Abstract
Potentiating neuroactive steroids are potent and efficacious modulators of the GABAA receptor that act by allosterically enhancing channel activation elicited by GABA. Steroids interact with the membrane-spanning domains of the α subunits of the receptor, whereas GABA binds to pockets in the interfaces between β and α subunits. Steroid interaction with a single site is known to be sufficient to produce potentiation, but it is not clear whether effects within the same β-α pair mediate potentiation. Here, we have investigated whether the sites for GABA and steroids are functionally linked (i.e., whether the occupancy of a steroid site selectively affects activation elicited by GABA binding to the transmitter binding site within the same β-α pair). For that, we used receptors formed of mutated concatenated subunits to selectively eliminate one of the two GABA sites and one of the two steroid sites. The data demonstrate that receptors containing a single functional GABA site are potentiated by the neurosteroid allopregnanolone regardless of whether the steroid interacts with the α subunit from the same or the other β-α pair. We conclude that steroids potentiate the opening of the GABAA receptor induced by either agonist binding site.
Introduction
Neuroactive steroids can act as powerful anesthetics, anticonvulsants, and neuroprotectants. The GABAA receptor, the major target of many exogenous neuroactive steroids, binds synaptically released or ambient GABA, resulting in the activation of an anion-selective channel. Interaction of steroid with the receptor enhances channel open probability through specific changes in channel open and closed times, resulting in increased flow of Cl− ions through the cell membrane (Akk et al., 2010).
The GABAA receptor is a pentameric protein. The major class of mammalian synaptic receptors consists of two α1 subunits, two β2 subunits, and a single γ2 subunit (McKernan and Whiting, 1996). The highly homologous subunits are organized pseudosymmetrically around the central channel. The arrangement of subunits around the central pore is βαγβα, counterclockwise when viewed from the outside of the cell (Baumann et al., 2002). The extracellular domain of the receptor contains two binding sites for the transmitter at the β-α subunit interfaces (Kash et al., 2004).
Potentiation by neuroactive steroids results from the interaction of steroid with the membrane-spanning domains of the two α subunits. Although the exact structural determinants of steroid binding are unknown, the actions of potentiating steroids are strongly reduced or eliminated by mutations to specific residues in the M1 and M4 transmembrane domains in the α1 subunit (Hosie et al., 2006; Akk et al., 2008; Li et al., 2009). Specifically, the α1Q241L mutation abolishes potentiation by the steroid allopregnanolone (Hosie et al., 2006; Akk et al., 2008). The GABAA receptor contains two α subunits and, presumably, two binding sites for steroids. Recent work using mutated concatameric subunits has demonstrated that receptors containing a single intact steroid site retain the ability to be potentiated by steroids (Akk et al., 2009; Bracamontes and Steinbach, 2009).
The α subunit is involved in binding both GABA and steroid. We sought to determine whether the binding of steroid selectively facilitates channel opening via occupancy of the transmitter binding site within the same β-α pair. In this hypothesis, steroid binding modifies GABA binding or signal transduction within the same β-α pair. An alternative hypothesis is that steroid actions are nonspecific with regard to which transmitter binding site is occupied and that the steroid acts through a global change in receptor conformation or channel gating.
For this purpose, we used concatenated GABAA receptors, allowing us to selectively mutate one of the two α and β subunits present in the receptor. The steroid (allopregnanolone) and agonist (GABA) binding were selectively disrupted by the α1(Q241L) and β2(Y205S) mutations, respectively, introduced to one of the two β-α pairs. The data indicate that the steroid effect is not selective, and that steroid binding to one α subunit essentially equally well potentiates activity from receptors binding GABA to the opposing as well as the same β-α pair.
Materials and Methods
The experiments were conducted on wild-type and mutated rat concatameric GABAA receptors. The receptors consisted of a triple β2-α1-γ2L (βαγ) construct and a β2-α1 (βα) tandem construct (Fig. 1). To eliminate GABA binding, the Y205S mutation (Amin and Weiss, 1993) was introduced to one or both β subunits. To eliminate steroid interaction with the receptor, the Q241L mutation (Hosie et al., 2006; Akk et al., 2008) was introduced to one or both α subunits. The α(Q241L) and β(Y205S) mutations were generated using the QuikChange site-directed mutagenesis kit (Stratagene, San Diego, CA). The mutated clones were fully sequenced to verify that only the desired mutation(s) had been produced.
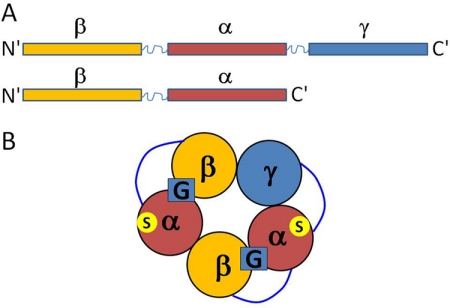
Fig. 1.
Concatameric receptors. A, the concatameric constructs were generated by linking the rat β2 and α1 subunits, carboxyl to amino termini, via a 23-amino acid residue long linker (see Materials and Methods for the sequence). The γ2L subunit was then linked to the carboxyl terminus of the β-α construct via a 26-amino acid residue long linker. B, view of the organization of the GABAA receptor from the extracellular side. The GABA binding sites (G) are located at the β-α subunit interfaces. The sites were disrupted by introducing the Y205S mutation to one or both β subunits. The binding sites for potentiating steroids are located within the α subunits (S). Steroid actions were disrupted by introducing the Q241L mutation to one or both α subunits.
The βα and βα(Q241L) concatamers have been reported previously (Bracamontes and Steinbach, 2009). The β(Y205S)α construct was made by subcloning a ClaI/AijI fragment from the β(Y205S) clone into the βα construct. The β(Y205S)α(Q241L) construct was created by subcloning the ClaI/AijI fragment from the β(Y205S) clone into βα(Q241L).
The triple construct βαγ was generated with the use of PCR overlap extension (Ho et al., 1989), using the γ2L single subunit and the βα construct as templates. The βα tandem was amplified with a forward oligonucleotide complementary to an internal sequence of the α1 subunit and a reverse oligonucleotide complementary to the 3′ end of the α1 coding region with additional sequence at the 3′ end to form part of the linker between the α and γ subunits. The γ2L subunit was amplified with the SP6 reverse oligonucleotide, and a forward oligonucleotide complementary to the 5′ coding region of the mature protein that excluded the signal peptide with additional sequence at the 5′ end encoding part of the linker sequence that overlaps the α1 oligo by 25 nucleotides. Both PCR products were purified by electrophoresis using a low melting temperature (lmt) agarose gel. PCR bands were excised from the gel and purified with QIAquick Gel Extraction Kit (QIAGEN, Valencia, CA). These purified PCR fragments were combined along with the previously used α1 forward and SP6 reverse oligonucleotides for overlap PCR, resulting in a partial α1-γ2L concatamer. This product was purified on an lmt gel as described above. The βα concatamer and the purified PCR product were digested with EcoNI (internal to α1) and XbaI (in pcDNA3) and subsequently purified on an lmt gel. The PCR fragment was ligated to the digested βα concatamer forming a βαγ concatamer. The triple concatamer was subsequently verified by sequencing. The β(Y205S)α(Q241L)γ construct was made as described above, using the β(Y205S)α(Q241L) construct as template. The β(Y205S)αγ and βα(Q241L)γ constructs were made by subcloning PflMI fragments of α1 α(Q241L) into β(Y205S)α(Q241L)γ and βαγ, respectively.
The amino acid sequence of the β-α linker in the triple concatamers β(Y205S)α(Q241L)γ and β(Y205S)αγ and in all βα tandems is Q5A3PAQ2A3PA2Q5. The β-α linker sequence in the triple concatamers βαγ and βα(Q241L)γ is Q5A3PAQ2AGP2A2Q5, and the nucleotide sequence includes an FseI restriction site. The α-γ linker sequence in all triple concatamers is Q5A3PTGQ2AQA3PA2Q5, and the nucleotide sequence includes a PinAI restriction site.
The receptors were expressed in Xenopus laevis oocytes. The cDNAs for the receptor subunits were subcloned into the pcDNA3 expression vector in the T7 orientation. The cDNA was linearized by XbaI (New England Biolabs, Ipswich, MA) digestion, and the cRNA was produced using mMessage mMachine (Ambion, Austin, TX). The oocytes were injected with 7 to 14 ng of cRNA per construct in a final volume of 20 to 60 nl, and incubated in ND96 (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 2.5 mM sodium pyruvate, and 5 mM HEPES, pH 7.4) at 16°C for 2 to 3 days before recording.
Standard two-electrode voltage clamp was used to record the currents. Both voltage and current electrodes were patch-clamp electrodes filled with 3 M KCl and had resistances of 0.5 to 1.5 MΩ. The oocytes were typically clamped at −60 mV. The chamber (RC-1Z; Warner Instruments, Hamden, CT) was perfused continuously at approximately 5 ml/min. Bath solution (92.5 mM NaCl, 2.5 mM KCl, 1 mM MgCl2, and 10 mM HEPES; pH 7.4) was perfused between all test applications.
Solutions were switched by pClamp using a Warner Instruments VC-8T valve controller. Solutions were applied from glass reservoirs via metal or Teflon tubing to reduce adsorption. A typical drug application protocol was to expose an oocyte to bath solution for 10 s, followed by a 20-s drug (GABA, pentobarbital, allopregnanolone) application and a switch back to bath solution. The washout period between successive drug applications was 1 to 3 min.
The current responses were amplified with an Axoclamp 900A amplifier (Molecular Devices, Sunnyvale, CA), digitized with a Digidata 1320 series digitizer (Molecular Devices) at a 100-Hz sampling rate, and stored using pClamp (Molecular Devices). The traces were analyzed with Clampfit (Molecular Devices). The GABA concentration-response curves were fitted using the program NFIT (The University of Texas Medical Branch at Galveston, Galveston, TX). Statistical analyses were carried out using Excel (Microsoft Corp., Redmond, WA).
Western blotting was conducted on extracts from X. laevis oocytes injected with various combinations of cRNA for concatameric constructs. The oocytes were injected with 10 ng of mRNA per construct. After 2 days of incubation at 15.8°C in ND96, the oocytes were put in a 1.5-ml microfuge tube and washed once with 0.5 ml of PBS. The PBS was removed and the oocytes were washed with 0.5 ml of PBS plus a protease inhibitor cocktail (P8465; Sigma-Aldrich, St. Louis, MO). The PBS was again aspirated and then 10 μl of lysis buffer (10 mM HEPES, pH 8.0, 100 mM NaCl, and 10 mM EDTA plus the protease inhibitor cocktail) per oocyte were added to the microfuge tube. The oocytes were homogenized by pipetting through a syringe needle. The homogenate was spun for 5 min at 1000 rpm, 4°C, and the supernatant was removed and spun again under identical conditions. Triton X-100 was added to the supernatant to a final concentration of 2% after which the mixture was rotated for 30 min at 4°C. The mixture was then spun at 14,000 rpm for 10 min at 4°C. The supernatant was added to 20 μl of FLAG agarose beads (Sigma-Aldrich, St. Louis, MO), and enough lysis buffer was added to dilute the concentration of Triton to 1% for an overnight immunoprecipitation reaction. The next day, the agarose beads were washed, and 5 μl of lysis buffer per oocyte were added to the washed agarose beads along with an equal volume of 2× Laemmli buffer. The solution was boiled for 5 min and spun at 2000 rpm to pellet the agarose beads. Fifty microliters of each sample were then loaded onto a precast 4 to 15% gradient Tris-glycine polyacrylamide gel (Bio-Rad, Hercules CA) and electrophoresed. The gel was then transferred to a nitrocellulose Hybond-ECL membrane (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). The membrane was preblocked in 100% Odyssey block solution (LI-COR Biosciences, Lincoln NE) at room temperature for 1 h, followed by overnight incubation at 4°C in a solution of 50% Odyssey block solution: 50% phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, and 1.4 mM KH2PO4, pH 7.3) containing 0.2% Tween 20 (Thermo Fisher Scientific, Waltham, MA) with primary antibody. The primary antibody was raised to the cytoplasmic loop of the α1 subunit (1:500 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight. The membrane was washed with PBS and four times with 0.2% Tween 20, then incubated with donkey anti-goat IRDye 680 (LI-COR Biosciences) at a 1:2000 dilution in a solution of 50% blocking buffer and 50% PBS with 0.2% Tween 20 at room temperature for 30 min. The membrane was washed as before followed by a rinse in PBS. Bands were visualized using the Odyssey system (LI-COR Biosciences).
The chemicals (salts, GABA, pentobarbital, and allopregnanolone) were purchased from Sigma-Aldrich. Stock solutions of allopregnanolone (10 mM) were made in dimethyl sulfoxide; dilutions to test concentrations were made on the day of the experiment. Stock solutions of pentobarbital (2 mM) were made in the bath solution.
Results
Concatameric Constructs Are Not Degraded.
We created concatameric GABAA receptors made of rat α1, β2, and γ2L subunits. The concatameric constructs (Fig. 1) were generated, amino- to carboxyl-terminal, in the β-α-γ (βαγ) and β-α configuration (βα). In some experiments, one or both β subunits contained the β2(Y205S) mutation, and one or both α subunits contained the α1(Q241L) mutation.
Proteolysis after expression can lead to partial (or full) degradation of the concatamers, potentially resulting in breakup of the constructs into single free subunits. If the degraded constructs retain the ability to assemble into functional receptors, the interpretation of the results presented below would be complicated. We performed Western blots of proteins extracted from injected oocytes (Fig. 2) to confirm that subunit concatamers are not appreciably degraded after expression in oocytes. Proteins were immunoprecipitated with antibody to a FLAG epitope placed near the amino terminus of the β2 subunit, and transfers were probed with antibody to the cytoplasmic region of the α1 subunit. The blots indicate that assembled receptors (i.e., receptors containing the β2 subunit) do not include detectable lower molecular mass material reacting with the anti-α1 antibody. Free α1 subunit migrates at ∼50 kDa, whereas the duplex concatameric constructs (βα) migrate at ∼120 kDa, comparable with results obtained previously [110–140 kD; (Baumann et al., 2001, 2003)]. The βαγ triple construct migrates at ∼210 kDa, somewhat slower than reported for the αβγ construct [∼170 kDa; (Baumann et al., 2003)].
Fig. 2.
Concatamers of subunits are not degraded in the oocytes. The figure shows a Western blot of immunoprecipitated proteins prepared from X. laevis oocytes (see Materials and Methods). Lane 1 shows material from uninjected oocytes, lane 2 from oocytes injected with free α1Flag plus β2 subunits, lane 3 with β*α + βα*γ, and lane 4 with βα* + β*αγ. The asterisk denotes presence of a mutation [α1(Q241L) or β2(Y205S)]. The two outside lanes show molecular mass standards (stated size in kilodaltons shown). The transfer was probed with antibody to the cytoplasmic region of the α1 subunit. The duplex tandems migrate at ∼120 kDa and the triplex at ∼210 kDa. Note that there is no indication that there is lower molecular mass material that reacts with anti-α1 antibody, indicating that receptor that contains the FLAG epitope (placed on the β2 subunit) does not contain detectable amounts of free α1 subunit. This indicates that the functional surface receptors that we study are composed of concatamers with minimal degradation.
Concatameric Receptors Are Functional.
Electrophysiological recordings demonstrate that all combinations of constructs tested are functional. Oocytes expressing the wild-type βαγ + βα receptors challenged with 1 to 3 mM GABA demonstrated peak currents of up to 6 μA. The concentration-response measurements were conducted by exposing the oocytes to 3 to 1000 μM GABA. The concentration-response curve fitted to the Hill equation yielded an EC50 of 33 ± 2 μM and a Hill coefficient of 2.0 ± 0.3 (n = 5 cells). For comparison, in our hands, the GABA EC50 for oocytes expressing free α1, β2, and γ2L subunits is 4.6 μM (Akk et al., 2011). Thus the linkage of subunits shifts the concentration-response curve to higher GABA concentrations, in agreement with previous data on concatameric GABAA receptors (Baumann et al., 2002; Akk et al., 2009). The fitting results for GABA concentration-response data for the concatameric receptors are summarized in Table 1. The concentration-response curves are given in Fig. 3.
TABLE 1.
GABA concentration-response properties of the concatameric receptors
The table shows the results from fitting the GABA concentration-response data to the Hill equation. The data were normalized to the response obtained at 1 mM GABA. The first row (αβγ) gives the results obtained from receptors containing free α1, β2. and γ2L subunits (from Akk et al., 2011). The lower range of peak responses is due to a type of chamber used in which only a portion of the cell membrane is exposed to drugs. Receptors containing two mutated β subunits showed negligible currents in the presence of 1 mM GABA (less than 10 nA).
| Receptor | Configuration | Maximal Response | EC50 | nH | No. of Cells | Range of Currents at Maximal GABA |
|---|---|---|---|---|---|---|
| μM | nA | |||||
| αβγ | GS/GS | 1.0 ± 0.03 | 4.6 ± 0.4 | 1.4 ± 0.1 | 7 | 675–1780 |
| βαγ-βα | GS/GS | 1.0 ± 0.03 | 33 ± 2 | 2.0 ± 0.3 | 5 | 4364–6075 |
| βαγ-β*α | GS/–S | 1.0 ± 0.02 | 95 ± 6 | 1.4 ± 0.1 | 6 | 827–2443 |
| βαγ-βα* | GS/G– | 1.0 ± 0.01 | 76 ± 3 | 1.9 ± 0.1 | 5 | 1831–5133 |
| βαγ-β*α* | GS/– – | 1.0 ± 0.02 | 79 ± 4 | 1.6 ± 0.1 | 5 | 1171–4403 |
| βα*γ-βα | G–/GS | 1.0 ± 0.02 | 53 ± 4 | 1.3 ± 0.1 | 5 | 3370–4592 |
| βα*γ-β*α | G–/–S | 1.1 ± 0.1 | 94 ± 23 | 1.0 ± 0.2 | 4 | 129–211 |
| βα*γ-βα* | G–/G– | 1.1 ± 0.03 | 156 ± 12 | 1.4 ± 0.1 | 5 | 487–1212 |
| βα*γ-β*α* | G–/– – | 1.1 ± 0.01 | 126 ± 5 | 1.2 ± 0.05 | 6 | 92–263 |
| β*αγ-βα | –S/GS | 1.0 ± 0.02 | 32 ± 2 | 1.1 ± 0.1 | 4 | 277–2823 |
| β*αγ-β*α | –S/–S | N.D. | N.D. | N.D. | 5 | <5 |
| β*αγ-βα* | –S/G– | 1.1 ± 0.02 | 140 ± 11 | 1.2 ± 0.1 | 7 | 35–142 |
| β*αγ-β*α* | –S/– – | N.D. | N.D. | N.D. | 5 | <5 |
| β*α*γ-βα | – –/GS | 1.1 ± 0.1 | 24 ± 6 | 1.0 ± 0.2 | 5 | 460–3851 |
| β*α*γ-β*α | – –/–S | N.D. | N.D. | N.D. | 6 | <10 |
| β*α*γ-βα* | – –/G– | 1.2 ± 0.02 | 187 ± 13 | 1.1 ± 0.1 | 5 | 50–148 |
| β*α*γ-β*α* | – –/– – | N.D. | N.D. | N.D. | 4 | <10 |
β*, β(Y205S); α*, α(Q241L); G, intact GABA site; S, intact steroid site; N.D., not determined.
Fig. 3.
GABA concentration-response curves of the concatameric receptors. A, receptors containing the wild-type βαγ construct in combination with four variants of the βα construct. The dashed line gives the GABA concentration-response curve for receptors containing free α1, β2, and γ2L subunits (from Akk et al., 2011). B, receptors containing the βα(Q241L)γ construct in combination with four variants of the βα construct. C, receptors containing the β(Y205S)αγ construct in combination with two variants of the βα construct. No responses to GABA were observed from β(Y205S)αγ + β(Y205S)α or β(Y205S)αγ + β(Y205S)α(Q241L) receptors. D, receptors containing the β(Y205S)α(Q241L)γ construct in combination with two variants of the βα construct. No responses to GABA were observed from β(Y205S)α(Q241L)γ + β(Y205S)α or β(Y205S)α(Q241L)γ + β(Y205S)α(Q241L) receptors. The correspondence between symbols and receptor types are given separately in each panel. The asterisk stands for the presence of mutation (in the β subunit, Y205S; in the α subunit, Q241L). The results from fitting are presented in Table 1.
The introduction of the α(Q241L) mutation had a relatively small effect on the maximal peak current and the concentration-response relationship. The maximal currents for receptors containing a single mutation were similar to those in receptors comprising unmutated concatamers (up to 5 μA), but the peak response from a receptor containing two mutated α subunits was reduced to ∼1 μA (Table 1). A previous single-channel study of the α(Q241L) mutation found that the presence of the mutation in both α subunits significantly reduces the maximal open probability (Akk et al., 2008). This suggests that the reduced peak response in concatameric receptors containing two mutated α subunits is due mainly to kinetic effects of the mutation rather than reduced expression. The introduction of the α(Q241L) mutation shifted the GABA concentration-response curves to higher drug concentrations. The EC50 values were 76 and 53 μM when the mutation was in the βα and βαγ constructs, respectively (Table 1). The GABA EC50 was shifted to 156 μM in the receptor containing the mutation in both constructs.
The β(Y205S) mutation has been shown to eliminate channel gating by GABA in receptors consisting of free subunits (Amin and Weiss, 1993). When the β(Y205S) mutation was introduced to both the βαγ and βα constructs, the receptors did not respond to application of up to 3 mM GABA (<5 nA; Table 1). Functional expression of the β(Y205S)αγ-β(Y205S)α receptors was demonstrated by current responses to pentobarbital (Fig. 4E). In four oocytes exposed to 2 mM pentobarbital, the maximal peak response was 280 nA, and the tail response upon the removal of the drug reached 700 nA. The peak response in the presence of a high concentration of pentobarbital is reduced by open channel block (Akaike et al., 1987), and the tail results from rapid unblocking of the channel. The peak of the tail response for receptors containing unmutated concatamers was 3 μA, suggesting that the total number of receptors was not greatly reduced by the presence of β(Y205S) in both concatamers.
Fig. 4.
Sample recordings from wild-type and mutant concatameric receptors. In each row responses are from the same cell. Row A shows traces from the wild-type receptor containing free α1β2γ2L subunits. Row B shows traces from the wild-type receptor containing concatenated subunits. Rows C and D show essential results for receptors in which the mutations to the GABA-binding site (β*) and steroid-binding site (α*) are placed in different β-α pairs. Note that the amplitude calibration bars in C and D for the responses to pentobarbital and GABA ± allopregnanolone differ. This is in agreement with the presence of the β(Y205S) mutation that is known to diminish responses to GABA but not pentobarbital. No potentiation by allopregnanolone was observed cells expressing receptors containing two mutated α subunits (E). GABA currents were not observed in cells expressing receptors containing two mutated β subunits (F).
Receptors containing a single β(Y205S) mutation responded to 1 mM GABA with peak responses of up to 2.5 μA. The presence of the mutation had a relatively small effect on the GABA concentration-response properties. The EC50 values were at 32 μM (no shift) or 95 μM (a 2.9-fold effect) when the β(Y205S) mutation was in the βαγ or βα construct, respectively. However, the mutation reduced the Hill coefficient from 2.0 in the wild-type concatameric receptor to 1.1 (mutation in the βαγ construct) or 1.4 (mutation in βα). At the concentrations of transmitter used here (<10 mM), the concentration-response curves were well characterized with a single component (Fig. 3).
Potentiation of Receptors Activated by GABA.
We examined allopregnanolone-mediated potentiation of concatameric receptors activated by GABA. The concentrations of GABA used in these experiments were selected on the basis of the concentration-response data presented in Fig. 3 to elicit approximately 20 to 30% of the maximal response. These are concentrations at which most of our previous work on steroid potentiation has been conducted (Akk et al., 2005, 2009; Li et al., 2007), thus offering the most material for comparison. Furthermore, the peak GABA currents were strongly reduced for several mutant combinations, probably as a result of the combined effects of the occupation of a single transmitter binding site (Baumann et al., 2003; Baur and Sigel, 2005) and the reduction in channel open probability that results from the α(Q241L) mutation (Akk et al., 2008). In these cases, the use of lower fractional GABA concentrations would have resulted in unreliable estimates for control currents.
The effect of 1 μM allopregnanolone was examined. This concentration elicits a maximal potentiating effect in wild-type receptors composed of free subunits (Li et al., 2007) as well as concatameric receptors containing wild-type subunits or receptors containing a single α1(Q241L) mutation (Akk et al., 2009; Bracamontes and Steinbach, 2009). The effect of steroid is given as multiples of the control peak response (1 means no effect).
Coapplication of 1 μM allopregnanolone with 15 to 20 μM GABA (EC22) potentiated the peak response by 4.2 ± 1.6-fold in the wild-type βαγ + βα receptor (Table 2; Fig. 4A). For comparison, allopregnanolone potentiates receptors containing free α1β2γ2 subunits by 2.9 ± 0.5-fold (Table 2). In agreement with previous data (Akk et al., 2009; Bracamontes and Steinbach, 2009), the presence of a single α1(Q241L) mutation allowed potentiation (2.2-fold for βαγ-βα(Q241L) and 3.4-fold for βα(Q241L)γ-βα). We note that the GABA fractional responses at which the steroid effects were measured differed slightly (Table 2). Hence, the comparative potentiating effects should be judged with care because they depend on the level of baseline activation. Receptors containing two mutated α subunits were not potentiated by allopregnanolone (1.0 ± 0.1 of control; Table 2; Fig. 4D).
TABLE 2.
Steroid modulation of the concatameric receptors activated by GABA
The table shows the effect of coapplication of 1 μM allopregnanolone with a submaximal concentration of GABA. Given are the effect of steroid (mean ± S.D.) in the presence of a low concentration of GABA as the ratio of the response in the presence of 1 μM allopregnanolone to the response of that cell (third column) in the absence of steroid (1 stands for no effect) the ratio of the peak responses to that concentration of GABA alone and saturating GABA (fourth column). The first row (αβγ) gives the results obtained from receptors containing free α1, β2, and γ2L subunits. Receptors containing two mutated β subunits showed negligible currents in the presence of GABA. Accordingly, steroid potentiation of GABA-elicited currents was not examined.
| Receptor | Configuration | Potentiation by Allopregnanolone | Fractional GABA Response | No of Cells |
|---|---|---|---|---|
| αβγ | GS/GS | 2.9 ± 0.5 | 0.26 ± 0.03 | 7 |
| βαγ-βα | GS/GS | 4.2 ± 1.6 | 0.22 ± 0.09 | 5 |
| βαγ-β*α | GS/–S | 10.4 ± 2.6 | 0.23 ± 0.03 | 5 |
| βαγ-βα* | GS/G– | 2.2 ± 0.3 | 0.27 ± 0.05 | 4 |
| βαγ-β*α* | GS/– – | 4.4 ± 0.5 | 0.23 ± 0.04 | 5 |
| βα*γ-βα | G–/GS | 3.4 ± 0.6 | 0.19 ± 0.04 | 4 |
| βα*γ-β*α | G–/–S | 2.7 ± 0.3 | 0.28 ± 0.05 | 5 |
| βα*γ-βα* | G–/G– | 1.0 ± 0.1 | 0.24 ± 0.15 | 4 |
| βα*γ-β*α* | G–/– – | 1.2 ± 0.2 | 0.27 ± 0.01 | 4 |
| β*αγ-βα | –S/GS | 4.9 ± 0.6 | 0.21 ± 0.05 | 5 |
| β*αγ-β*α | –S/–S | N.D. | N.D. | N.D. |
| β*αγ-βα* | –S/G– | 2.5 ± 0.6 | 0.29 ± 0.04 | 6 |
| β*αγ-β*α* | –S/– – | N.D. | N.D. | N.D. |
| β*α*γ-βα | – –/GS | 4.2 ± 2.0 | 0.26 ± 0.08 | 3 |
| β*α*γ-β*α | – –/–S | N.D. | N.D. | N.D. |
| β*α*γ-βα* | – –/G– | 1.1 ± 0.1 | 0.26 ± 0.03 | 4 |
| β*α*γ-β*α* | – –/– – | N.D. | N.D. | N.D. |
β*, β(Y205S); α*, α(Q241L); G, intact GABA site; S, intact steroid site; N.D., not determined.
Receptors containing the β(Y205S) mutation in a single concatameric construct were strongly potentiated by steroid. When the mutation was in the βαγ construct, 1 μM allopregnanolone potentiated the peak response to 4.9-fold of control. When the mutation was in βα, the effect was 10.4-fold (Table 2). These relatively large values for potentiation are an indication that the β(Y205S) mutation depresses the maximal open probability in the presence of GABA, thus allowing modulation to levels above the maximal current seen with GABA. This kind of effect has been described previously for δ subunit-containing receptors, which have an intrinsically low maximal open probability when activated by GABA (Bianchi and Macdonald, 2003). Receptors containing two β(Y205S) mutations demonstrated no currents in the presence of up to 3 mM GABA. Accordingly, steroid potentiation of GABA responses from these receptors could not be studied.
Combinations of α and β subunit mutations could be divided into two classes. In the first combination, the mutations are within the same β-α pair, thus eliminating one of the GABA binding sites as well as the steroid site associated with the same pair. This leaves the other β-α pair intact to bind both GABA and steroid. In the second combination, the mutations are made in opposite β-α pairs, and the intact GABA and steroid sites lie within different β-α pairs.
Receptors containing the GABA and steroid site mutations in the same β-α pairs (βαγ + β(Y205S)α(Q241L) and β(Y205S)α(Q241L)γ + βα) were potentiated by allopregnanolone. The effect was 4.2-fold when the mutations were in the βαγ construct, and 4.4-fold when the mutations were in βα (Table 2).
Receptors containing the β and α subunit mutations in opposite β-α pairs were also potentiated by steroid. The potentiating effect of 1 μM allopregnanolone was 2.7-fold in βα(Q241L)γ + β(Y205S)α and 2.5-fold in β(Y205S)αγ + βα(Q241L) receptors (Table 2; Fig. 4, B and C). From here, we infer that steroid modulation does not occur by an effect confined to a single α subunit.
We also conducted control experiments in which one of the β-α pairs contained the GABA site mutation and both steroid sites in the two α subunits were mutated. As expected, no potentiation was detected in βα(Q241L)γ + β(Y205S)α(Q241L) (1.2-fold) or β(Y205S)α(Q241L)γ + βα(Q241L) receptors (1.1-fold; Table 2).
Potentiation of Receptors Activated by Pentobarbital.
Receptors containing two β(Y205S) mutations could not be tested for potentiation of currents elicited by GABA because of very small responses. However, such receptors remain responsive to the allosteric activator pentobarbital (Amin and Weiss, 1993). Accordingly, to verify that the β(Y205S) mutation does not interfere with steroid actions when it is present in both β subunits, we tested steroid-mediated potentiation of currents elicited by submaximal concentrations of pentobarbital.
To quantify the potentiating effect of allopregnanolone, we initially exposed the receptors to a low concentration (100–400 μM) of pentobarbital. This concentration was selected to produce approximately 15 to 25% of the tail response observed in the presence of 2 mM pentobarbital. In the next step, 1 μM allopregnanolone was coapplied with the low concentration of pentobarbital.
Receptors lacking mutations to the steroid site [i.e., β(Y205S)αγ and β(Y205S)α receptors] were potentiated by allopregnanolone with an average effect of 6.9-fold (Fig. 4E; Table 3). The introduction of a single α(Q241L) mutation to the double β(Y205S) mutant has little effect on potentiation by allopregnanolone. When the α(Q241L) mutation is in the βαγ, the effect of allopregnanolone is 6.6-fold, and when the α(Q241L) mutation is in the βα construct, the effect of steroid is 4.8-fold. When both α subunits contain the mutation, no potentiation of pentobarbital-elicited currents is observed. The results are summarized in Table 3. From these experiments, we conclude that the β(Y205S) mutations do not interfere with the ability of allopregnanolone to produce channel potentiation.
TABLE 3.
Steroid modulation of concatameric receptors with no intact GABA-binding sites, activated by pentobarbital
The table shows the effect of coapplication of 1 μM allopregnanolone with a submaximal concentration of pentobarbital. Given are the effect of steroid (mean ± S.D.) in the presence of a low concentration (100–400 μM) of pentobarbital (1 stands for no effect) (third column) and the ratio of the peak response to a low concentration of pentobarbital and the tail current after the application of 2 mM pentobarbital (fourth column).
| Receptor | Configuration | Potentiation by Allopregnanolone | Fractional Pentobarbital Response | No. of Cells |
|---|---|---|---|---|
| β*αγ-β*α | –S/–S | 6.9 ± 1.7 | 0.20 ± 0.07 | 4 |
| β*αγ-β*α* | –S/– – | 4.8 ± 1.2 | 0.25 ± 0.10 | 5 |
| β*α*γ-β*α | – –/–S | 6.6 ± 1.5 | 0.14 ± 0.06 | 4 |
| β*α*γ-β*α* | – –/– – | 1.2 ± 0.3 | 0.21 ± 0.06 | 4 |
β*, β(Y205S); α*, α(Q241L); S, intact steroid site.
Discussion
We present results from a study on the interactions between the primary orthosteric transmitter binding sites and the allosteric binding sites for potentiating neurosteroids in the α1β2γ2L GABAA receptor. We employed a set of concatenated subunits in which each of the two transmitter sites and each of the two steroid sites was selectively disrupted by the β(Y205S) and α(Q241L) point mutations, respectively. We confirm previous data demonstrating that a single intact steroid site is sufficient to confer sensitivity to the actions of potentiating steroids. Furthermore, we show that receptors containing a single intact GABA binding site can be activated by GABA and potentiated by the neurosteroid allopregnanolone and that potentiation can be mediated by steroid interactions with its site within the same β-α pair that mediates receptor activation as well as the opposite β-α pair.
We examined the potentiation of responses to pentobarbital in the absence of functional GABA-binding sites. In this case, it also appears that the presence of a single steroid-binding site confers full ability to potentiate activation by an agonist that does not bind to the GABA-binding site and that also apparently induces somewhat different conformational changes in the extracellular domain of the receptor (Muroi et al., 2009; Akk et al., 2011).
The use of concatenated subunits is an effective way to constrain the stoichiometry and order of subunits. Furthermore, it allows a selective introduction of mutations to one of the two α or β subunits present in most GABAA receptors. We previously created and characterized concatameric receptors containing γ2L-β2-α1 (γβα) and β2-α1 (βα) constructs (Akk et al., 2009; Bracamontes and Steinbach, 2009). To avoid potential issues with constraining the amino terminus of the β subunit, the present work was conducted on β2-α1-γ2L (βαγ) + β2-α1 (βα) receptors. We find that receptors containing the γβα triple construct have a GABA concentration-response curve shifted to higher agonist concentrations compared with βαγ-containing receptors or receptors consisting of α1, β2, γ2L free subunits, potentially reflecting reduced flexibility of the aminoterminus of the β subunit. In other work, Baumann et al. (2002) found that the GABA EC50 estimates for βαγ + βα receptors were the closest to the unlinked receptor. The activation curves for γβα + βα or αβα + γβ receptors were further right-shifted. It is thus clear that the linkage of subunits quantitatively affects receptor function. However, the majority of the macroscopic and single-channel biophysical and pharmacological properties remain unaltered in concatameric receptors (Baumann et al., 2002; Boileau et al., 2005; Akk et al., 2009), and the approach remains a powerful tool for selective and focused manipulation of the receptor structure.
A previous study (Baumann et al., 2003), which used concatameric αβα + γβ constructs and in which the β(Y205S) mutation was selectively introduced to one of the constructs, found that the GABA EC50 was right-shifted by ∼2-fold when the mutation was in the γβ construct [a configuration similar to βαγ + β(Y205S)α in the present study]. When the β(Y205S) mutation was introduced to the αβα construct (a configuration similar to the mutation being in the βαγ construct), the GABA EC50 was shifted by 5-fold to higher concentrations (Baumann et al., 2003). It was proposed that these values reflect activation due to occupation of the unmutated site, whereas a second component, with estimated EC50 values at 1 to 10 M, arises from the occupation of the mutated GABA binding site. From these data, Baumann et al. (2003) estimated that the β-α pair flanked by the γ and β subunits (defined as site 1) has a 3-fold lower affinity to GABA than the β-α pair flanked by the α and γ subunits (site 2). Our data show the opposite relationship between the position of the mutation and receptor activation by GABA. Introduction of the β(Y205S) mutation to the βα construct (analogous to a mutated site 1) leads to a larger shift in the GABA concentration-response curve than when the mutation is introduced to the βαγ construct. The underlying reason for the difference is unclear to us.
Our data demonstrating that receptors containing intact transmitter and steroid sites within different β-α subunit pairs can be potentiated by allopregnanolone indicate that the conformational changes induced by the occupation of either steroid-binding site are equivalently transduced to both transmitter-binding sites. Alternatively, the occupation of a steroid site may lead to general conformational changes in the gate domain that, in turn, stabilize the open channel state of the receptor. The present work does not distinguish between the two possibilities. In any case, there seems to be no selective linkage between a steroid site and a transmitter binding site. The present study focused on potentiation by the endogenous neurosteroid allopregnanolone. It is probable that other steroids (e.g., pregnanolone, tetrahydrodeoxycorticosterone) the actions of which depend on the α1Q241 residue demonstrate behavior qualitatively similar to that observed in the present study.
There have been relatively few previous studies of linkage between allosteric modulator sites and agonist sites. A previous study examining the linkage between the site for benzodiazepines positioned at the γ-α subunit interface and the transmitter binding sites found that the occupation of the benzodiazepine site similarly affected channel opening induced by the occupation of either GABA site (Baur and Sigel, 2005).
The neuronal-type nicotinic α4β2 receptor is potentiated by 17β-estradiol, with a critical set of amino acids at the extreme carboxyl-terminal end of the α4 subunit (Paradiso et al., 2001; Curtis et al., 2002). A recent study has found that these amino acids can be placed on either a transmitter-binding or the structural subunit to confer potentiation (Jin and Steinbach, 2011). In combination with the present results, these findings suggest that allosteric potentiators act globally on Cys-loop receptors to enhance the probability of being open, rather than preferentially through “preferred” partner subunits.
Acknowledgments
We thank Chuck Zorumski and Steve Mennerick (Washington University School of Medicine) for providing X. laevis oocytes and Amanda Taylor for technical help with oocyte harvest.
This work was supported by the National Institutes of Health National Institute of General Medicine [Grant GM47969]. J.H.S. is the Russell and Mary Shelden Professor of Anesthesiology.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.111.071662.
- PCR
- polymerase chain reaction
- lmt
- low melting temperature
- PBS
- phosphate-buffered saline.
Authorship Contributions
Participated in research design: Bracamontes, Steinbach, and Akk.
Conducted experiments: Bracamontes, McCollum, Esch, Li, Ann, and Akk.
Performed data analysis: McCollum, Ann, and Akk.
Wrote or contributed to the writing of the manuscript: Steinbach and Akk.
References
- Akaike N, Maruyama T, Tokutomi N. (1987) Kinetic properties of the pentobarbitone-gated chloride current in frog sensory neurones. J Physiol 394:85–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Covey DF, Evers AS, Mennerick S, Zorumski CF, Steinbach JH. (2010) Kinetic and Structural Determinants for GABA-A Receptor Potentiation by Neuroactive Steroids. Curr Neuropharmacol 8:18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Li P, Bracamontes J, Reichert DE, Covey DF, Steinbach JH. (2008) Mutations of the GABA-A receptor α1 subunit M1 domain reveal unexpected complexity for modulation by neuroactive steroids. Mol Pharmacol 74:614–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Li P, Bracamontes J, Steinbach JH. (2009) Activation and modulation of concatemeric GABAA receptors expressed in human embryonic kidney cells. Mol Pharmacol 75:1400–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Li P, Bracamontes J, Wang M, Steinbach JH. (2011) Pharmacology of structural changes at the GABAA receptor transmitter binding site. Br J Pharmacol 162:840–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Shu HJ, Wang C, Steinbach JH, Zorumski CF, Covey DF, Mennerick S. (2005) Neurosteroid access to the GABAA receptor. J Neurosci 25:11605–11613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin J, Weiss DS. (1993) GABAA receptor needs two homologous domains of the β-subunit for activation by GABA but not by pentobarbital. Nature 366:565–569 [DOI] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. (2001) Subunit arrangement of γ-aminobutyric acid type A receptors. J Biol Chem 276:36275–36280 [DOI] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. (2002) Forced subunit assembly in α1β2γ2 GABAA receptors. Insight into the absolute arrangement. J Biol Chem 277:46020–46025 [DOI] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. (2003) Individual properties of the two functional agonist sites in GABA(A) receptors. J Neurosci 23:11158–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur R, Sigel E. (2005) Benzodiazepines affect channel opening of GABAA receptors induced by either agonist binding site. Mol Pharmacol 67:1005–1008 [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. (2003) Neurosteroids shift partial agonist activation of GABAA receptor channels from low- to high-efficacy gating patterns. J Neurosci 23:10934–10943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau AJ, Pearce RA, Czajkowski C. (2005) Tandem subunits effectively constrain GABAA receptor stoichiometry and recapitulate receptor kinetics but are insensitive to GABAA receptor-associated protein. J Neurosci 25:11219–11230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracamontes JR, Steinbach JH. (2009) Steroid interaction with a single potentiating site is sufficient to modulate GABAA receptor function. Mol Pharmacol 75:973–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis L, Buisson B, Bertrand S, Bertrand D. (2002) Potentiation of human α4β2 neuronal nicotinic acetylcholine receptor by estradiol. Mol Pharmacol 61:127–135 [DOI] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51–59 [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. (2006) Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature 444:486–489 [DOI] [PubMed] [Google Scholar]
- Jin X, Steinbach JH. (2011) A portable site: the binding for 17β-estradiol can be placed on any subunit of a nicotinic α4β2 receptor. J Neurosci 31:5045–5054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Trudell JR, Harrison NL. (2004) Structural elements involved in activation of the gamma-aminobutyric acid type A (GABAA) receptor. Biochem Soc Trans 32:540–546 [DOI] [PubMed] [Google Scholar]
- Li P, Bandyopadhyaya AK, Covey DF, Steinbach JH, Akk G. (2009) Hydrogen bonding between the 17β-substituent of a neurosteroid and the GABAA receptor is not obligatory for channel potentiation. Br J Pharmacol 158:1322–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Shu HJ, Wang C, Mennerick S, Zorumski CF, Covey DF, Steinbach JH, Akk G. (2007) Neurosteroid migration to intracellular compartments reduces steroid concentration in the membrane and diminishes GABAA receptor potentiation. J Physiol 584:789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. (1996) Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci 19:139–143 [DOI] [PubMed] [Google Scholar]
- Muroi Y, Theusch CM, Czajkowski C, Jackson MB. (2009) Distinct structural changes in the GABAA receptor elicited by pentobarbital and GABA. Biophys J 96:499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso K, Zhang J, Steinbach JH. (2001) The C terminus of the human nicotinic α4β2 receptor forms a binding site required for potentiation by an estrogenic steroid. J Neurosci 21:6561–6568 [DOI] [PMC free article] [PubMed] [Google Scholar]