Abstract
Activation of group II metabotropic glutamate receptors (mGlu2 and -3 receptors) has shown a potential antipsychotic activity, yet the underlying mechanism is only partially known. Altered epigenetic mechanisms contribute to the pathogenesis of schizophrenia and currently used medications exert chromatin remodeling effects. Here, we show that systemic injection of the brain-permeant mGlu2/3 receptor agonist (−)-2-oxa-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY379268; 0.3–1 mg/kg i.p.) increased the mRNA and protein levels of growth arrest and DNA damage 45-β (Gadd45-β), a molecular player of DNA demethylation, in the mouse frontal cortex and hippocampus. Induction of Gadd45-β by LY379268 was abrogated by the mGlu2/3 receptor antagonist (2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid (LY341495; 1 mg/kg i.p.). Treatment with LY379268 also increased the amount of Gadd45-β bound to specific promoter regions of reelin, brain-derived neurotrophic factor (BDNF), and glutamate decarboxylase-67 (GAD67). We directly assessed gene promoter methylation in control mice and in mice pretreated for 7 days with the methylating agent methionine (750 mg/kg i.p.). Both single and repeated injections with LY379268 reduce cytosine methylation in the promoters of the three genes, although the effect on the GAD67 was significant only in response to repeated injections. Single and repeated treatment with LY379268 could also reverse the defect in social interaction seen in mice pretreated with methionine. The action of LY379268 on Gadd45-β was mimicked by valproate and clozapine but not haloperidol. These findings show that pharmacological activation of mGlu2/3 receptors has a strong impact on the epigenetic regulation of genes that have been linked to the pathophysiology of schizophrenia.
Introduction
Metabotropic glutamate (mGlu) receptors form a family of eight receptor subtypes that are subdivided into three groups. Group II receptors (subtypes mGlu2 and mGlu3) are preferentially located in the preterminal region of nerve endings, where their activation decreases cAMP formation and negatively modulates neurotransmitter release (for review, see Niswender and Conn, 2010). The development of mGlu2/3 receptor agonists as antipsychotic agents moved from the observation that (1S,2S,5R,6S)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (LY354740), an orthosteric mGlu2/3 receptor agonist endowed with high receptor affinity and brain penetration, inhibits presynaptic glutamate release (Battaglia et al., 1997), allowing for testing the hyperglutamatergic hypothesis of schizophrenia (SZ) (Aghajanian and Marek, 2000). Moghaddam and Adams (1998) found that LY354740 attenuates the disruptive effect of phencyclidine on cognition, motor behavior, and cortical glutamate efflux, suggesting that this drug ameliorates the behavioral effects of phencyclidine by attenuating presynaptic glutamatergic activity. “Dual” mGlu2/3 receptor agonists and selective mGlu2 receptor enhancers have shown robust activity in preclinical models used to predict the efficacy of potential antipsychotic agents (Gewirtz and Marek, 2000; Kłodzinska et al., 2002; Schoepp and Marek, 2002; Conn et al., 2008, 2009). In a phase II clinical trial, the novel mGlu2/3 receptor agonist (−)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY404039) showed good efficacy in improving both positive and negative symptoms in patients with SZ (Patil et al., 2007). LY404039 had a good safety and tolerability profile and caused neither extrapyramidal side effects nor increased body weight and blood triglyceride levels (Patil et al., 2007). The mGlu3 receptor seems to have a role in the overall antipsychotic action of mGlu2/3 receptor agonists, because mutations in the GRM3 gene (encoding human mGlu3 receptors) and an abnormal dimerization of mGlu3 receptors in the prefrontal cortex of patients with SZ are associated with a risk for SZ and lack of response to conventional antipsychotics (Egan et al., 2004; Bishop et al., 2005). Thus, drugs that activate mGlu2 and mGlu3 receptors are promising candidates as novel antipsychotic agents potentially of use in patients who are drug-resistant or who do not tolerate the adverse effects induced by conventional antipsychotic agents. Several lines of evidence suggest that altered epigenetic mechanisms contribute to the pathogenesis of GABAergic/glutamatergic network dysfunction in SZ patients (Benes et al., 2007; Huang et al., 2007; Sharma et al., 2008; Grayson et al., 2009; Guidotti et al., 2009). We and others have found that drugs used in the treatment of psychosis, such as atypical antipsychotics (e.g., clozapine, quetiapine, olanzapine) and valproate (VPA), induce chromatin structure remodeling by promoting mechanisms of histone covalent modifications and DNA demethylation in GABAergic or glutamatergic neurons (Dong et al., 2008; Guidotti et al., 2009). In neurons, demethylation of DNA is mediated at least in part by a base-excision repair mechanism that first requires the conversion of 5-methylcytosine into thymine (T) through deamination, followed by T removal by means of a guanine (G)/T mismatch DNA-glycosylase (Ooi and Bestor, 2008; Ma et al., 2009; Zhu, 2009). Evidence suggests that in the brain the correct coupling between 5-methylcytosine deaminase and G/T mismatch DNA glycosylase is facilitated by Gadd45-β (growth arrest and DNA-damage-inducible protein 45 types-β) (Ma et al., 2009). Gadd45-β is a member of the Gadd45 family of small nuclear acidic proteins (∼17 kDa) (Gadd45-α and -γ) that is rapidly and transiently induced in mature hippocampal neurons in response to neuronal circuit activation and ionotropic glutamate receptor stimulation (Barreto et al., 2007; Ma et al., 2009). For example, hippocampal neuronal circuit activation elicited by electroconvulsive treatment in mice induces the expression of Gadd45-β and increases DNA demethylation at promoters of genes encoding the brain-derived neurotrophic factor (BDNF) and type-1 fibroblast growth factor (Ma et al., 2009). No increase in DNA demethylation is seen in mice with a genetic deletion of Gadd45-β (Ma et al., 2009). Because treatment with conventional antipsychotic drugs is known to reverse the hypermethylation of genes involved in the regulation of GABAergic transmission by promoting DNA demethylation (Guidotti et al., 2009), we examined whether this mechanism is shared by pharmacological activation of mGlu2/3 receptors.
Materials and Methods
Materials.
(−)-2-Oxa-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY379268) and (2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid (LY341495) were purchased from Tocris (Ellisville, MO). VPA, methionine, and haloperidol were purchased from Sigma-Aldrich (St. Louis, MO). Clozapine (CLZ) was purchased from Sandoz Pharmaceutical (San Diego, CA).
Treatment.
Adult male Swiss Albino mice (Harlan, Indianapolis, IN) of 18 to 22 g of body weight were housed with a 12-h light/dark cycle, and food and water were given ad libitum. Different groups of animals were treated with VEH, Met, VPA, CLZ, LY379268, and LY341495 as follows: for Gadd45 expression, mice were treated with a single injection of LY379268 (0.5 mg/kg i.p.) or LY341495 (1 mg/kg i.p.) alone or in combination. Animals were killed 2 h after drug injection. Various doses of LY379268 (ranging from 0.125 to 2 mg/kg) were used to construct a dose-response curve (Fig. 1b). For the cytosine methylation studies, mice were pretreated for 7 days with Met (750 mg/kg i.p. twice a day). For Gadd45-β ChIP experiments, mice were treated with either 1) LY379268 (0.5 mg/kg i.p.) alone or in combination with LY341495 (1 mg/kg i.p.) or 2) VPA (70 mg/kg i.p.) alone or in combination with LY379268. In another group of experiments, VPA (70 mg/kg i.p.), CLZ (5 mg/kg s.c.), and haloperidol (1.5 mg/kg i.p.) were administered as a single or repeated treatment to evaluate the Gadd45-β mRNA levels or gene promoter methylation. VPA, Met, LY379268, and LY341495 were dissolved in saline; CLZ was dissolved in sesame oil. Haloperidol was dissolved with a drop of glacial acetic acid brought to pH 6 with the addition of sodium hydroxide.
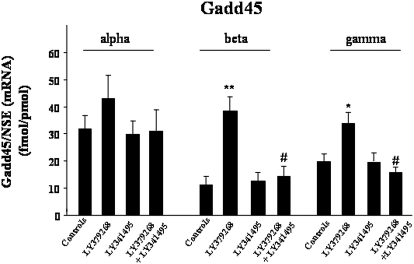
Fig. 1.
The increase of Gadd45-β and Gadd45-γ mRNA levels induced by a single injection of LY379268 (0.5 mg/kg i.p., 2 h before animal killing) in mouse FC was blocked by the coadministration of LY341495 (1 mg/kg i.p.). Values represent the mean ± S.E. of five mice. *, p < 0.05; **, p < 0.01 vs. control group. #, p < 0.01 versus the group treated with LY379268 (one-way ANOVA + Newman-Keuls).
Measurement of Gadd45α, -β, and -γ mRNA by Quantitative-Competitive PCR.
Total RNA was extracted from the mouse frontal cortex, hippocampus, and cerebellum with TRIzol reagent (Invitrogen, Carlsbad, CA). Gadd45 mRNA content was measured by reverse transcription-PCR with colinear internal standards generated by deletion (Auta et al., 2007). Amplification of the three isoforms of Gadd45 cDNA was carried out employing the primers indicated in Table 1. Concentrations of mRNA were calculated from a known amount of internal standards and corrected for NSE using the primers indicated in Table 1.
TABLE 1.
Primers
| Primer | Sequence |
|---|---|
| Amplification | |
| Gadd45α | |
| Forward | 5′-GCGAGAACGACATCAACATCCTGC-3′ |
| Reverse | 5′-TCGGCCCCTTGACATCAGTTTCTG-3′ |
| Gadd45β | |
| Forward | 5′-AGTCGTTCTGCTGCGACAATGACA-3′ |
| Reverse | 5′-GTATCACGGGTAGGGTAGCCTTTG-3′ |
| Gadd45γ | |
| Forward | 5′-TCTGGAAGAAGTCCGTGGCCAGGA-3′ |
| Reverse | 5′-ATGCAATGCAGGTCTCCCGGCGCG-3′ |
| NSE | |
| Forward | 5′-AATCCAAGTTTGGGGCCAATGCCA-3′ |
| Reverse | 5′-TCTTTTCCGTGTAGCCAGCCTTGT-3′ |
| reelin | |
| Forward | 5′-GGGCGGCGGGCCCCGAGG-3′ |
| Reverse | 5′-AGAGACCGACGGGCTGCC-3′ |
| GAD67 | |
| Forward | 5′-GAGGAGAGCGGGCCAAGA-3′ |
| Reverse | 5′-GTGCCGCTCCACACGCC-3′ |
| BDNF-IX | |
| Forward | 5′-CATGAGACCGGGCAAGTC-3′ |
| Reverse | 5′-CCTTGGGAGGAATGTGTGAT-3′ |
| GAD65 | |
| Forward | 5′-AATGAGTTCGTTGGTGTGGAAGCG-3′ |
| Reverse | 5′-TTGGCTCATTGTTTGAGGGCTGTC-3′ |
Western Blot Analysis.
Tissue were homogenized at 4°C in a radioimmunoprecipitation assay lysis buffer containing 1 mM protease inhibitor cocktail (Sigma-Aldrich), pH 7.4. Twenty micrograms of proteins were resuspended in SDS-bromphenol blue reducing buffer. Western blot analyses were carried out using 18% Tris-glycine gel (Invitrogen). After blotting onto a nitrocellulose filter (0.2-μm pore size; Invitrogen), the blots were incubated overnight at 4°C with primary polyclonal antibody directed against Gadd45-β (1 μg/ml; Aviva Systems Biology, San Diego, CA) in Tris-buffered saline/Tween 20 buffer (100 mM Tris-HCl, 0.9% NaCl, and 0.1% Tween 20, pH 7.4). After three washes with Tris-buffered saline/Tween 20 buffer, blots were incubated for 1 h with the secondary antibody (peroxidase-coupled anti-rabbit) (Sigma-Aldrich). The same blots were incubated for 1 h at room temperature with mouse monoclonal anti-β-actin (Sigma-Aldrich). The analysis was performed by using a Storm 860 PhosphorImager (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) with ImageQuant analysis software, and the values were expressed as an optical density ratio with respect to β-actin. After blotting, only a major band of ∼17 kDa was recognized by the Gadd45-β antibody. To establish the specificity of the antibody, immunoblots were carried out using brain homogenates obtained from Gadd45-β KO mice (generous gift from Dr. Dan Liebermann's lab, Fels Institute for Cancer Research and Molecular Biology, Temple University, Philadelphia, PA).
Himmunohistochemistry: Gadd45-β Staining.
Tissues were fixed with 4% paraformaldehyde in PBS. Floating sections (30 μm) were immunostained for Gadd45-β as follows: tissue slides were rinsed with PBS, then blocked for 30 min with 3% normal goat serum in PBS. Sections were incubated overnight at 4°C with anti-Gadd45-β antibody (Aviva Systems Biology) diluted (1:1000) in PBS containing 1% normal goat serum. Afterward, sections were washed in PBS and incubated with a biotinylated-secondary antibody for an hour at room temperature. Slices were then reacted with diaminobenzidine ammonium sulfate according to the procedures described by Rodriguez et al. (2002).
Methylated reelin, GAD67, and BDNF-IX Promoter Assay (5-Methylcytosine DNA Immunoprecipitation).
We analyzed the ratio of 5′-methylated cytosines to the unmethylated cytosines of murine reelin, GAD67, or BDNF (promoter region IX) CpG-enriched promoter sequences.
Genomic DNA was extracted from the mouse frontal cortex (FC) and sonicated to produce a fragment size of 200 to 600 bp. After ethanol precipitation, 3 μg of sonicated DNA were diluted to 300 μl in Tris-EDTA buffer and heat-denatured at 95°C for 10 min. Thirty microliters of sonicated solution were removed and stored at −20°C to be used as input to quantify the total amount of promoter before immunoprecipitation. The remaining solution was incubated overnight at 4°C with a mouse anti-5-methylcytosine monoclonal antibody (MagMeDIP kit; Diagenode, Denville, NJ). The immunoprecipitated DNA was released from the antibody complex by proteinase-K digestion. After phenol-chloroform extraction and ethanol precipitation, the DNA pellet was resuspended in 20 μl of diethyl pyrocarbonate-treated water. A CpG-rich GAD67 promoter fragment (from −840 to −768 bp), reelin fragment (from −423 to −252 bp), and BDNF-IX promoter fragment (Ma et al., 2009) were measured by quantitative PCR using the primers indicated in Table 1. The percentage of methylated versus unmethylated promoter was calculated by using the following equation: % (meDNA-IP/total input) = 2[(Ct(10% input) − 3.32) − Ct(meDNA − IP)] × 100% (MagMeDIP kit instruction manual; Diagenode).
An immunoprecipitation negative control (no antibody added) was included in each assay and produced no detectable signal. For further details, see Satta et al. (2008).
ChIP Assay: Measurements of Gadd45-β Binding to the reelin, GAD67, GAD65, and BDNF-IX Gene Promoters.
Approximately 10 mg of FC tissue was used for this procedure. Tissue was incubated with 500 μl of PBS containing 1% formaldehyde at 37°C for 10 min, supplemented with a protease inhibitor cocktail (Sigma-Aldrich). After being washed three times with ice-cold PBS, tissue was homogenized in 300 μl of SDS lysis buffer (supplied by ChIP kit; Millipore, Billerica, MA). To obtain consistent chromatin fragmentation, the lysates were sonicated for 15 min on ice (Sonic Dismembrator, model 500; Thermo Fisher Scientific, Waltham, MA). The ChIP procedure was carried out by using the ChIP assay kit and protocol (Millipore). The concentration of the antibody for Gadd45-β used was 1 μg/ml. An aliquot (2%) of the sonicated lysate without antibody (Input) was used to quantify the total amount of DNA in the sample extracts before immunoprecipitation. At the end of ChIP procedure, the protein/DNA cross-linked nucleosomal chromatin complex immunoprecipitated by the specific antibody was reverse cross-linked with NaCl at a final concentration of 100 mM at 65°C overnight. Samples were then treated with proteinase-K. Protein-free DNA was extracted in phenol/chloroform and precipitated and washed in ethanol. The extract was used for detection and quantification of reelin, GAD67, GAD65, and BDNF-IX gene promoters as described in the previous paragraph.
Behavioral Test: Social Interaction in a Novel Environment.
All animals were housed in the experimental room for an hour before the test session for habituation. We used the experimental paradigm described by Tremolizzo et al. (2005). In brief, each male mouse was placed in a novel cage together with a nonaggressive male mouse used as an intruder, and the interaction between the two mice was recorded for 10 min with a digital video camera (Samsung, Seoul, Korea). The total duration of the interaction (seconds per 10 min) was scored by two blinded well trained operators. The interaction was defined by body contact, including inspection and anogenital sniffing. Reliability of measurements was assessed by correlating the scores of the two operators. In a first group of experiments, mice were injected with Met (750 mg/kg i.p., twice a day) for 7 days. A single injection of LY379268 (0.5 mg/kg i.p.) was administered immediately, 16 and 22 h after the last dose of Met. All the mice were tested 24 h after methionine withdrawal. In another set of experiment, mice were pretreated with Met for 7 days and with repeated injections of LY379268 (0.5 mg/kg i.p., twice a day, for 3 days) in combination with Met in the last 3 days of Met pretreatment. The control group received saline for 7 days.
Statistical Analysis.
All results were expressed as means ± S.E. One-way ANOVA followed by the Newman-Keuls multiple comparison test were used to assess the significance of the differences between groups. The criterion for significance was p < 0.05 or 0.01.
Results
mGlu2/3 Receptors Activation Increased the Expression of Gadd45 in the Mouse FC.
We first examined the expression of Gadd45α, -β, and -γ in the mouse FC in response to the stimulation of mGlu2/3 receptors by systemic administration of LY379268. We selected LY379268 because this compound shares the pharmacological properties of LY404039 (Schoepp et al., 1999), which is under clinical development for the treatment of SZ (Patil et al., 2007).
A single injection of LY379268 (0.5 mg/kg i.p.) induced a 3- to 4-fold increase in Gadd45-β mRNA levels (Fig. 1). The increase peaked at 2 h and progressively declined after 6 h (not shown). In the same samples, we detected a slight increase in Gadd45-γ mRNA but no significant changes in Gadd45-α mRNA levels (Fig. 1). The increase in Gadd45-β and -γ mRNA levels induced by LY379268 was abolished by coadministration of the preferential mGlu2/3 receptor antagonist LY341495 (1 mg/kg i.p.), which was inactive on its own (Fig. 1).
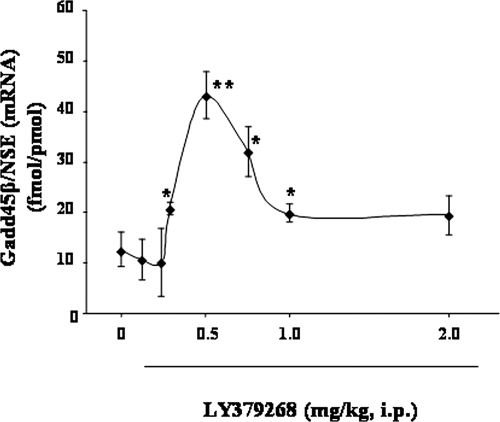
The effect of LY379268 on Gadd45-β mRNA levels in the mouse FC showed an inverse-U shaped dose-response curve, a maximal increase being observed at relatively low doses (0.3–0.75 mg/kg) and a smaller increase at higher doses (1 or 2 mg/kg) (Fig. 2). Because the therapeutic response to antipsychotic drugs required protracted treatment, we studied whether the action of LY379268 persisted after repeated injections. We found that the increase in Gadd45-β mRNA levels in the FC remained unchanged after repeated injections of LY379268 (0.5 mg/kg i.p. once daily for 7 days) (13 ± 2.1 and 2 ± 6.0 fmol of Gadd45-β/pmol of NSE mRNA in control animals and 2 h after the last injection of LY379268, respectively, n = 5). This increase was reduced by coadministration of 1 mg/kg the mGlu2/3 receptor antagonist LY341495 (22 ± 3.5 fmol Gadd45-β/pmol NSE mRNA, n = 5). A 3- to 4-fold increase in Gadd45-β mRNA expression was also observed in the hippocampus and cerebellum of mice injected with LY379268 (Fig. 3).
Fig. 2.
Inverted U-shape dose-response curve of LY379268 (0.125–2 mg/kg) treatment on Gadd45-β mRNA expression. Values are the mean ± S.E. of five determinations. *, p < 0.05; **, p < 0.01 versus control group (0) (one-way ANOVA + Newman-Keuls).
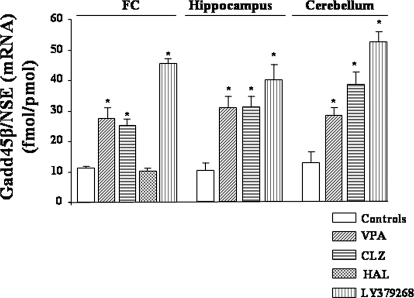
Fig. 3.
Increased expression of Gadd45-β mRNA in mouse FC, hippocampus, and cerebellum after single injection of VPA (70 mg/kg i.p.), CLZ (5 mg/kg, s.c.), and LY379268 (0.5 mg/kg i.p.) but not HAL (1.5 mg/kg i.p.), *, p < 0.05 versus control (one-way ANOVA + Newman-Keuls).
We also studied the expression of Gadd45-β protein by immunoblotting. Densitometric analysis showed that a single injection of LY379268 (0.5 mg/kg i.p.) induced an approximate 2-fold increase in Gadd45-β protein levels in the FC compared with control mice injected with saline. Similar increase in Gadd45-β levels was also observed with LY379268 1 mg/kg. There were changes with LY379268 0.25 mg/kg injection (data not shown).
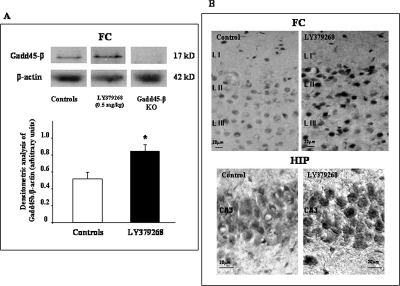
Gadd45-β has a molecular weight and structural homology to Gadd45-α and Gadd45-γ; to demonstrate the specificity of the antibody, we compared FC extracts obtained from wild-type mice with FC extracts obtained fron Gadd45-β KO mice. As shown in Fig. 4A, no immunoreactive bands were observed in brain extracts obtained from Gadd45-β KO mice. In addition, as shown in Fig. 4B, 2 h after an injection of 0.5 mg/kg i.p. LY379268, the intensity and number of Gadd45-β-positive neurons was increased compared with VEH-treated mice in cerebral motor cortex and hippocampus. In the cerebral cortex, the increase in intensity and number of Gadd45-β positive neurons was especially evident in nuclei of layer II and III pyramidal neurons as suggested by colocalization of Gadd45-β with glutamate vesicular transport (D. P. Gavin, R. Sharma, K. Chase, F. Matrisciano, E. Dong, and A. Guidotti, submitted). In the hippocampus, the increase is particularly evident in pyramidal neurons of cornu ammonis. As described above, the results are not contaminated by cross reactivity with Gadd45-α and -γ because the antibodies used for the immunohistochemical analysis failed to recognize any other protein in brains of Gadd45-β KO mice (see Fig. 4A).
Fig. 4.
A, a representative immunoblot of Gadd45-β protein extracted from the mouse FC treated 2 h earlier with an injection of LY379268 (0.5 mg/kg i.p.). No bands are observed in FC of Gadd45-β KO mice. The values for densitometric analysis are means ± S.E. of five individual determinations. *, p < 0.05 (one-way ANOVA + Newman-Keuls versus control mice). B, immunohistochemistry analysis of Gadd45-β: LY379268 (0.5 mg/kg i.p. 2 h before) elicits an increase of neuronal Gadd45-β immunoreactivity in the FC (layer II and III; 20× magnification) and hippocampus (CA3) (right), compared with controls (left) (40× magnification).
Gadd45-β Binding to reelin, GAD67, and BDNF CpG-rich Promoter Regions.
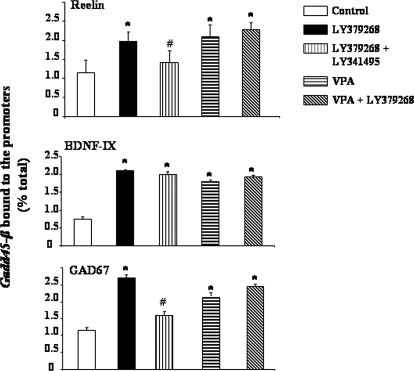
To examine whether Gadd45-β induced by the activation of mGlu2/3 receptors participates in chromatin structure remodeling, we focused on the promoters of three genes that are targets for epigenetic regulation and are apparently down-regulated in SZ: reelin, GAD67, and BDNF (Benes et al., 1992; Fatemi et al., 2000; Guidotti et al., 2000; Lewis et al., 2005; Guo et al., 2010). We measured CpG-rich promoter regions of reelin, GAD67, and BDNF-IX genes immunoprecipitated with a specific Gadd45-β antibody (see Materials and Methods). Animals received a repeated treatment with LY379268 (0.5 mg/kg i.p., 3 days, twice a day) alone or in combination with LY341495 (1 mg/kg i.p.). Quantitative PCR analysis showed that LY379268 induced an increase in reelin, GAD67, and BDNF-IX gene promoter fractions precipitated by the Gadd45-β antibody (Fig. 5). However, not all the genes were immunoprecipitated by the Gadd45-β antibody. For example, only a small percentage (< 0.1%) of the total GAD65 promoter was immunoprecipitated by the Gadd45-β antibody in vehicle- or LY379268-treated mice. The effect of LY379268 on reelin and GAD67 (but not BDNF-IX) promoters was significantly reduced in a group of animals treated with the mGlu2/3 receptor antagonist LY341495 (Fig. 5).
Fig. 5.
LY379268 and valproate (VPA) increase Gadd45-β binding to reelin, BDNF-IX, and GAD67 promoters. The effect of LY379268 (0.5 mg/kg i.p., 2 h before) is reduced by the coadministration of LY341495 (1 mg/kg i.p.) except for the binding of Gadd45-β to BDNF-IX promoter. VPA (70 mg/kg s.c., 2 h before) also increases the binding of Gadd45-β to reelin, GAD67, and BDNF-IX promoters. However, the increased binding of Gadd45-β to reelin, GAD67, and BDNF-IX promoters elicited by LY379268 is not potentiated by coadministration of VPA, suggesting overlapping mechanisms of both drugs in inducing Gadd45-β protein binding to promoters. Values represent the mean ± S.E. of five mice. *, p < 0.05 versus control group; #, p < 0.05 versus LY379268 group (one-way ANOVA + Newman-Keuls).
mGlu2/3 Receptor Activation Induced Demethylation in reelin-, GAD67-, and BDNF-IX CpG-rich Promoter Regions.
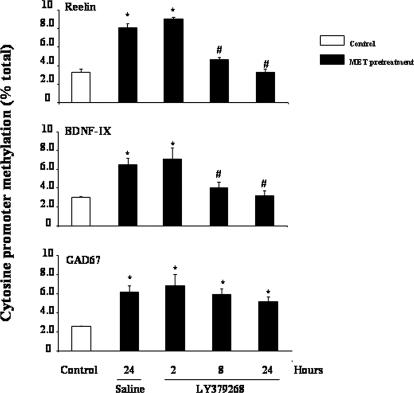
To examine whether the mGlu2/3 receptor agonist-induced increase of Gadd45-β binding to target promoters was associated with DNA demethylation, we performed MeDIP analysis of reelin, GAD67, and BDNF-IX promoters. Mice were treated for 7 days with VEH or Met (750 mg/kg i.p., twice a day). Met treatment alone induced hypermethylation of reelin, GAD67, and BDNF-IX promoters (Fig. 6). However, not all genes were hypermethylated by Met. For example, the methylation status of GAD65 and β-globulin promoters was not affected by Met pretreatment, and global ChIP-on-chip promoter analyses revealed that approximately 5% of the gene promoters are heavily hypermethylated (Dong et al., 2008). Measurements of promoter methylation were carried out 24 h after the end of Met treatment in mice receiving either saline or LY379268 (0.5 mg/kg i.p.) 2, 8, or 24 h before promoter methylation measurements. LY379268 induced a time-dependent decrease in both reelin and BDNF-IX promoter methylation with a significant effect detected when LY379268 was administered 8 and 24 h before the assay (Fig. 6). However, a single injection of LY379268 did not reduce the hypermethylation of the GAD67 gene promoter (Fig. 6).
Fig. 6.
A single injection of LY379268 facilitates reelin and BDNF-IX but not GAD67 promoter demethylation. Mice were pretreated with methionine (Met) (750 mg/kg s.c., twice a day for 7 consecutive days) to induce hypermethylation of the promoters. At day 7, Met treatment was discontinued and mice were injected at different times (hours) before killing with saline or LY379268. Control mice received saline i.p. for 7 days (open bar). Promoter methylation was measured 24 h after the last saline or Met injection. Values are the ratios between the amount of the methylated promoter fragment immunoprecipitated by 5-methylcytosine antibodies and the promoter fragment present in the initial total extract (input) (percentage of total). Values represent the mean ± S.E. of five mice. *, p < 0.01 versus control group. #, p < 0.05 versus saline- or LY379268-treated mice (one-way ANOVA + Newman-Keuls).
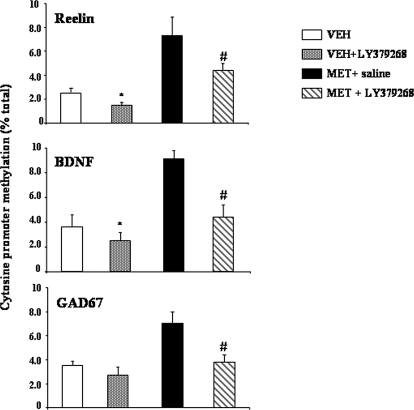
In another series of experiments, we examined the methylation of reelin, GAD67, and BDNF-IX promoters after repeated treatments with LY379268 (0.5 mg/kg i.p., twice daily, 3 days). We used this treatment schedule because in previous studies, Dong et al. (2008) demonstrated that VPA and clozapine induced a marked DNA demethylation when the drugs are injected for 3 days. Under these conditions, LY379268 markedly decreased the extent of the Met-induced hypermethylation of reelin, GAD67, and BDNF-IX promoters (Fig. 7). Reduced methylation of reelin and BDNF gene promoters was also seen under basal conditions; (i.e., in mice receiving repeated injections of LY379268 without Met pretreatment) (Fig. 7).
Fig. 7.
Repeated injections of LY379268 (0.5 mg/kg i.p., twice a day, 3 consecutive days) decrease the number of methylated cytosines in reelin, GAD67, and BDNF-IX CpG-rich promoter regions. The study compared saline-injected animals (open bar, control) with mice pretreated with Met (750 mg/kg s.c., twice a day, 7 consecutive days) (closed bars). Reelin, GAD67, and BDNF-IX promoter methylation was measured 24 h after Met treatment termination and the last injection of LY379268. Values are the ratios between the amount of the gene promoter fragment immunoprecipitated by 5-methylcytosine antibodies and the promoter fragment present in the initial total extract (input) (percentage of total). Values represent the mean ± S.E. of five mice. *, p < 0.05, versus control group; #, p < 0.01 versus Met-treated mice receiving saline instead of LY379268 (one-way ANOVA + Newman-Keuls).
Conventional Drugs Used in the Treatment of Psychosis Also Increase Gadd45-β Levels and Induce Promoter Demethylation.
To support the idea that the epigenetic changes we observed with LY379268 are common to other drugs used clinically for the treatment of psychosis, we also measured Gadd45-β mRNA levels, Gadd45-β binding to reelin-, GAD67-, and BDNF-IX promoters, and reelin promoter methylation in mice treated with CLZ, haloperidol (HAL), or VPA. VPA (a histone deacetylase inhibitor that also induces DNA demethylation) is a mood stabilizer used in combination with antipsychotic drugs to treat patients with bipolar disorders. CLZ, a drug that induces histone hyperacetylation and activates DNA demethylation, is a prototype of atypical antipsychotic drugs (Guidotti et al., 2000), whereas HAL belongs to a class of typical antipsychotics and fails to modify histones or induce DNA demethylation (Dong et al., 2008; Guidotti et al., 2009). In these experiments, Gadd45-β mRNA brain levels were measured 2 h after a single injection of VPA (70 mg/kg i.p.), CLZ (5 mg/kg s.c.), and HAL (1.5 mg/kg i.p.) in mice. We observed a 2- to 3-fold increase of Gadd45-β mRNA levels in the FC, hippocampus, and cerebellum of mice treated with VPA and CLZ, whereas no changes were observed with HAL in the FC (Fig. 3). We also examined the effect of a single injection of VPA on Gadd45-β mRNA levels in combination with LY379268 (0.5 mg/kg i.p.). When the two drugs were combined, no additive effects were observed (15.6 ± 2.1, 28.0 ± 2.1, 46.28 ± 3.2, and 49.0 ± 2.1 fmol of Gadd45-β/pmol of NSE mRNA in control animals, VPA, LY379268, and VPA+LY379268, respectively; n = 5) whereas LY341495 (1 mg/kg i.p.) failed to decrease the Gadd45-β mRNA levels when combined with VPA (28 ± 2.1 and 25.6 ± 3.0 fmol Gadd45-β/pmol NSE mRNA for VPA and VPA+LY341495, respectively; n = 5) suggesting a different mechanism of action between VPA and LY379268 in increasing Gadd45-β mRNA levels.
Similar to LY379268, VPA Increased the Binding of Gadd45-β to the Target Gene Promoters.
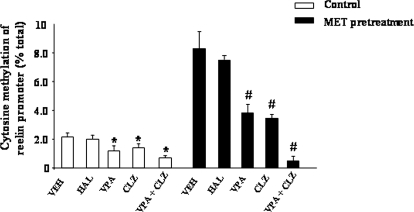
Furthermore, using the MeDIP assay, we confirmed our previous observation that VPA and CLZ decrease the number of methylated cytosines at the reelin promoter, whereas HAL was inactive (Fig. 8). The decrease in methylation at reelin promoter was synergistically potentiated when VPA and CLZ were used in combination. VPA and CLZ decreased the number of methylated cytosines in the FC of mice pretreated with Met and in naive mice (Fig. 8).
Fig. 8.
VPA and CLZ, but not HAL, induce cytosine demethylation of reelin promoter. Mice were pretreated with Met (750 mg/kg s.c., twice a day, 7 days) to induce hypermethylation of the promoters. Measurements were conducted 24 h after Met withdrawal. VPA (70 mg/kg s.c., twice a day, 3 days); CLZ (5 mg/kg s.c., twice a day, 3 days); HAL (1.5 mg/kg i.p., twice a day, 3 days). Promoter methylation was measured as the ratios between the amount of the methylated promoter fragment immunoprecipitated by 5-mC antibodies and the promoter fragment present in the initial total extract (input) (percentage of total). Values represent the mean ± S.E. of five mice. *, #, p < 0.05 versus vehicle (control) group (one-way ANOVA + Newman-Keuls).
mGlu2/3 Receptor Activation Reversed the Methionine-Induced Decrease in Social Interaction Time.
As expected from our previous study (Tremolizzo et al., 2005), mice receiving 7 days of Met treatment displayed a significant reduction of social interaction time when presented with an intruder mouse.
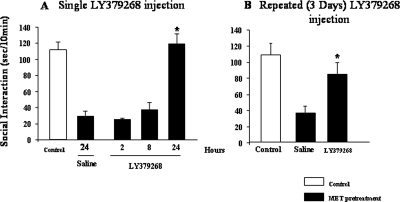
A single injection of LY379268 (0.5 mg/kg i.p.), reversed the Met-induced social interaction deficit with a maximum effect after 24 h (Fig. 9A). As shown in Fig. 9B, also a repeated treatment (3 days, twice a day) with LY379268 (0.5 mg/kg,i.p.) was able to reverse the Met-induced social interaction deficit.
Fig. 9.
LY379268 prevents the methionine-induced decrease in social interaction time. A, mice received Met (750 mg/kg i.p.) for 7 days twice daily. The group of controls was treated with saline. After Met withdrawal, animals were treated with a single injection of LY379268 (0.5 mg/kg i.p.) or saline (0.1 ml/10 g) 2, 8, and 24 h before testing. All mice were tested 24 h after Met withdrawal. B, mice received Met for 7 days. LY379268 (0.5 mg/kg i.p.) or vehicle were administered in combination with Met during the last 3 days, twice a day. Repeated exposure to LY379268 elicits an increase of social interaction time compared with controls (Met-pretreated + VEH group). Values are the mean ± S.E. of eight mice. *, p < 0.05 versus control group (one-way ANOVA + Newman-Keuls).
Discussion
Agonists of mGlu2/3 receptors and enhancers of mGlu2 receptor are among the first medications without a direct action on monoaminergic transmission developed for the treatment of SZ (for review, see Conn et al., 2009; Krystal et al., 2010; Marek, 2010; Mezler et al., 2010; Niswender and Conn, 2010). Our study provides the first evidence that activation of mGlu2/3 receptors by LY379268 elicits a cascade of nuclear events characterized by an almost immediate and transient increase of Gadd45b associated with the binding of Gadd45b to reelin, GAD67, and BDNF-IX promoters and subsequent delayed (≥8 h) reduction of reelin, GAD67, and BDNF-IX promoter methylation.
The increase in Gadd45-β expression and the binding of Gadd45-β to the reelin and GAD67 promoters induced by LY379268 was mediated by the activation of mGlu2/3 receptors because it was blocked by the Glu2/3 receptor antagonist, LY341495. However, LY341495 unexpectedly failed to reduce binding of Gadd45-β to the BDNF promoter. This might reflect an off-target action of LY341495 at the BDNF promoter, as suggested by the evidence that LY341495 enhances 1-(2,5-dimethoxy-4-iodophenethyl)-2-aminopropane-induced transcription of cortical BDNF (Gewirtz et al., 2002).
It is noteworthy that the dose-response curve of LY379268 showed an inverted U-shape, which might reflect a different affinity and intrinsic activity of LY379268 at native mGlu2 and mGlu3 receptors, respectively. In addition, the effect of LY379268 was specific for Gadd45-β because the drug failed to increase Gadd45-α and only marginally increased Gadd45-γ mRNA.
Recent evidence suggests that in the nervous system, Gadd45-β acts as a neuronal activity-dependent immediate early gene that promotes epigenetic DNA demethylation in neurons by coupling 5-cytosine deaminase and G/T mismatch repair glycosylase, thereby facilitating DNA demethylation via activation of DNA excision repair mechanisms (Barreto et al., 2007; Ma et al., 2009). Mice subjected to electroconvulsive treatment showed a transient increase in Gadd45-β mRNA and protein levels followed by long-lasting activation of BDNF promoter demethylation in the hippocampus, which are not seen in Gadd45-β KO mice (Barreto et al., 2007; Ma et al., 2009). Taken together, these data suggest that the early increase of Gadd45-β may be related to the delayed demethylation of reelin, GAD67, and BDNF promoters.
The delay between the Gadd45-β increase that peaks at 2 h and promoter demethylation, which is evident at 8 h and reach a maximum at 24 h after LY379268 administration, is probably due to the fact that under physiological conditions, promoter demethylation is an inefficient process with a half-life of several hours and even days (Dong et al., 2008). In line with this observation, Di Liberto et al. (2009) have shown that systemic administration of LY379268 increases BDNF mRNA levels after 3 h and BDNF protein levels after 24 h in the mouse brain. Here, gene promoter demethylation induced by LY379268 in mice pretreated with Met was already evident at 8 h, thus preceding the behavioral effect on social interaction, which was seen at 24 h. Ma et al. (2009) reported that Gadd45-β is rapidly induced in hippocampal neurons in response to neuronal circuit activation and ionotropic glutamate receptor stimulation.
Because LY379268 attenuates presynaptic glutamate release in phencyclidine-treated rats (Moghaddam and Adams 1998, Cartmell et al., 1999; Imre et al., 2006), it is not intuitively apparent how LY379268 might induce Gadd45-β in all the mouse brain areas studied. It could thus be speculated that LY379268, attenuating presynaptic glutamate release at NMDA receptors located on GABAergic interneurons, could lead to an attenuation of the feedback GABAergic inhibitory tone on glutamatergic principal neurons (i.e., pyramidal neurons in the cortex and hippocampus). The consequent activation of corticolimbic neuronal circuits may explain the hyperfunction of the Gadd45-β gene in glutamatergic neurons as suggested by the immunohistochemical evidence that Gadd45-β is primarily increased in pyramidal neurons of the cortex and hippocampus (Fig. 4B). An alternative explanation is that LY379268, like VPA, acts intracellularly, activating a still unknown cascade of biochemical events, including histone tail covalent acetylation or methylation that leads to the induction of Gadd45-β, also in the absence of activity-dependent depolarization.
We have reported previously that VPA and the atypical antipsychotic CLZ but not the typical antipsychotic HAL induce GABAergic gene promoter demethylation (Detich et al., 2003; Dong et al., 2008; Guidotti et al., 2009). Here, we show that VPA and CLZ but not HAL elicit a robust increase of Gadd45-β associated with ensuing activation of DNA demethylation. The VPA-induced increase of Gadd45-β mRNA (Fig. 3) or protein (Guidotti et al., 2011) is not blocked by the mGlu2/3 receptor antagonist LY341495, suggesting a nonreceptorial mechanism of action. Presumably, VPA increases histone tail covalent acetylation in inducing a transcriptional activation of chromatin. This may involve a 5-cytosine deaminase such as the methyl-DNA binding protein 4, which we found to be increased after a single injection of LY379268 in our mice (F. Matrisciano, unpublished observations). Hence, drugs used to treat psychiatric disorders such as SZ or bipolar disorders may alter the expression of Gadd45-β, perhaps leading to a modification of gene transcription through promoter demethylation.
Clinical and preclinical studies suggest that mGlu2/3 receptors located in the prefrontal cortex and limbic regions may play a critical role in modulating GABA/glutamate neuronal circuit interactions that control cognitive and executive functions and are altered in SZ (Conn et al., 2008; Roth et al., 2009; Chaki, 2010; Gordon, 2010; Marek, 2010). Recent work indicates that epigenetic mechanisms, including covalent acetylation, methylation of histone tails, or DNA-promoter methylation, can be altered in SZ and bipolar disorders (Huang et al., 2007; Mill et al., 2008; Guidotti et al., 2009). Thus, the enzymatic machinery that regulates epigenetic mechanisms is an attractive molecular target for the study and development of a novel epigenetic treatment.
Further investigation is needed to 1) establish the neuronal location of the increase of Gadd45-β after LY379268 administration; 2) clarify whether the mGlu2 or the mGlu3 receptor subtype is primarily involved in the increase of Gadd45-β using positive allosteric modulator subtype-specific or knock-out mice for mGlu2 or mGlu3; 3) identify the molecular mechanism underlying the increase of Gadd45-β by the activation of mGlu2/3 receptors, and 4) identify the signaling pathways that link the increase of Gadd45-β to the activation of DNA demethylation, using Gadd45-β knock-out mice.
In summary, considering the possible contribution of maladaptive epigenetic mechanisms in the pathogenesis of SZ and bipolar disorders, our observations that LY379268 induces demethylation of reelin, GAD67, and BDNF promoters can explain the potential antipsychotic effects of mGlu2/3 receptor agonists and open new fields of investigation aimed at treating cognitive dysfunction in psychotic patients.
This work was supported in part by the National Institutes of Health National Institute of Mental Health [Grant MH070855].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.070896.
- mGlu
- metabotropic glutamate
- LY354740
- (1S,2S,5R,6S)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid
- SZ
- schizophrenia
- LY404039
- (−)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic acid
- VPA
- valproate
- Gadd45
- growth arrest and DNA-damage-inducible protein 45
- BDNF
- brain-derived neurotrophic factor
- LY379268
- (−)-2-Oxa-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylic acid
- LY341495
- (2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid
- VEH
- vehicle
- CLZ
- clozapine
- ChIP
- chromatin immunoprecipitation
- PCR
- polymerase chain reaction
- NSE
- neuron-specific enolase
- KO
- knockout
- PBS
- phosphate-buffered saline
- FC
- frontal cortex
- bp
- base pair(s)
- MeDIP
- 5-methyl-cytosine DNA immunoprecipitation
- ANOVA
- analysis of variance
- HAL
- haloperidol.
Authorship Contributions
Participated in research design: Matrisciano, Nicoletti, and Guidotti.
Conducted experiments: Matrisciano, Dong, and Gavin.
Wrote or contributed to the writing of the manuscript: Matrisciano, Nicoletti, and Guidotti.
Other: Guidotti coordinated research.
References
- Aghajanian GK, Marek GJ. (2000) Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Brain Res Rev 31:302–312 [DOI] [PubMed] [Google Scholar]
- Auta J, Chen Y, Ruzicka WB, Grayson DR. (2007) Nucleic acid quantitation using the competitive polymerase chain reaction: Practical neurochemistry methods. The Handbook of Neurochemistry and Molecular Neurobiology, eds Baker G, Dunn S, Holt A, Lajtha A. (Springer, New York: ), pp 341–361 [Google Scholar]
- Barreto G, Schäfer A, Marhold J, Stach D, Swaminathan SK, Handa V, Döderlein G, Maltry N, Wu W, Lyko F, et al. (2007) Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature 445:671–675 [DOI] [PubMed] [Google Scholar]
- Battaglia G, Monn JA, Schoepp DD. (1997) In vivo inhibition of veratridine-evoked release of striatal excitatory amino acids by the group II metabotropic glutamate receptor agonist LY354740 in rats. Neurosci Lett 229:161–164 [DOI] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. (2007) Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci USA 104:10164–10169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Alsterberg G, Bird ED, SanGiovanni JP. (1992) Increased GABAA receptor binding in superficial layers of cingulate cortex in schizophrenics. J. Neurosci 12:924–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop JR, Ellingrod VL, Moline J, Miller D. (2005) Association between the polymorphic GRM3 gene and negative symptom improvement during olanzapine treatment. Schizophr Res 77:253–260 [DOI] [PubMed] [Google Scholar]
- Cartmell J, Monn JA, Schoepp DD. (1999) The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus d-amphetamine motor behaviors in rats. J Pharmacol Exp Ther 291:161–170 [PubMed] [Google Scholar]
- Chaki S. (2010) Group II metabotropic glutamate receptor agonists as a potential drug for schizophrenia. Eur J Pharmacol 639:59–66 [DOI] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Jones CK. (2009) Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci 30:25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Tamminga C, Schoepp DD, Lindsley C. (2008) Schizophrenia: moving beyond monoamine antagonists. Mol Interv 8:99–107 [DOI] [PubMed] [Google Scholar]
- Detich N, Bovenzi V, Szyf M. (2003) Valproate induces replication-independent active DNA demethylation. J Biol Chem 278:27586–27592 [DOI] [PubMed] [Google Scholar]
- Di Liberto V, Bonomo A, Frinchi M, Belluardo N, Mudò G. (2009) Group II metabotropic glutamate receptor activation by agonist LY379268 treatment increases the expression of brain derived neurotrophic factor in the mouse brain. Neuroscience 165:863–873 [DOI] [PubMed] [Google Scholar]
- Dong E, Nelson M, Grayson DR, Costa E, Guidotti A. (2008) Clozapine and sulpiride but not haloperidol or olanzapine activate brain DNA demethylation. Proc Natl Acad Sci USA 105:13614–13619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Straub RE, Goldberg TE, Yakub I, Callicott JH, Hariri AR, Mattay VS, Bertolino A, Hyde TM, Shannon-Weickert C, et al. (2004) Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. Proc Natl Acad Sci USA 101:12604–12609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Earle JA, McMenomy T. (2000) Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol Psychiatry 5:654–663 [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Marek GJ. (2000) Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology 23:569–576 [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Chen AC, Terwilliger R, Duman RC, Marek GJ. (2002) Modulation of DOI-induced increases in cortical BDNF expression by group II mGlu receptors. Pharmacol Biochem Behav 73:317–326 [DOI] [PubMed] [Google Scholar]
- Gordon JA. (2010) Testing the glutamate hypothesis of schizophrenia. Nat Neurosci 13:2–4 [DOI] [PubMed] [Google Scholar]
- Grayson DR, Chen Y, Dong E, Kundakovic M, Guidotti A. (2009) From trans-methylation to cytosine methylation: evolution of the methylation hypothesis of schizophrenia. Epigenetics 4:144–149 [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Chen Y, Davis JM, Dong E, Gavin DP, Grayson DR, Matrisciano F, Pinna G, Satta R, et al. (2011) Epigenetic GABAergic targets in schizophrenia and bipolar disorder. Neuropharmacology 60:1007–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, et al. (2000) Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry 57:1061–1069 [DOI] [PubMed] [Google Scholar]
- Guidotti A, Dong E, Kundakovic M, Satta R, Grayson DR, Costa E. (2009) Characterization of the action of antipsychotic subtypes on valproate-induced chromatin remodeling. Trends Pharmacol Sci 30:55–60 [DOI] [PubMed] [Google Scholar]
- Guo C, Yang Y, Su Y, Si T. (2010) Postnatal BDNF expression profiles in prefrontal cortex and hippocampus of a rat schizophrenia model induced by MK-801 administration. J Biomed Biotechnol 2010:783297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Matevossian A, Whittle C, Kim SY, Schumacher A, Baker SP, Akbarian S. (2007) Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. J Neurosci 27:11254–11262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imre G, Salomons A, Jongsma M, Fokkema DS, Den Boer JA, Ter Horst GJ. (2006) Effects of the mGluR2/3 agonist LY379268 on ketamine-evoked behaviours and neurochemical changes in the dentate gyrus of the rat. Pharmacol Biochem Behav 84:392–399 [DOI] [PubMed] [Google Scholar]
- Kłodzinska A, Bijak M, Tokarski K, Pilc A. (2002) Group II mGlu receptor agonists inhibit behavioural and electrophysiological effects of DOI in mice. Pharmacol Biochem Behav 73:327–332 [DOI] [PubMed] [Google Scholar]
- Krystal JH, Mathew SJ, D'Souza DC, Garakani A, Gunduz-Bruce H, Charney DS. (2010) Potential psychiatric applications of metabotropic glutamate receptor agonists and antagonists. CNS Drugs 24:669–693 [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. (2005) Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 6:312–324 [DOI] [PubMed] [Google Scholar]
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H. (2009) Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science 323:1074–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek GJ. (2010) Metabotropic glutamate2/3 (mGlu2/3) receptors, schizophrenia and cognition. Eur J Pharmacol 639:81–90 [DOI] [PubMed] [Google Scholar]
- Mezler M, Geneste H, Gault L, Marek GJ. (2010) LY-2140023, a prodrug of the group II metabotropic glutamate receptor agonist LY-404039 for the potential treatment of schizophrenia. Curr Opin Investig Drugs 11:833–845 [PubMed] [Google Scholar]
- Mill J, Tang T, Kaminsky Z, Khare T, Yazdanpanah S, Bouchard L, Jia P, Assadzadeh A, Flanagan J, Schumacher A, et al. (2008) Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am J Hum Genet 82:696–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. (1998) Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 281:1349–1352 [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. (2010) Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol 50:295–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi SK, Bestor TH. (2008) The colorful history of active DNA demethylation. Cell 133:1145–1148 [DOI] [PubMed] [Google Scholar]
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, et al. (2007) Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med 13:1102–1107 [DOI] [PubMed] [Google Scholar]
- Rodriguez MA, Caruncho HJ, Costa E, Pesold C, Liu WS, Guidotti A. (2002) In Patas monkey, glutamic acid decarboxylase-67 and reelin mRNA coexpression varies in a manner dependent on layers and cortical areas. J Comp Neurol 451:279–288 [DOI] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Sodhi M, Kleinman JE. (2009) Epigenetic mechanisms in schizophrenia. Biochim Biophys Acta 1790:869–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta R, Maloku E, Zhubi A, Pibiri F, Hajos M, Costa E, Guidotti A. (2008) Nicotine decreases DNA methyltransferase 1 expression and glutamic acid decarboxylase 67 promoter methylation in GABAergic interneurons. Proc Natl Acad Sci USA 105:16356–16361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp DD, Jane DE, Monn JA. (1999) Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology 38:1431–1476 [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Marek GJ. (2002) Preclinical pharmacology of mGlu2/3 receptor agonists: novel agents for schizophrenia? Curr Drug Targets CNS Neurol Disord 1:215–225 [DOI] [PubMed] [Google Scholar]
- Sharma RP, Grayson DR, Gavin DP. (2008) Histone deacetylase 1 expression is increased in the prefrontal cortex of schizophrenia subjects: analysis of the National Brain Databank microarray collection. Schizophr Res 98:111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremolizzo L, Doueiri MS, Dong E, Grayson DR, Davis J, Pinna G, Tueting P, Rodriguez-Menendez V, Costa E, Guidotti A. (2005) Valproate corrects the schizophrenia-like epigenetic behavioral modifications induced by methionine in mice. Biol Psychiatry 57:500–509 [DOI] [PubMed] [Google Scholar]
- Zhu JK. (2009) Active DNA demethylation mediated by DNA glycosylases. Ann Rev Genet 43:143–166 [DOI] [PMC free article] [PubMed] [Google Scholar]