Abstract
Recent studies on cannabinoid-induced analgesia implicate certain transient receptor potential (TRP) channels as a therapeutic target along with metabotropic cannabinoid receptors. Although TRP ankyrin 1 (TRPA1)-selective cannabinoids, such as (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl) pyrrolo-[1,2,3-d,e]-1,4-benzoxazin-6-yl]-1-naphthalenyl-methanone (WIN55,212), are effective at desensitizing TRPA1 and TRP vanilloid 1 (TRPV1), there is a gap in knowledge in understanding the opposite situation, namely whether TRPV1-selective cannabinoids desensitize TRPA1. We selected the TRPV1-specific synthetic cannabinoid, arachidonoyl-2 chloroethanolamine (ACEA), to study peripheral antihyperalgesic properties because ACEA is known to activate TRPV1. Hence, we used in vitro as well as in vivo assays to evaluate the following: 1) the effects of ACEA on the TRPA1-selective agonist, mustard oil (MO), for calcitonin gene-related peptide (CGRP) release from rat hindpaw skin in vitro; 2) the effects of a peripherally selective dose of ACEA on MO-induced nocifensive behavior in vivo; and 3) the effects of five ACEA-insensitive TRPV1 mutations on ACEA-inhibition of MO-evoked calcium accumulation using a Chinese hamster ovary cell expression system. Our results demonstrate that 1) ACEA significantly attenuated (∼40%) MO-evoked CGRP release from rat hindpaw skin, and this effect was not antagonized by the TRPV1 antagonist, capsazepine; 2) ACEA significantly inhibited (∼40%) MO-induced nocifensive behavior in wild-type mice but not in TRPV1 knockout mice; and 3) all TRPV1 mutations insensitive to ACEA lacked the ability to inhibit MO-evoked calcium accumulation in Chinese hamster ovary cells transfected with TRPV1 and TRPA1. Taken together, the results indicate that a TRPV1-selective cannabinoid, ACEA, inhibits MO-evoked responses via a TRPV1-dependent mechanism. This study strengthens the hypothesis that cannabinoids mediate their peripheral analgesic properties, at least in part, via the TRP channels.
Introduction
The desensitization of transient receptor potential vanilloid 1 (TRPV1) and TRP ankyrin 1 (TRPA1) activities is a logical target for development of analgesics because gene deletion studies have shown that mice with genetic deletion of either TRPV1 (Caterina et al., 2000; Davis et al., 2000; Szabó et al., 2005) or TRPA1 (Bautista et al., 2006; Kwan et al., 2006) display significantly reduced nociceptive behaviors in several preclinical pain models. In addition, both TRPV1 and TRPA1 are present on 20 to 40% of sensory neurons, including a substantial proportion that responds to noxious stimuli (Caterina et al., 2000; Bautista et al., 2005). Thus, both TRPV1 and TRPA1 are important integrators of peripheral noxious stimuli.
Numerous studies have demonstrated that both synthetic and plant-derived cannabinoids produce robust antinociception in preclinical pain models. However, their precise mechanism(s) of action remains unclear. The traditional hypothesis is that cannabinoids mediate antihyperalgesia and antinociception via activation of the G protein-coupled cannabinoid receptors, CB1 and CB2. For example, many studies have reported that (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl) pyrrolo-[1,2,3-d,e]-1,4-benzoxazin-6-yl]-1-naphthalenyl-methanone (WIN55,212) evokes antinociception and antihyperalgesia via the G protein-coupled CB1 receptor using pharmacological blockade or gene deletion studies (Zimmer et al., 1999; Johanek et al., 2001; Johanek and Simone, 2004; Agarwal et al., 2007). In addition, the antihyperalgesic properties of (2-iodo-5-nitrophenyl)-[1-(1-methylpiperidin-2-ylmethyl)-1H-indol-3-yl]-methanone (AM1241) and cannabilactones have been attributed exclusively to the CB2 receptor (Malan et al., 2001, 2003; Ibrahim et al., 2003, 2005; Quartilho et al., 2003; Gutierrez et al., 2007; Khanolkar et al., 2007). However, recent studies have demonstrated an alternative mechanism by which cannabinoids modulate nociceptive transmission via ionotropic receptors. Agonist-induced desensitization of the TRPA1 channel can result in the peripheral antinociceptive effect of certain cannabinoids, such as WIN55,212 (Akopian et al., 2008, 2009). Additional studies have demonstrated that cannabinoid inhibition of TRPA1 activities leads to a heterologous desensitization of TRPV1 (Patwardhan et al., 2006; Akopian et al., 2007, 2009; Ruparel et al., 2008), similar to the cross-desensitization observed with prototypical TRPA1 and TRPV1 agonists such as mustard oil (MO) and capsaicin (Akopian et al., 2007; Ruparel et al., 2008). In addition, six different phytocannabinoids, such as cannabidiol, tetrahydrocannabinol, cannabichromene, and cannabigerol, have been shown to activate TRPA1 but antagonize responses of TRP melastatin 8 in vitro; moreover, cannabidiol serves as a cannabinoid agonist of TRPV2 (De Petrocellis et al., 2008; Qin et al., 2008; Di Marzo and De Petrocellis, 2010).
Although TRPA1-selective cannabinoids such as WIN55,212 and AM1241 are effective at desensitizing TRPV1 via a calcium-dependent calcineurin pathway (Patwardhan et al., 2006), there is a gap in knowledge in understanding whether the reverse is true, namely whether TRPV1-selective cannabinoids lead to the heterologous desensitization of TRPA1. Accordingly, we have evaluated whether arachidonyl-2 chloroethanolamine (ACEA), a TRPV1-selective cannabinoid, triggers heterologous desensitization of TRPA1.
Materials and Methods
Animals.
All animal study protocols were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio and conformed to the International Association for the Study of Pain and U.S. government guidelines. Animals were housed for 1 week before the experiment, and food and water were available ad libitum. Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA), weighing 250 to 300 g, were used for the skin superfusion assays for release of calcitonin gene-related peptide (CGRP), and C57BL/6J wild-type (WT) and C57BL/6J TRPV1(−/−) mice were used for the behavioral experiments.
Neuropeptide Release Assay.
The release of immunoreactive CGRP (iCGRP) from isolated hindpaw skin biopsies was performed as described previously (Kilo et al., 1997; Ruparel et al., 2008), except that the skin biopsies (6–7-mm diameter) dissected from glabrous rat hindpaws were simply immersed into 2-ml wells containing various treatment conditions in a modified Hanks' buffer (containing 10.9 mM HEPES, 4.2 mM sodium bicarbonate, 10 mM dextrose, and 0.1% bovine serum albumin, pH adjusted to 7.4 at 303 mOsmol/kg). The TRPA1-selective agonist MO (Sigma-Aldrich, St. Louis, MO) (Jeske et al., 2006; Akopian et al., 2007), ACEA and capsazepine (CPZ; both from Tocris Bioscience, Ellisville, MO) were diluted with Hanks' buffer for the assays.
For the desensitization experiments, two biopsies per well were placed into 24-well plates containing 1.2 ml of Hanks' buffer (37°C) and, after a wash period and collection of two 20-min baseline samples, were exposed to either vehicle or 100 μM CPZ for 20 min. The biopsies were then treated for 20 min with either vehicle, 100 μM ACEA, 100 μM CPZ, or the combination of 100 μM CPZ and 100 μM ACEA. The skin was then exposed to a 10-min vehicle-washout period, which was followed by a 2-min application of 0.1% MO (Ruparel et al., 2008). The total evoked iCGRP release was measured by pooling the 2-min exposure sample with the subsequent 18-min vehicle-exposure sample. Biopsies were used only once.
iCGRP Radioimmunoassay.
The CGRP radioimmunoassay was conducted essentially as described previously (Kilo et al., 1997; Patwardhan et al., 2006; Ruparel et al., 2008). In brief, 100 μl of primary antibody against CGRP (final dilution, 1:106; kindly donated by Dr. M. J. Iadarola, National Institutes of Health, Bethesda, MD) was added to 1-ml aliquots and incubated at 4°C for 48 h. After this incubation, 100 μl of [125I]-Tyr0-CGRP28–37 (∼2 × 104 cpm) and 50 μl of goat anti-rabbit antisera coupled to ferric beads (Applied Biosystems, Foster City, CA) were added to the tubes and incubated at 4°C for an additional 24 h. The reaction was stopped using immunomagnetic separation of bound from the free tracer. The minimal detectable levels for CGRP for this assay were ∼3 fmol/ml and the 50% displacement was ∼30 fmol. All test compounds were evaluated for potential interference in the radioimmunoassay.
Behavioral Assays.
On the day of the experiment, MO was diluted in mineral oil and ACEA [stock made in 100% methyl-2-pyrrolidinone solution) (MPL); Sigma-Aldrich] was diluted to 27% MPL/saline. Preliminary studies demonstrated no differences between mineral oil and MPL/saline vehicle-treated animals, and therefore the final vehicle control consisted of MPL/saline. All animals were acclimatized in empty cages for 20 to 30 min, and all studies were conducted by observers blinded to treatment allocation.
WT and TRPV1(−/−) mice were given a 15-μl intraplantar injection of either vehicle or ACEA (100 μg) followed by a 10-μl intraplantar injection of 0.1% MO 15 min later. The duration (in seconds) spent grooming and flinching the injected hindpaw over a 5-min period for MO-induced behavior was measured. For experiments evaluating the peripheral action of ACEA, 15 μl of either vehicle or ACEA (100 μg) was injected into the contralateral hindpaw, whereas 15 μl of vehicle and then (after 15 min) 10 μl of MO (0.1%) was injected into the ipsilateral paw.
Constructs and Heterologous Expression in Chinese Hamster Ovary Cells.
Expression plasmids of TRPV1 (GenBank accession no. NM031982) in pcDNA3 (Invitrogen, Carlsbad, CA) and of TRPA1 (GenBank accession no. NM177781) in pcDNA5/FRT (Invitrogen) were used. The TRPV1 mutants Y511A and S512Y in pcDNA3 were kindly provided by Dr. David Julius (University of California, San Francisco, San Francisco, CA). All other TRPV1 mutations were custom made in pcDNA3 (TOP Gene, Montreal, QC, Canada). Expression constructs with a visual marker (green fluorescent protein expressing pEGFP-N1; Clontech, Mountain View, CA) were delivered into Chinese hamster ovary (CHO) cells using PolyFect (QIAGEN, Valencia, CA) according to the manufacturers' protocols. CHO cells were subjected to experimental procedures within 24 to 48 h after transfection.
Ca2+ Imaging in CHO Cells.
The Ca2+ imaging experiments were performed as described previously (Diogenes et al., 2006; Akopian et al., 2007). All drugs and compounds were dissolved in modified 1× Hanks' buffer containing 10.9 mM HEPES, 4.2 mM sodium bicarbonate, 10 mM dextrose, and 1.8 mM calcium chloride (pH adjusted to 7.4) as described previously (Ruparel et al., 2008). Only green fluorescent protein-positive cells were selected for experiments. The net changes in Ca2+ accumulation were calculated by subtracting the basal [Ca2+]i (mean value collected for 60 s before agonist application) from the peak [Ca2+]i value achieved after exposure to the agonists. Data are presented as an absorption ratio of 340/380. A ratio greater than 0.02 was considered positive because this value represents the mean +2 S.D. of basal values observed from >300 untreated cells.
For experiments evaluating the effects of ACEA on TRPV1 mutants, CHO cells transfected with either WT TRPV1 alone or any of the mutations were first washed in Hanks' buffer followed by collection of baseline values for 60 s. The cells were then treated with either vehicle (Hanks' buffer) or 100 μM ACEA for 2 min and washed again with buffer.
For the desensitization experiments, CHO cells were doubly transfected with TRPA1 and either WT TRPV1 or a selected TRPV1 mutant. Cells were washed for 60 s to collect baseline values, after which they were treated with either vehicle or 100 μM ACEA for 2 min. The cells were then washed for 4 min and then treated with a 25-μM MO for 2 min. Finally, the cells were washed again for approximately 2 to 3 min.
Electrophysiology.
Recordings were made in perforated patch voltage-clamp configuration [holding potential (Vh) of −60 mV] at 22–24°C from CHO cells. Data were acquired and analyzed using an Axopatch 200B amplifier and pCLAMP 9.0 software (Molecular Devices, Sunnyvale, CA). Recording data were filtered at 0.5 kHz and sampled at 2 kHz. Borosilicate pipettes (Sutter, Novato, CA) were polished to resistances of 7 to 10 MΩ in the perforated patch pipette solution. Access resistance (Rs) was compensated (40–80%) when appropriate up to the value of 15 to 20 MΩ. Data were rejected when leak currents were >50 pA or input resistance was <300 MΩ. Currents were considered positive when their amplitudes were 5-fold larger than displayed noise (in root mean square). Standard external solution contained the following: 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM d-glucose, and 10 mM HEPES, pH 7.4. The standard pipette solution for the perforated patch whole-cell configurations contained the following: 140 mM KCl, 1 mM MgCl2, 10 mM d-glucose, 10 mM HEPES, pH 7.3, and 250 μg/ml amphotericin B.
Data Analysis.
All iCGRP release desensitization experiments were conducted with n = 4 to 6 wells/group and repeated at least three times for statistical analysis. Overall, a sample size (n) of 12 to 18 was used for statistical analysis for these experiments. The data are presented as percentage above basal levels of iCGRP (mean ± S.E.M.), and the measured basal CGRP levels (i.e., in femtomoles per mole) are provided in the figure legends. The behavioral experiments were performed with n = 6 to 8 mice/group, and the data are presented as time (seconds) spent displaying nocifensive behavior (mean ± S.E.M.). All calcium-imaging experiments were performed three times, and a sample size of 50 to 100 cells after pooling data was used for statistical analysis. For experiments comparing only two groups, an unpaired Student's t test was performed; all other experiments were analyzed by two-way analysis of variance with Dunnett's multiple comparison test. Data were analyzed using Prism software version 4.0 (GraphPad Software Inc., San Diego, CA).
Results
ACEA Cross-Desensitizes TRPA1 In Vitro.
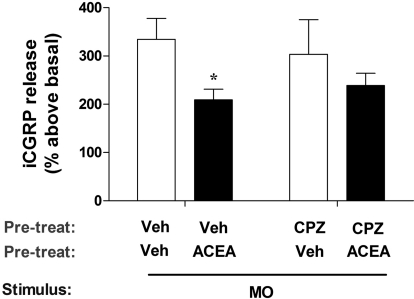
We have shown previously that the TRPA1/CB1/CB2 cannabinoid agonist WIN55,212 heterologously inhibits capsaicin-induced responses in vitro as well as in vivo via activation of TRPA1 (Patwardhan et al., 2006; Akopian et al., 2008). In the present study, we tested the opposite hypothesis, namely that a TRPV1-selective cannabinoid could heterologously desensitize a TRPA1-mediated effect. To conduct these studies, we used the synthetic cannabinoid, ACEA, which is a TRPV1-selective cannabinoid and has no effect on TRPA1 (Price et al., 2004; Akopian et al., 2009), and tested for inhibition of MO, a TRPA1-selective agonist (Jeske et al., 2006; Akopian et al., 2009). Figure 1 demonstrates that pretreatment of skin biopsies with ACEA significantly inhibits MO-evoked CGRP release by approximately 40% (Veh/Veh/MO = 334.6 ± 43.1% versus Veh/ACEA/MO = 209 ± 21.9%). ACEA inhibited MO-evoked CGRP release by ∼40% in vehicle pretreatment (p < 0.05); the ACEA effect was not significantly reversed in biopsies pretreated with the TRPV1 antagonist CPZ (CPZ/Veh/MO = 303.3 ± 71.81% versus CPZ/ACEA/MO = 239 ± 25.2%). Control experiments verified that application of ACEA alone did not trigger iCGRP release (Veh = 2.2 ± 0.4 fmol versus ACEA = 2.3 ± 0.19 fmol; p is nonsignificant). Taken together, these data are consistent with the hypothesis that ACEA desensitizes peripheral terminals of MO-responsive peptidergic fibers.
Fig. 1.
ACEA inhibits MO-evoked CGRP release. Skin biopsies were collected from male rat hindpaws, exposed to pretreatment with either Veh or CPZ (100 μM), followed by either Veh or ACEA (100 μM); all groups were then exposed to mustard oil (0.1%) for 2 min, which was combined with a subsequent 18-min exposure to a modified Hanks' buffer. iCGRP was measured by radioimmunoassay, and mean basal levels (= 100%) were 5 to 6 fmol/ml. n = 12 to 18; error bars = S.E.M.; *, p < 0.05.
ACEA Cross-Desensitizes TRPA1 In Vivo.
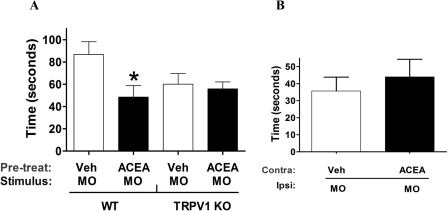
We next examined the physiological significance of this finding by performing in vivo nocifensive behavioral experiments. The assay was based on previously published reports (Caterina et al., 2000; Bautista et al., 2006; Kwan et al., 2006). In brief, mouse hindpaws were first given intraplantar injections of ACEA (no distinct nocifensive behavior was observed compared to the vehicle group) followed by an injection of 0.1% MO 15 min later. The amount of time spent by the animal in displaying nocifensive behavior over the first 5 min after MO was recorded. A 100-μg dose of ACEA significantly inhibited MO-induced nocifensive behavior in WT mice (Fig. 2A). In control experiments, the injection of ACEA alone did not induce a significant nocifensive response (Veh = 0.2 ± 0.2 s versus ACEA = 3.6 ± 2.2 s; p is nonsignificant). To confirm that the effects of ACEA were mediated via TRPV1, we repeated the study in TRPV1(−/−) mice and observed that ACEA inhibition of MO was abolished in these animals (Fig. 2A).
Fig. 2.
Effect of ACEA on MO-induced nocifensive behavior. A, evaluation of the effect of preinjection of ACEA (100 μg) on MO-induced nocifensive behavior (0.01% MO 15 min post-ACEA) in WT and TRPV1(−/−) mice. B, evaluation of the effect of preinjection of ACEA (100 μg) in the contralateral paw to desensitize MO-induced nocifensive behavior in the ipsilateral MO-induced paw after 15 min. n = 6 to 8; error bars = S.E.M.; *, p < 0.05.
To evaluate the site of ACEA action (i.e., central versus peripheral), we injected 100 μg of ACEA into the contralateral paw and MO into the ipsilateral paw. Our results indicate that a 100-μg ACEA dose injected contralaterally did not inhibit MO-induced nocifensive behavior (Fig. 2B). This result clearly demonstrates that the inhibitory effect of ACEA is peripheral and is mediated at the local site of injection. It is noteworthy that the response of MO-induced nocifensive behavior was lower in TRPV1 knockout mice compared with the WT mice (Fig. 2A). This finding could be attributed to the absence of TRPV1 in null-mutant animals that leads to attenuation in TRPA1-mediated responses in sensory neurons (Akopian et al., 2007; Salas et al., 2009). Taken together, our data, for the first time, demonstrate that a TRPV1-selective cannabinoid agonist heterologously cross-desensitizes TRPA1 responses in vitro as well as in vivo.
ACEA-Insensitive TRPV1 Mutants Do Not Desensitize TRPA1.
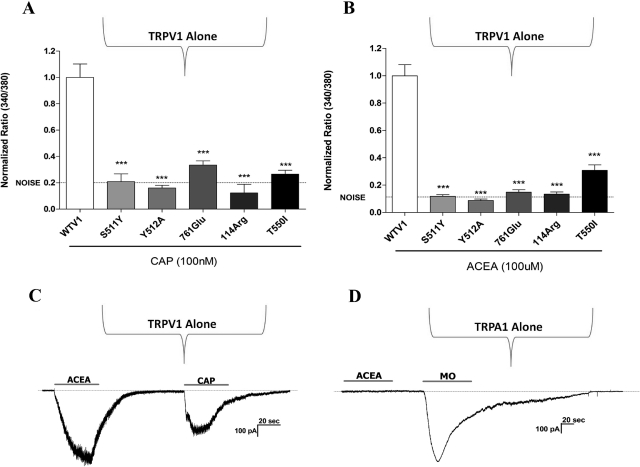
We next used calcium imaging of CHO expression systems to confirm and extend these findings. To confirm the involvement of TRPV1, we first compared WT TRPV1 responses to five different TRPV1 mutants (Fig. 3). Because ACEA has structural similarities to anandamide and capsaicin (Zygmunt et al., 1999; Jordt and Julius, 2002), we selected several capsaicin-insensitive TRPV1 mutants (Jordt and Julius, 2002; Jung et al., 2002; Gavva et al., 2004) as likely channels that would be also insensitive to ACEA. Our data confirmed that these selected TRPV1 mutations were indeed insensitive to capsaicin (Fig. 3A) as well as insensitive to ACEA (Fig. 3B). To confirm the selectivity of ACEA, we used whole-cell voltage-clamp to measure ACEA-activated current in CHO cells transfected with either TRPV1 (Fig. 3C) or TRPA1 (Fig. 3D). It is noteworthy that the application of ACEA induced an inward current in TRPV1-positive but not TRPA1-positive cells.
Fig. 3.
Effect of TRPV1 mutations on ACEA-evoked calcium accumulation. A, evaluation of WT and TRPV1 mutations on the effects of capsaicin- (CAP; 100 nM) evoked calcium accumulation using Fura 2 imaging in CHO expression systems with net increases in the 340/380 nm absorption ratio plotted on the ordinate. Data are normalized to WT. WT TRPV1 ratio (340/380) = 0.099548. B, evaluation of WT and TRPV1 mutations on the effects of ACEA- (100 μM) evoked calcium accumulation. Data are normalized to WT. WT TRPV1 ratio (340/380) = 0.176668. C, and D, representative traces show ACEA-activated current (100 μM) in TRPV1- (C) but not in TRPA1 (D)-expressing CHO cells. Capsaicin (100 nM) and mustard oil (25 μM) were used as positive controls to identify TRPV1- and TRPA1-expressing cells, respectively. Horizontal bars (approximately 40 s) denote durations of agonist applications. n = 75 to 140; ***, p < 0.001.
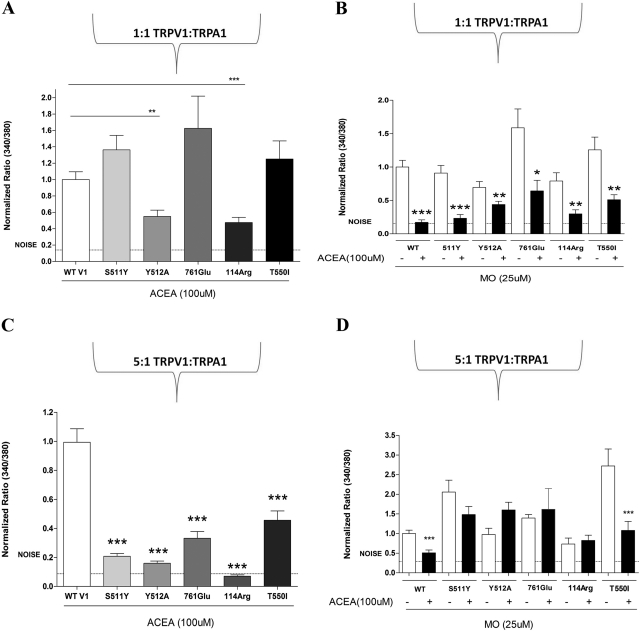
Next, we studied the effect of these ACEA-insensitive TRPV1 mutants on the inhibition of MO-evoked calcium accumulation in the CHO expression system. Our initial experiments coexpressed equimolar amounts of WT TRPA1 DNA with either WT TRPV1 DNA or a selected TRPV1 mutant DNA (Fig. 4, A and B). However, because quantification was not done, the “equimolar” ratio is an assumption based on the amount of cDNA given to cells. It is noteworthy that the cotransfection of an equimolar amount of TRPA1 partially “rescued” the TRPV1 mutants such that they now demonstrated responsiveness to ACEA (Fig. 4A versus Fig. 3B) as well as ACEA-evoked inhibition of MO-evoked calcium accumulation (Fig. 4B). In general, the coexpression of TRPA1 with the TRPV1 mutants produced two patterns of responses to ACEA (Fig. 4A), some mutants (i.e., S511Y, 761Glu, and T550I) demonstrating increased responsiveness relative to WT TRPV1 and other mutants (i.e., Y512A and 114Arg) only partially rescuing ACEA responses. These effects can be attributed to the copresence of TRPA1 in CHO cells (Fig. 4A versus Fig. 3B).
Fig. 4.
Effect of a 1:1 and 5:1 transfection of TRPV1 mutations and TRPA1 on ACEA inhibition of MO. A, evaluation of the effect of ACEA (100 μM) on WT and TRPV1 mutants transfected in a 1:1 ratio with TRPA1. Data are normalized to WT. WT TRPV1 ratio (340/380) = 0.14205. B, evaluation of the effect of ACEA (100 μM) pretreatment on MO- (0.1%) evoked calcium accumulation in WT and TRPV1 mutants transfected in a 1:1 ratio with TRPA1. Data are normalized to WT. WT TRPV1 ratio (340/380) = 0.13088. C, evaluation of the effect of ACEA (100 μM) on WT and TRPV1 mutations transfected in a 5:1 ratio with TRPA1. Data are normalized to WT. WT TRPV1 ratio (340/380) = 0.22836. B, evaluation of the effect of ACEA (100 μM) pretreatment on MO- (0.1%) evoked calcium accumulation in WT and TRPV1 mutations transfected in a 5:1 ratio with TRPA1. Data are normalized to WT. WT TRPV1 ratio (340/380) = 0.06874. n = 95 for WT, n = 60 to 95 for WT; ***, p < 0.001. n = 120 for WT, n = 50 to 75; *, p < 0.05, **, p < 0.01, and ***, p < 0.001.
Several TRP channels are known to physically interact, leading to key conformational changes in the final protein assembly (Xu et al., 2000; Hofmann et al., 2002; Hellwig et al., 2005). It is possible that transfection of equimolar amounts of TRPA1 DNA and TRPV1 DNA permits a physical interaction between TRPV1 and TRPA1 that leads to conformational changes in the TRPV1 mutants (Salas et al., 2009; Staruschenko et al., 2010), thereby restoring their sensitivity to ACEA. To test this hypothesis, we designed a coexpression experiment in which the dose of WT TRPA1 DNA was five times greater than WT TRPV1 DNA, or with various TRPV1 mutant DNAs (i.e., a TRPV1/TRPA1 DNA dose ratio of 5:1). Under these transfection conditions, all five mutations showed significantly reduced sensitivity to ACEA (Fig. 4, C versus A). Accordingly, in the same experimental set, all five mutations that were ACEA-insensitive then failed to desensitize MO-evoked calcium accumulation, with the exception of TRPV1 mutation T550I (Fig. 4, B versus D).
Taken together, these results confirm our CGRP data that ACEA heterologously desensitizes MO responses in vitro and that this effect requires activation of TRPV1 by ACEA. In addition, our data suggest that an interaction between TRPV1 and TRPA1 modifies the biophysical properties of the heteromer, leading to alteration of TRPV1 mutant responsiveness to agonists, namely capsaicin and ACEA.
Discussion
The peripheral antinociceptive and antihyperalgesic effects of cannabinoids have been well documented and recognized (Richardson et al., 1998), but the mechanism(s) mediating this effect is still not elucidated in detail. Nevertheless, there is an agreement that mechanisms of cannabinoid peripheral effects could be dependent on types of cannabinoids, types of involved cannabinoid receptors, direct inhibition of sensory neurons, and modulations of non-neuronal peripheral cells that will eventually result in inhibition of sensory neurons. We have demonstrated that WIN55,212, a combined CB1/CB2 agonist, inhibits TRPV1 responses via activation of the TRPA1 channel on sensory neurons (Jeske et al., 2006; Patwardhan et al., 2006; Akopian et al., 2008).
The current study tested the hypothesis that a CB1-activating cannabinoid, ACEA (Akopian et al., 2009), triggers the heterologous desensitization of TRPA1 via a TRPV1 pathway. Our results indicate that 1) ACEA significantly attenuates MO-evoked CGRP release from rat hindpaw; 2) ACEA significantly inhibits MO-induced short-term nociception in WT mice, and this effect is abolished in TRPV1 knockout mice; and 3) ACEA is capable of inhibiting MO-induced calcium accumulation in vitro, but only under conditions in which TRPV1 is functionally responsive to ACEA. Taken together, these data demonstrate that ACEA inhibits TRPA1 responses via a TRPV1-dependent mechanism. The TRPV1 mutant experiments were designed to test whether ACEA inhibition of MO-evoked calcium accumulation required the coexpression of cannabinoid-responsive TRPV1 protein. The results provided strong support for this possibility because ACEA was able to suppress MO effects only under conditions in which either the WT or mutant TRPV1 was responsive to ACEA (the only exception was T550I).
It is noteworthy that the application of an equimolar (1:1) amount of TRPA1 DNA with TRPV1 mutant DNA partially rescued the ACEA responsiveness not observed in homomeric TRPV1 mutant channels (Figs. 4A versus 3B). Considering that ACEA is not able to activate TRPA1 (Fig. 4D), one plausible explanation for this effect is that TRPA1 interaction with TRPV1 could create conformational changes in the TRPV1 channel, which will lead to its new pharmacological properties (Salas et al., 2009; Staruschenko et al., 2010). Thus, it was shown previously that AM1241 is more potent in TRPA1-TRPV1 expressing sensory neurons than in cells expressing TRPA1 alone (Akopian et al., 2008).
To begin to address this possibility, we designed an overexpression experiment using the TRPV1 mutants with TRPA1 (5:1 dose ratio), reasoning that this might increase the probability of forming ACEA-insensitive homomeric channels consisting of TRPV1 mutants. As predicted, the results demonstrate reduced ACEA responsiveness under these conditions. Overall, these studies provide additional evidence that complex formation between TRPV1 and TRPA1 could lead to different pharmacological properties of individual channels. This result could have important implications during pathological pain states when each channel is activated by endogenous ligands.
Cannabinoids can exert their effects via either metabotropic or ionotropic receptors. These mechanisms are complex because some cannabinoid agonists can act on both GPCR receptors and channels. This multiplicity of mechanisms may be somewhat restricted, considering that cannabinoids act on channels in sensory neurons and GPCR receptors on mainly peripheral nonneuronal cells (Akopian et al., 2008, 2009). Thus, ACEA is likely to mediate its peripheral analgesic effects via the TRPV1 and CB1 receptors, targeting TRPV1 on sensory neurons and CB1 on non-neuronal peripheral cells. However, there are data that point toward expression of CB1 expression in sensory neurons. In such circumstances, cross-talk between GPCRs and ionotropic cannabinoid receptors could be considered. Indeed, recent reports suggest that TRPV1 responses may be sensitized by the CB1 agonist, HU-210, and that constitutive CB1 activity maintains TRPV1 in a sensitized state (Hermann et al., 2003; Fioravanti et al., 2008). Thus, cross-talk among CB1 and TRP channels may occur when colocalized on the cellular domain. However, although we did not perform studies with CB1-null mice or CB1 antagonists, the data obtained here in CHO cells, in which CB1 receptors are not expressed, allow us to suggest that no such CB1-TRPV1 cross-talk is involved in the effect of ACEA on MO-induced activation of TRPA1.
In conclusion, this study supports and strengthens the hypothesis that the peripheral effects of cannabinoids are mediated, at least in part, by ionotropic TRP channels. Although the findings do not exclude a potential role for metabotropic cannabinoid receptors in peripheral cannabinoid analgesia, they do reveal that cannabinoids can desensitize TRPV1 and TRPA1 via homologous and heterologous mechanisms. Furthermore, the results provide further support to a growing body of evidence of the possible formation of TRPV1/TRPA1 heteromeric channels.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant DA019585]; the National Institutes of Health National Institute of Dental and Craniofacial Research [Grants DE017696, DE019311]; and the National Institutes of Health National Center for Research Resources [Grant U54-RR02438] (Clinical Translational Science Award).
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.068940.
- TRP
- transient receptor potential
- WIN55,212
- (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl) pyrrolo-[1,2,3-d,e]-1,4-benzoxazin-6-yl]-1-naphthalenyl-methanone
- ACEA
- arachidonoyl-2 chloroethanolamine
- CB1
- cannabinoid receptor subtype 1
- AM1241
- (2-iodo-5-nitrophenyl)-[1-(1-methylpiperidin-2-ylmethyl)-1H-indol-3-yl]-methanone
- CGRP
- calcitonin gene-related peptide
- WT
- wild type
- iCGRP
- immunoreactive CGRP
- CHO
- Chinese hamster ovary
- CPZ
- capsazepine
- MO
- mustard oil
- MPL
- methyl-2-pyrrolidinone solution
- Veh
- vehicle
- TRPV
- TRP vanilloid
- TRPA
- TRP ankyrin.
Authorship Contributions
Participated in research design: Ruparel, Patwardhan, Akopian, and Hargreaves.
Conducted experiments: Ruparel.
Performed data analysis: Ruparel, Patwardhan, Akopian, and Hargreaves.
Wrote or contributed to the writing of the manuscript: Ruparel, Patwardhan, Akopian, and Hargreaves.
References
- Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, et al. (2007) Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci 10:870–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. (2007) Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J Physiol 583:175–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopian AN, Ruparel NB, Jeske NA, Patwardhan A, Hargreaves KM. (2009) Role of ionotropic cannabinoid receptors in peripheral antinociception and antihyperalgesia. Trends Pharmacol Sci 30:79–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopian AN, Ruparel NB, Patwardhan A, Hargreaves KM. (2008) Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation. J Neurosci 28:1064–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124:1269–1282 [DOI] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Högestätt ED, Julius D, Jordt SE, Zygmunt PM. (2005) Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA 102:12248–12252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288:306–313 [DOI] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, et al. (2000) Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 405:183–187 [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Vellani V, Schiano-Moriello A, Marini P, Magherini PC, Orlando P, Di Marzo V. (2008) Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J Pharmacol Exp Ther 325:1007–1015 [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L. (2010) Endocannabinoids as regulators of transient receptor potential (TRP) channels: a further opportunity to develop new endocannabinoid-based therapeutic drugs. Curr Med Chem 17:1430–1449 [DOI] [PubMed] [Google Scholar]
- Diogenes A, Patwardhan AM, Jeske NA, Ruparel NB, Goffin V, Akopian AN, Hargreaves KM. (2006) Prolactin modulates TRPV1 in female rat trigeminal sensory neurons. J Neurosci 26:8126–8136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravanti B, De Felice M, Stucky CL, Medler KA, Luo MC, Gardell LR, Ibrahim M, Malan TP, Jr, Yamamura HI, Ossipov MH, et al. (2008) Constitutive activity at the cannabinoid CB1 receptor is required for behavioral response to noxious chemical stimulation of TRPV1: antinociceptive actions of CB1 inverse agonists. J Neurosci 28:11593–11602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavva NR, Klionsky L, Qu Y, Shi L, Tamir R, Edenson S, Zhang TJ, Viswanadhan VN, Toth A, Pearce LV, et al. (2004) Molecular determinants of vanilloid sensitivity in TRPV1. J Biol Chem 279:20283–20295 [DOI] [PubMed] [Google Scholar]
- Gutierrez T, Farthing JN, Zvonok AM, Makriyannis A, Hohmann AG. (2007) Activation of peripheral cannabinoid CB1 and CB2 receptors suppresses the maintenance of inflammatory nociception: a comparative analysis. Br J Pharmacol 150:153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwig N, Albrecht N, Harteneck C, Schultz G, Schaefer M. (2005) Homo- and heteromeric assembly of TRPV channel subunits. J Cell Sci 118:917–928 [DOI] [PubMed] [Google Scholar]
- Hermann H, De Petrocellis L, Bisogno T, Schiano Moriello A, Lutz B, Di Marzo V. (2003) Dual effect of cannabinoid CB1 receptor stimulation on a vanilloid VR1 receptor-mediated response. Cell Mol Life Sci 60:607–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann T, Schaefer M, Schultz G, Gudermann T. (2002) Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci USA 99:7461–7466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, Vanderah TW, Lai J, Porreca F, Makriyannis A, et al. (2003) Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci USA 100:10529–10533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, Davar G, Makriyannis A, Vanderah TW, Mata HP, et al. (2005) CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci USA 102:3093–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske NA, Patwardhan AM, Gamper N, Price TJ, Akopian AN, Hargreaves KM. (2006) Cannabinoid WIN 55,212-2 regulates TRPV1 phosphorylation in sensory neurons. J Biol Chem 281:32879–32890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanek LM, Heitmiller DR, Turner M, Nader N, Hodges J, Simone DA. (2001) Cannabinoids attenuate capsaicin-evoked hyperalgesia through spinal and peripheral mechanisms. Pain 93:303–315 [DOI] [PubMed] [Google Scholar]
- Johanek LM, Simone DA. (2004) Activation of peripheral cannabinoid receptors attenuates cutaneous hyperalgesia produced by a heat injury. Pain 109:432–442 [DOI] [PubMed] [Google Scholar]
- Jordt SE, Julius D. (2002) Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell 108:421–430 [DOI] [PubMed] [Google Scholar]
- Jung J, Lee SY, Hwang SW, Cho H, Shin J, Kang YS, Kim S, Oh U. (2002) Agonist recognition sites in the cytosolic tails of vanilloid receptor 1. J Biol Chem 277:44448–44454 [DOI] [PubMed] [Google Scholar]
- Khanolkar AD, Lu D, Ibrahim M, Duclos RI, Jr, Thakur GA, Malan TP, Jr, Porreca F, Veerappan V, Tian X, George C, et al. (2007) Cannabilactones: a novel class of CB2 selective agonists with peripheral analgesic activity. J Med Chem 50:6493–6500 [DOI] [PubMed] [Google Scholar]
- Kilo S, Harding-Rose C, Hargreaves KM, Flores CM. (1997) Peripheral CGRP release as a marker for neurogenic inflammation: a model system for the study of neuropeptide secretion in rat paw skin. Pain 73:201–207 [DOI] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. (2006) TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50:277–289 [DOI] [PubMed] [Google Scholar]
- Malan TP, Jr, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah T, Porreca F, Makriyannis A. (2001) CB2 cannabinoid receptor-mediated peripheral antinociception. Pain 93:239–245 [DOI] [PubMed] [Google Scholar]
- Malan TP, Jr, Ibrahim MM, Lai J, Vanderah TW, Makriyannis A, Porreca F. (2003) CB2 cannabinoid receptor agonists: pain relief without psychoactive effects? Curr Opin Pharmacol 3:62–67 [DOI] [PubMed] [Google Scholar]
- Patwardhan AM, Jeske NA, Price TJ, Gamper N, Akopian AN, Hargreaves KM. (2006) The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin. Proc Natl Acad Sci USA 103:11393–11398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Patwardhan A, Akopian AN, Hargreaves KM, Flores CM. (2004) Modulation of trigeminal sensory neuron activity by the dual cannabinoid-vanilloid agonists anandamide, N-arachidonoyl-dopamine and arachidonyl-2-chloroethylamide. Br J Pharmacol 141:1118–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin N, Neeper MP, Liu Y, Hutchinson TL, Lubin ML, Flores CM. (2008) TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci 28:6231–6238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartilho A, Mata HP, Ibrahim MM, Vanderah TW, Porreca F, Makriyannis A, Malan TP., Jr (2003) Inhibition of inflammatory hyperalgesia by activation of peripheral CB2 cannabinoid receptors. Anesthesiology 99:955–960 [DOI] [PubMed] [Google Scholar]
- Richardson JD, Kilo S, Hargreaves KM. (1998) Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain 75:111–119 [DOI] [PubMed] [Google Scholar]
- Ruparel NB, Patwardhan AM, Akopian AN, Hargreaves KM. (2008) Homologous and heterologous desensitization of capsaicin and mustard oil responses utilize different cellular pathways in nociceptors. Pain 135:271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas MM, Hargreaves KM, Akopian AN. (2009) TRPA1-mediated responses in trigeminal sensory neurons: interaction between TRPA1 and TRPV1. Eur J Neurosci 29:1568–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staruschenko A, Jeske NA, Akopian AN. (2010) Contribution of TRPV1-TRPA1 interaction to the single channel properties of the TRPA1 channel. J Biol Chem 285:15167–15177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó A, Helyes Z, Sándor K, Bite A, Pintér E, Németh J, Bánvölgyi A, Bölcskei K, Elekes K, Szolcsányi J. (2005) Role of transient receptor potential vanilloid 1 receptors in adjuvant-induced chronic arthritis: in vivo study using gene-deficient mice. J Pharmacol Exp Ther 314:111–119 [DOI] [PubMed] [Google Scholar]
- Xu XZ, Chien F, Butler A, Salkoff L, Montell C. (2000) TRPgamma, a drosophila TRP-related subunit, forms a regulated cation channel with TRPL. Neuron 26:647–657 [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. (1999) Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA 96:5780–5785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, Julius D, Högestätt ED. (1999) Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400:452–457 [DOI] [PubMed] [Google Scholar]