release of 1 μmol AMC/min under the specified reaction conditions.
Independent predictors
Body composition
Body weight was measured after an overnight fast and the morning void and while the participant was wearing only undergarments, a hospital gown, and no jewelry. Habitual body weight was measured at screening, when a research nutritionist assessed habitual protein and energy intake. Research body weight was measured on day 3 of the controlled meal plan. Whole-body and regional lean and fat mass were quantified by using dual energy X-ray absorptiometry (DXA). DXA scanners at each center were cross-calibrated by scanning the same soft tissue phantom. Scans were analyzed at the USC Reading Center by an experienced DXA-certified bionutritionist (4, 5).
Aerobic capacity
At baseline, peak oxygen consumption (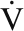 O2) was assessed by using cycle ergometry (4, 5). Peak
O2) was assessed by using cycle ergometry (4, 5). Peak 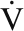 O2 was the highest oxygen consumption attained when participants could not maintain a pedaling rate >55 rpm.
O2 was the highest oxygen consumption attained when participants could not maintain a pedaling rate >55 rpm.
Hormone assays
For screening, total serum testosterone was measured by using immunoassays in the local university hospital laboratories and IGF-I at Quest Diagnostics (San Juan Capistrano, CA). Insulin concentrations were analyzed by using an automated enzyme immunoassay (Tosoh AIA 600 II analyzer; Tosoh Bioscience Inc, South San Francisco, CA) (4, 5). Insulin resistance was evaluated by using the homeostasis model assessment of insulin resistance (HOMA-IR) index (39), and insulin sensitivity was evaluated by using the quantitative insulin sensitivity check index (QUICKI; 40).
Physical activity and quality of life
Self-reported leisure-time physical activity in the 7 d before study was assessed by using the Physical Activity Scale for the Elderly (PASE) questionnaire. Self-reported quality of life was assessed by using the Short-Form Health Survey (SF-12).
Statistical analyses
Baseline characteristics are presented as means ± SDs and medians and ranges. Change in protein intake was calculated as the difference between controlled research protein intake (g · kg−1 · d−1) and habitual protein intake (g · kg−1 · d−1). Participants were divided into 4 groups, those with reduced or increased protein intake while consuming the controlled research meals and those with positive or negative nitrogen balance. Two-sample t tests were performed to compare the physiologic and metabolic variables between these groups. Relations between change in protein intake, positive compared with negative nitrogen balance, and protein metabolism outcomes were assessed by using linear regression and were illustrated by using scatter plots. Spearman's correlations were used for these nonnormally distributed variables. Five outcomes were measured in the 4 groups; therefore, post hoc analyses were Bonferroni adjusted to account for multiple comparisons (P = 0.05/5 = 0.01).
Other potential predictors of baseline protein metabolism measures were identified by using univariate regression (P < 0.20). Candidate variables were further screened by stepwise regression (entry α = 0.10). Partial r2 values were calculated for each predictor based on the final model for each protein metabolism outcome. Statistical analyses were conducted by using the Statistical Analysis System (version 9.2; SAS Institute Inc, Cary, NC).
RESULTS
Participant characteristics
Of the 122 men enrolled, 112 completed all aspects of the study and provided baseline nutrition, metabolic, and physiologic data (Table 1). The average difference between habitual body weight at screening (85.2 ±12.7 kg) and on day 3 of the research meals, just before testing began (84.7 ± 12.5 kg), was small (−0.5 ± 1.7 kg; median:−0.4; minimum: −5.4; maximum: 5.8) but statistically significant (P = 0.0003; Table 1). The average time interval between measures of habitual and research body weight was 55 ± 30 d (median: 48; minimum: 6; maximum: 178). The results reported here were not adjusted for this small weight loss because average weight changed (from −1.1 to + 0.1 kg) when groups transitioned from habitual to research meals (Table 2), and statistical adjustment for this did not alter the findings.
Deviations in protein intake
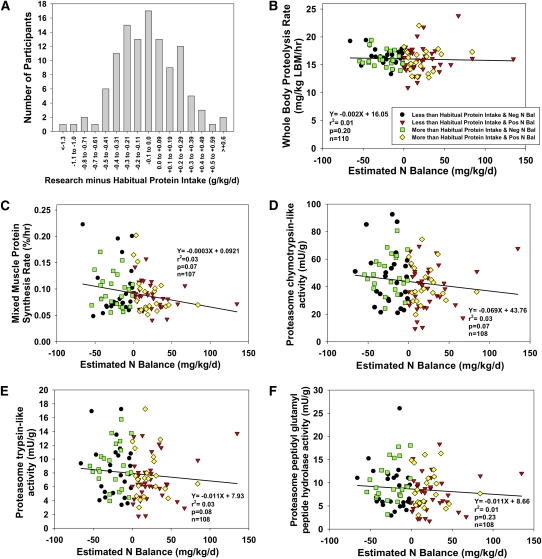
The difference in daily protein intake (g protein/kg) between the controlled research meals and habitual consumption varied widely, but was normally distributed (Figure 2A). On average, the differences in daily protein and energy intake between the controlled research and habitual meals were −5 ± 25 g protein/d (range: −101 to 54 g protein/d) and +129 ± 375 kcal/d (range: −943 to 1362 kcal/d). This provided an opportunity to examine whether an acute change from habitual to controlled protein intake was related to changes in whole-body and muscle protein metabolism in these older men. Fifty men were in negative nitrogen balance despite consuming research meals that matched or exceeded their reported habitual protein and energy intakes (Tables 1 and 2).
FIGURE 2.
A: Frequency distribution for the difference in protein intake (research minus habitual protein; g · kg−1 · d−1) in older men. The number of men who consumed less protein with the research meal plan than they consumed habitually and those who consumed more protein with the research meal plan than they consumed habitually was normally distributed. B–F: Correlates of whole-body and muscle protein metabolism in older men. Univariate correlation analyses among protein intakes that were lower or higher than habitual and induced negative or positive nitrogen balance, and whole-body proteolysis rate (B), mixed muscle protein fractional synthesis rate (C), and muscle proteolytic enzyme activities [chymotrypsin-like (D), trypsin-like (E), and peptidyl glutamyl peptide hydrolase (F) activities]. No significant relations were observed (all Spearman regression P values >0.01) regardless of how the change in protein intake was expressed (% of total energy or g protein · kg−1 · d−1). LBM, lean body mass: Neg, negative; Pos, positive; Bal, balance.
Effects of deviations in protein intake on protein metabolism
The 112 men were divided into 4 groups based on their protein consumption and nitrogen balance status (Table 2). Despite sizable deviations from habitual to research meal protein intakes (median: −3.8 to 2.1% of total energy; minimum: −16%; maximum: 6%) and changes in nitrogen balance, on average, whole-body and muscle protein kinetic parameters were not different between the 4 groups (Table 2), regardless of habitual or research protein intakes and nitrogen balance status in these older men.
As previously reported, the muscle free pool [13C2]Leu enrichment was 76 ± 5% of the plasma [13C2]KIC enrichment (22, 24) (Table 3). When muscle tissue free pool [13C2]Leu was used to represent the precursor pool for protein synthesis, mixed muscle protein synthesis rates were 24% higher than when plasma [13C2]αKIC values were used, but the relations between muscle protein synthesis rate and all variables were the same and our conclusions confirmed it, regardless of whether muscle free pool [13C2]Leu or plasma [13C2]αKIC was used to represent the precursor pool for muscle protein synthesis. For this reason, we report MMP values based on plasma [13C2]αKIC.
TABLE 3.
13C-Enrichment values in various precursor pools and mixed muscle protein1
| Mean ± SD | Median (minimum, maximum) | |
| Plasma [1,2-13C2]Leu (mol % excess) | 4.9 ± 0.6 | 4.9 (3.3, 6.6) |
| Plasma [1,2-13C2]αKIC (mol % excess) | 3.6 ± 0.4 | 3.5 (2.4, 5.1) |
| Muscle free pool [1,2-13C2]Leu (mol % excess) | 2.7 ± 0.4 | 2.6 (1.8, 4.3) |
| Mixed muscle protein [1,2-13C2]Leu (atom % excess) | 0.0392 ± 0.0141 | 0.0353 (0.0132, 0.0804) |
Plasma values represent the average measured during the last 2 h of the tracer infusion. Muscle free pool represents the average measured 1.5 and 14 h after the tracer infusion. Mixed muscle protein values represent the increment from 1.5 to 14 h of the tracer infusion. KIC, -keto-isocaproic acid.
The between-group means may mask correlative relations between changes in habitual and research protein intakes, changes in nitrogen balance, and protein metabolism outcomes. Using Spearman's correlation analyses, we examined relations between changes (both decrease and increase) in protein intake from habitual to research meals, nitrogen balance status (negative or positive), and each of the protein metabolism outcomes (Figure 2). This tested whether greater reductions in protein intake and a more negative nitrogen balance predicted higher proteolytic rates and lower muscle protein synthesis rates. It also tested whether greater increases in protein intake and a more positive nitrogen balance predicted lower rates of proteolysis and higher muscle protein synthesis rates. No significant relations were observed (Figure 2, B–F) regardless of whether we expressed the changes in protein intake as a percentage of total energy intake or g · kg−1 · d−1 (data not shown). Thus, acute changes in protein intake along with changes in nitrogen balance did not predict any of the whole-body or muscle protein metabolism variables. Regardless of the magnitude of the reduction in protein intake between habitual and research meals and the magnitude of negative nitrogen balance, there was no evidence of higher proteolytic rates or lower muscle protein synthesis rates. Likewise, the magnitude of the increase in protein intake between habitual and research meals and the magnitude of positive nitrogen balance did not predict changes in proteolytic or muscle protein synthesis rates.
Predictors of protein metabolism
We examined relations between traditional regulators of muscle protein mass (Table 4) and whole-body and muscle protein metabolism outcomes. The strongest predictors of whole-body proteolysis rate were insulin resistance (HOMA-IR) and insulin sensitivity (QUICKI) indexes; these indexes were closely related (r = −0.87, P < 0.0001). Better insulin sensitivity was significantly associated with lower whole-body proteolysis rates. Based on partial r2, QUICKI explained 21% of the variance in whole-body proteolysis rate. However, for all other protein metabolism outcomes, no covariate identified by stepwise linear regression analysis explained >7% of the variance associated with that measure, despite the fact that several of these associations were expected (Table 4). For example, a higher total testosterone concentration was associated with a lower muscle proteasome trypsin-like enzyme activity (partial r2 = 0.07, P = 0.01). Furthermore, greater insulin resistance (HOMA-IR) was associated with higher proteasome peptidyl glutamyl peptide hydrolase enzyme activity (partial r2 = 0.05, P = 0.02). Some unexpected weak associations were also noted. Greater insulin resistance (HOMA-IR) was associated with higher muscle protein synthesis rates (partial r2 = 0.04, P = 0.02), and higher albumin concentrations were associated with lower muscle protein synthesis rates (partial r2 = 0.03, P = 0.05). The physiologic relevance of the stepwise linear associations with small partial r2 values ≤7% is questionable despite statistical significance.
TABLE 4.
Univariate and multivariate predictors of baseline protein metabolism measures in older men1
| Dependent and independent variables | P (stepwise regression)2 | β Regression coefficient | Partial r2 |
| Whole-body proteolysis rate3 | |||
| QUICKI | <0.001 | −77.3 | 0.21 |
| Mixed muscle protein synthesis rate4 | |||
| Serum albumin | 0.05 | −0.023 | 0.03 |
| HOMA-IR | 0.02 | 0.008 | 0.04 |
| Total lean mass | 0.02 | −0.001 | 0.04 |
| Muscle proteasome chymotrypsin-like activity5 | |||
| Age | 0.06 | 0.79 | 0.04 |
| Creatinine clearance | 0.02 | 0.21 | 0.03 |
| Muscle proteasome trypsin-like activity6 | |||
| Total testosterone | 0.01 | −0.005 | 0.07 |
| HOMA-IR | 0.03 | 0.81 | 0.04 |
| Muscle proteasome PGPD activity7 | |||
| Total testosterone | 0.08 | −0.004 | 0.03 |
| HOMA-IR | 0.02 | 0.90 | 0.05 |
HOMA-IR, homeostasis model assessment of insulin resistance; QUICKI, quantitative insulin sensitivity check index; PGPD, peptidyl glutamyl peptide hydrolase.
All potential predictors represent baseline values. Entry level for stepwise: P < 0.20.
Covariates: HOMA-IR (P < 0.0001), QUICKI (P < 0.0001), BMI (P = 0.08), and total lean mass (P = 0.10).
Covariates: total energy intake (P = 0.06), HOMA-IR (P = 0.08), total lean mass (P = 0.04), appendicular lean mass (P = 0.04), serum albumin (P = 0.13), and age (P = 0.14).
Covariates: creatinine clearance (P = 0.06), HOMA-IR (P = 0.10), trunk fat mass (P = 0.06), age (P = 0.16), testosterone (P = 0.13), total energy intake (P = 0.20), difference in total energy intake between research and habitual intakes (P = 0.17), Physical Activity Scale for the Elderly (P = 0.18), and BMI (P = 0.15).
Covariates: total testosterone (P = 0.01), creatinine clearance (P = 0.04), HOMA-IR (P = 0.05), trunk fat mass (P = 0.05), total energy intake (P = 0.14), difference in total energy intake between research and habitual intakes (P = 0.17), Physical Activity Scale for the Elderly (P = 0.19), QUICKI (P = 0.17), and BMI (P = 0.12).
Covariates: total testosterone (P = 0.06), creatinine clearance (P = 0.05), HOMA-IR (P = 0.01), QUICKI (P = 0.06), trunk fat mass (P = 0.03), BMI (P = 0.08), and appendicular fat mass (P = 0.11).
On the basis of the principal finding from the stepwise linear regression analysis that better insulin sensitivity (QUICKI) was significantly associated with lower whole-body proteolysis rates, we adjusted the univariate correlation analysis between reductions and increases in protein intake from habitual to research meals and whole-body proteolysis rate to determine whether this altered our original finding. After adjustment for QUICKI, there was still no evidence of a relation between changes in protein intake and whole-body proteolysis rate (r2 = 0.004, NS).
DISCUSSION
The findings indicate that controlled research meals that provide protein intakes at Recommended Dietary Allowance levels that deviate from habitual protein intake by ±4% and induce changes in nitrogen balance (±25 mg · kg−1 · d−1) do not significantly alter measurements of fasting whole-body proteolysis rates, mixed muscle protein synthesis rates, or muscle proteolytic enzyme activities in older men. This suggests that controlling dietary protein intakes for several days before quantifying muscle protein synthesis does not artifactually lower the measured rate of fasting muscle protein synthesis in older men and does not account for advanced age-associated lower rates of fasting muscle protein synthesis reported by several (2, 15–18, 24) but not all investigators (19, 20). The findings indicate that the practice of controlling protein intake and nitrogen balance for several days (eg, 3 d) before the quantification of fasting [13C]Leu kinetics and muscle proteasome enzyme activities in older men may not be necessary, as long as body weight is maintained (±4%), protein intake is within 0.9–1.1 g · kg−1 · d−1, and nitrogen balance is ±25 mg · kg−1 · d−1. We found the magnitude of the difference between habitual and controlled protein intakes was not associated with significant changes in fasting [13C]Leu kinetics or muscle proteolytic enzyme activities in older men. In stepwise linear regression analyses using many potential regulators of human protein metabolism, changes in protein and energy intakes were not associated with resting whole-body proteolysis rates, mixed muscle protein synthesis rates, or muscle proteolytic enzyme activities. Instead, the strongest predictor of fasting whole-body proteolysis rate was an index of insulin sensitivity. This implies that knowledge of an older man's insulin sensitivity is a more important potential covariate than controlling protein energy intake for several days before quantifying fasting [13C]Leu kinetics or muscle proteolytic enzyme activities.
Our finding that the insulin sensitivity index was more important than other traditional regulators of whole-body proteolysis supports and extends previous findings (14, 18, 19, 34, 41, 42). Volpi et al (10, 14, 34, 41) and Rennie et al (19, 42) have exquisitely shown in older persons that skeletal muscle proteins are refractory to acute increases in amino acid and insulin concentrations that normally stimulate protein synthesis and inhibit proteolysis in younger adults. This suggests that age-related muscle protein loss may be accelerated by 1) a blunted anabolic response to acute increases in circulating amino acid and insulin concentrations during feeding, and 2) a relative insensitivity to the antiproteolytic actions of modest (≈15 μU/mL) or higher insulin concentrations. In the current study, it is possible that the lack of association observed among changes in daily protein intake (from habitual to research meals), nitrogen balance, and fasting whole-body and muscle protein metabolism (Figure 2) resulted from blunted protein anabolic and catabolic responses to short-term (3 d) changes in dietary protein/amino acid intakes in these older men. Perhaps 3 d of controlled protein and energy research meals was of insufficient duration to alter whole-body and muscle protein metabolism in these older men. Despite the blunted antiproteolytic response to insulin in older compared with younger men, an index of fasting insulin sensitivity remained the strongest predictor of the whole-body proteolysis rate. This reinforces the importance of fasting insulin concentrations to regulate proteolysis relative to other variables that influence muscle protein mass in older men (eg, protein intake, digestion, and absorption; testosterone and IGF-I concentrations; body composition; and renal function).
On average, the fasting mixed muscle protein synthesis rates measured in these older men are higher than those in our previous studies, which used a similar stable-isotope tracer method ([1-13C]Leu tracer). Still, 33% of the men had low fasting muscle protein synthesis rates (0.041–0.070%/h) (2, 15–18, 24). As suggested, quantification of in vivo resting muscle protein synthesis rates is limited by measurement sensitivity and large intersubject variability (43). Despite the infusion of a double-labeled tracer ([1,2-13C2]Leu), our CV for mixed muscle protein synthesis was 39%, which suggested that this variation is not due to analytic/methodologic variation but rather to biological variation that remains unaccounted for. A potential explanation for the higher mean fasting fractional muscle protein synthesis rates observed here include the fact that the men were relatively healthy, independent, and physically active. In previous studies, sedentary older men and women were studied, some of whom had comorbidities (eg, insulin resistance) and lived in congregate assisted-living facilities (44). The resting mixed muscle protein synthesis rate was not related to peak aerobic capacity. This finding differs from that of Henderson et al (18); in univariate and multivariate analyses, the natural logarithm of 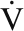 O2 predicted the mixed muscle protein synthesis rate in 144 healthy men and women aged 63–73 y (mean peak
O2 predicted the mixed muscle protein synthesis rate in 144 healthy men and women aged 63–73 y (mean peak 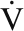 O2 for men: 27.5 mL · kg−1 · min−1), but it only accounted for a small proportion (4–5%) of the variance in these models.
O2 for men: 27.5 mL · kg−1 · min−1), but it only accounted for a small proportion (4–5%) of the variance in these models.
This study had some limitations. The controlled meals were consumed for only 3 d, and a longer exposure to this protein intake or nitrogen balance status may be necessary to reveal correlations with whole-body and muscle protein metabolism. On average, body weight during the research meals was lower than habitual, so we may have underestimated protein and energy requirements for these men. This may induce an energy deficit large enough to obscure any effect of acutely altering protein intake on the muscle protein metabolism variables. However, the difference between habitual and research body weight was small (−0.5 ± 1.7 kg), these measures were separated by 55 ± 30 d, and the actual energy deficit was only −82 ± 510 kcal/d. Whole-body protein kinetics reflect a composite of all body proteins and may be insensitive to changes in protein and energy intakes. For this reason, we measured mixed muscle protein synthesis and muscle proteolytic enzyme activities. We only studied relatively healthy, older men, so our findings cannot be generalized to other populations (eg, older men and women with severe sarcopenia, physical frailty, and/or comorbidities or healthy younger men and women). An alternative study design would involve quantifying protein metabolic variables during both habitual and research meals, but this was not fiscally feasible or practical for participants enrolled in a complex metabolic study (4, 5). We only quantified fasting mixed muscle protein synthesis rates. Because of the large number of participants, it was not feasible to quantify synthesis rates for other muscle proteins (eg, cytosolic, mitochondrial, or nuclear) under fasting and postprandial conditions, but these may respond differently to acute dietary changes. Intake of specific amino acids may be a more important regulator of protein metabolism than dietary protein or energy intakes. This would require individually removing and supplementing research meals with select amino acids. Overall, when comparing muscle protein metabolism variables between older and younger participants, it is advisable to compare those consuming very similar habitual protein and energy intakes rather than changing their intakes for a short period (3 d) before conducting the protein metabolism measures.
The strengths of the study included a relatively large sample of well-characterized older men combined with baseline whole body and muscle protein/amino acid kinetics quantified under tightly regulated dietary conditions. Dietary deviations were acute, but normally distributed; therefore, valid and meaningful comparisons between changes in protein intake (compared with habitual) and nitrogen balance could be made.
In summary, acute increases and decreases in protein intake within 4% of habitual intake were not associated with changes in resting whole-body and muscle protein metabolism in 65–90-y-old free-living men. Previous reports of lower mixed muscle protein synthesis rates in older men cannot be explained as an artifact due to reductions in the protein content of the controlled research meals consumed for several days before quantifying whole-body and muscle protein metabolism. We confirmed that an index of insulin sensitivity, among several potential regulators, is a strong predictor of the whole-body proteolysis rate in older men and may be a more important regulator of whole-body and muscle protein metabolism than is controlling protein intake for several days before quantifying protein/amino acid kinetic parameters in older men.
Acknowledgments
We thank the participants for their time and effort during the conduct of these studies. Sam Smith and Jennifer (Xianghong) Wang provided mass spectrometry analytical expertise. Batch testing (insulin and IGF-I) was performed by the research staff in the USC General Clinical Research Center Core Endocrine Laboratory.
The authors' responsibilities were as follows—FRS, SB, KEY, RR, CC-S, and ETS: were responsible for the hypotheses, specific aims, and study design; CC-S, EFB, ETS, RR, and FRS: were responsible for data acquisition; and SPA, MK, and JH: conducted data quality control, audits, summaries, and statistical analyses. RR is now an employee of Novartis Institutes for Biomedical Research. All authors reviewed the analyses and contributed to and reviewed all manuscript versions. None of the authors reported a conflict of interest.
REFERENCES
- 1.Greiwe JS, Cheng B, Rubin DC, Yarasheski KE, Semenkovich CF. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J 2001;15:475–82 [DOI] [PubMed] [Google Scholar]
- 2.Yarasheski KE, Zachwieja JJ, Bier DM. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol 1993;265:E210–4 [DOI] [PubMed] [Google Scholar]
- 3.Giannoulis MG, Jackson N, Shojaee-Moradie F, et al. The effects of growth hormone and/or testosterone on whole body protein kinetics and skeletal muscle gene expression in healthy elderly men: a randomized controlled trial. J Clin Endocrinol Metab 2008;93:3066–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sattler FR, Castaneda-Sceppa C, Binder EF, et al. Testosterone and growth hormone improve body composition and muscle performance in older men. J Clin Endocrinol Metab 2009;94:1991–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroeder ET, Castaneda-Sceppa C, Wang Y, et al. Hormonal regulators of muscle and metabolism in aging (HORMA): design and conduct of a complex, double masked multicenter trial. Clin Trials 2007;4:560–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmeli E, Reznick AZ. The physiology and biochemistry of skeletal muscle atrophy as a function of age. Proc Soc Exp Biol Med 1994;206:103–13 [DOI] [PubMed] [Google Scholar]
- 7.Welle S. Cellular and molecular basis of age-related sarcopenia. Can J Appl Physiol 2002;27:19–41 [DOI] [PubMed] [Google Scholar]
- 8.Morley JE. Anorexia, sarcopenia, and aging. Nutrition 2001;17:660–3 [DOI] [PubMed] [Google Scholar]
- 9.Boirie Y, Gachon P, Beaufrere B. Splanchnic and whole-body leucine kinetics in young and elderly men. Am J Clin Nutr 1997;65:489–95 [DOI] [PubMed] [Google Scholar]
- 10.Fujita S, Volpi E. Amino acids and muscle loss with aging. J Nutr 2006;136:277S–80S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujita S, Dreyer HC, Drummond MJ, et al. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol 2007;582:813–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung HY, Cesari M, Anton S, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev 2009;8:18–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castaneda C, Gordon PL, Parker RC, Uhlin KL, Roubenoff R, Levey AS. Resistance training to reduce the malnutrition-inflammation complex syndrome of chronic kidney disease. Am J Kidney Dis 2004;43:607–16 [DOI] [PubMed] [Google Scholar]
- 14.Fujita S, Rasmussen BB, Cadenas JG, Grady JJ, Volpi E. Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am J Physiol Endocrinol Metab 2006;291:E745–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci USA 1996;93:15364–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welle S, Thornton C, Jozefowicz R, Statt M. Myofibrillar protein synthesis in young and old men. Am J Physiol 1993;264:E693–8 [DOI] [PubMed] [Google Scholar]
- 17.Yarasheski KE. Exercise, aging, and muscle protein metabolism. J Gerontol A Biol Sci Med Sci 2003;58:M918–22 [DOI] [PubMed] [Google Scholar]
- 18.Henderson GC, Dhatariya K, Ford GC, et al. Higher muscle protein synthesis in women than men across the lifespan, and failure of androgen administration to amend age-related decrements. FASEB J 2009;23:631–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuthbertson D, Smith K, Babraj J, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 2005;19:422–4 [DOI] [PubMed] [Google Scholar]
- 20.Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA 2001;286:1206–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rand WM, Pellett PL, Young VR. Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults. Am J Clin Nutr 2003;77:109–27 [DOI] [PubMed] [Google Scholar]
- 22.Hasten DL, Pak-Loduca J, Obert KA, Yarasheski KE. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78-84 and 23-32 yr olds. Am J Physiol Endocrinol Metab 2000;278:E620–6 [DOI] [PubMed] [Google Scholar]
- 23.Yarasheski KE, Smith SR, Powderly WG. Reducing plasma HIV RNA improves muscle amino acid metabolism. Am J Physiol Endocrinol Metab 2005;288:E278–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am J Physiol 1997;273:E790–800 [DOI] [PubMed] [Google Scholar]
- 25.Schwarz HP, Karl IE, Bier DM. The alpha-keto acids of branchedchain amino acids: simplified derivatization for physiological samples and complete separation as quinoxalinols by packed column gas chromatography. Anal Biochem 1980;108:360–6 [DOI] [PubMed] [Google Scholar]
- 26.Yarasheski KE, Zachwieja JJ, Gischler J, Crowley J, Horgan MM, Powderly WG. Increased plasma Gln and Leu Ra and inappropriately low muscle protein synthesis rate in AIDS wasting. Am J Physiol 1998;275:E577–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reeds DN, Cade WT, Patterson BW, Powderly WG, Klein S, Yarasheski KE. Whole-body proteolysis rate is elevated in HIV-associated insulin resistance. Diabetes 2006;55:2849–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthews DE, Motil KJ, Rohrbaugh DK, Burke JF, Young VR, Bier DM. Measurement of leucine metabolism in man from a primed, continuous infusion of L-[13C]leucine. Am J Physiol 1980;238:E473–9 [DOI] [PubMed] [Google Scholar]
- 29.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism.1949. Nutrition 1990;6:213–21 [PubMed] [Google Scholar]
- 30.Balagopal P, Ford GC, Ebenstein DB, Nadeau DA, Nair KS. Mass spectrometric methods for determination of [13C]leucine enrichment in human muscle protein. Anal Biochem 1996;239:77–85 [DOI] [PubMed] [Google Scholar]
- 31.Nair KS, Halliday D, Griggs RC. Leucine incorporation into mixed skeletal muscle protein in humans. Am J Physiol 1988;254:E208–13 [DOI] [PubMed] [Google Scholar]
- 32.Yarasheski KE, Smith K, Rennie MJ, Bier DM. Measurement of muscle protein fractional synthetic rate by capillary gas chromatography/ combustion/ isotope ratio mass spectrometry. Biol Mass Spectrom 1992;21:486–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rennie MJ, Edwards RH, Halliday D, Matthews DE, Wolman SL, Millward DJ. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci (Lond) 1982;63:519–23 [DOI] [PubMed] [Google Scholar]
- 34.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab 2000;85:4481–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yarasheski KE, Zachwieja JJ, Campbell JA, Bier DM. Effect of growth hormone and resistance exercise on muscle growth and function in older men. Am J Physiol 1995;268:E268–76 [DOI] [PubMed] [Google Scholar]
- 36.Watt PW, Lindsay Y, Scrimgeour CM, et al. Isolation of aminoacyl-tRNA and its labeling with stable-isotope tracers: use in studies of human tissue protein synthesis. Proc Natl Acad Sci USA 1991;88:5892–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solomon V, Goldberg AL. Importance of the ATP-ubiquitin-proteasome pathway in the degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J Biol Chem 1996;271:26690–7 [DOI] [PubMed] [Google Scholar]
- 38.Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ, Schreiber SL. Inhibition of proteasome activities and subunit-specific amino terminal threonine modification by lactacystin. Science 1995;268:726–31 [DOI] [PubMed] [Google Scholar]
- 39.Lansang MC, Williams GH, Carroll JS. Correlation between the glucose clamp technique and the homeostasis model assessment in hypertension. Am J Hypertens 2001;14:51–3 [DOI] [PubMed] [Google Scholar]
- 40.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000;85:2402–10 [DOI] [PubMed] [Google Scholar]
- 41.Rasmussen BB, Fujita S, Wolfe RR, et al. Insulin resistance of muscle protein metabolism in aging. FASEB J 2006;20:768–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkes EA, Selby AL, Atherton PJ, et al. Blunting of insulin inhibition of proteolysis in legs of older subjects may contribute to age-related sarcopenia. Am J Clin Nutr 2009;90:1343–50 [DOI] [PubMed] [Google Scholar]
- 43.Koopman R, van Loon LJ. Aging, exercise, and muscle protein metabolism. J Appl Physiol 2009;106:2040–8 [DOI] [PubMed] [Google Scholar]
- 44.Yarasheski KE, Pak-Loduca J, Hasten DL, Obert KA, Brown MB, Sinacore DR. Resistance exercise training increases mixed muscle protein synthesis rate in frail women and men ≥ 76 yr old. Am J Physiol 1999;277:E118–25 [DOI] [PubMed] [Google Scholar]