Abstract
Allosteric agonists are powerful tools for exploring the pharmacology of closely related G protein-coupled receptors that have nonselective endogenous ligands, such as the short chain fatty acids at free fatty acid receptors 2 and 3 (FFA2/GPR43 and FFA3/GPR41, respectively). We explored the molecular mechanisms mediating the activity of 4-chloro-α-(1-methylethyl)-N-2-thiazolylbenzeneacetamide (4-CMTB), a recently described phenylacetamide allosteric agonist and allosteric modulator of endogenous ligand function at human FFA2, by combining our previous knowledge of the orthosteric binding site with targeted examination of 4-CMTB structure-activity relationships and mutagenesis and chimeric receptor generation. Here we show that 4-CMTB is a selective agonist for FFA2 that binds to a site distinct from the orthosteric site of the receptor. Ligand structure-activity relationship studies indicated that the N-thiazolyl amide is likely to provide hydrogen bond donor/acceptor interactions with the receptor. Substitution at Leu173 or the exchange of the entire extracellular loop 2 of FFA2 with that of FFA3 was sufficient to reduce or ablate, respectively, allosteric communication between the endogenous and allosteric agonists. Thus, we conclude that extracellular loop 2 of human FFA2 is required for transduction of cooperative signaling between the orthosteric and an as-yet-undefined allosteric binding site of the FFA2 receptor that is occupied by 4-CMTB.
Introduction
Many G protein-coupled receptors (GPCRs) share either the same endogenous ligand or respond to a group of ligands with overlapping selectivity, making efforts to ascribe individual biology particularly challenging. This is especially problematic when the “orthosteric” binding pocket for endogenous ligands is very highly conserved between receptor subtypes, thereby limiting selectivity at these GPCRs. Selectivity issues at related GPCRs have been circumvented by the use of “allosteric” ligands, which, as their name suggests, bind to a distinct or “other” site on the receptor (Christopoulos, 2002; Kenakin, 2009; Smith and Milligan, 2010; Smith et al., 2011). Thought to have been under less evolutionary pressure to remain conserved (Soudijn et al., 2004), allosteric binding sites provide a novel means of selectively regulating and therefore functionally characterizing related GPCRs.
In cases where selectivity has been achieved through allosteric binding sites [e.g., for the muscarinic acetylcholine receptors (Christopoulos and Kenakin, 2002; Eglen, 2005; Antony et al., 2009; Conn et al., 2009)], efforts have been aided by the vast array of molecular and pharmacological tools available to researchers. This is not the case, however, for many recently deorphanized GPCRs that are paired with endogenous ligands possessing only low or moderate potency for their cognate receptor. Because of their poor potency, radiolabeling these ligands to examine receptor binding has not been possible, and maxima of concentration-response curves are often not clearly defined within the concentration range practical to employ. Thus, identification of selective synthetic ligands at these receptors is imperative before characterization of receptor activation is possible.
Of a number of recently deorphanized GPCRs, a family of receptors attracting interest is the free fatty acid receptors (nomenclature as in Alexander et al., 2008), FFA1, FFA2, and FFA3 (Stoddart et al., 2008b; Milligan et al., 2009), historically named GPR40, GPR43, and GPR41, respectively (Brown et al., 2003; Kotarsky et al., 2003; Le Poul et al., 2003; Nilsson et al., 2003). In particular, FFA2 and FFA3 are both activated by short-chain fatty acids (SCFAs) of chain lengths C1 to C5, and we have recently demonstrated a high degree of similarity in the orthosteric binding pockets of these closely related receptors (Stoddart et al., 2008a). Although little is known about the physiological and pathological roles of these receptors, FFA2 knock-out mice have implicated the receptor in the regulation of inflammation in models of colitis, arthritis and asthma (Maslowski et al., 2009; Sina et al., 2009). Furthermore, colonic effects of SCFAs generated via fermentation processes may play a role in maintaining energy homeostasis, particularly via FFA2 (Sleeth et al., 2010).
Lee et al., (2008) have reported the phenylacetamide (S)-4-chloro-α-(1-methylethyl)-N-2-thiazolylbenzeneacetamide (S-4-CMTB) as the first selective ligand for FFA2. S-4-CMTB was shown to be an “ago-allosteric” modulator in that it was both a direct agonist at FFA2 and also a positive allosteric modulator of the actions of SCFAs at the receptor (Lee et al., 2008). Given the potential utility of a selective ligand at FFA2, herein we have examined the molecular determinants for allosterism and agonism of this and related compounds at FFA2. We report that allosteric communication between 4-CMTB and the SCFA propionate was dependent upon the nature of the second extracellular loop (ECL2), whereby replacement of this region of FFA2 with the equivalent region of FFA3 entirely eliminated allosteric communication at the receptor without limiting direct agonism by either ligand, and even mutation of the single amino acid Leu173 was sufficient to disrupt allosterism. Thus, ECL2 of FFA2 acts as a molecular switch to transmit conformational changes between orthosteric and allosteric binding sites of the FFA2 SCFA receptor.
Materials and Methods
Materials.
Tissue culture reagents were from Invitrogen (Paisley, Strathclyde, UK). Experimental reagents were from Sigma-Aldrich (Poole, Dorset, UK). Ligands HWD001–HWD009, HWD011–HWD013, (S)-4-CMTB and (R)-4-CMTB were prepared in our laboratories as described in Supplemental File 1. Commercially obtained ligands were from Enamine (Kiev, Ukraine) [HWD014–HWD018, (S)-HWD020, (R)-HWD020] and Pharmeks (Moscow, Russia) (HWD019). Absence of major secondary products and purity (83–99%) of purchased ligands was confirmed by high-performance liquid chromatography-photodiode array, high-performance liquid chromatography-mass spectroscopy (electrospray ionization) and 1H-NMR spectroscopy by Laia Miret Casals and Fernando Albericio (Universitat de Barcelona, Barcelona, Spain). The radiochemical [35S]GTPγS was from PerkinElmer Life and Analytical Sciences (Beaconsfield, Buckinghamshire, UK).
Site-Directed Mutagenesis and Generation of ECL2 Swap.
Human (h)FFA2 or FFA3 was fused via the C terminus to enhanced yellow fluorescent protein (eYFP) and subcloned into pcDNA5/FRT/TO plasmid (Invitrogen), as described previously (Stoddart et al., 2008a). Primers for polymerase chain reaction of FFA2 (FFA3 ECL2)-eYFP chimeric receptor were designed around the conserved regions within transmembrane domains 4 and 5 (see Fig. 8). Receptor cDNA was amplified using primers annealing at the N-terminal and conserved region for one receptor and the conserved region and C-terminal for the other. These fragments were then combined in a single polymerase chain reaction where they were allowed to anneal at the conserved region and act as “primer-templates” to synthesize the complete chimera. This was then amplified using the N-terminal primer from the first receptor and the C-terminal primer from the second. Restriction sites built into the primers were used to subclone the chimera into pcDNA5/FRT/TO with eYFP as described above.
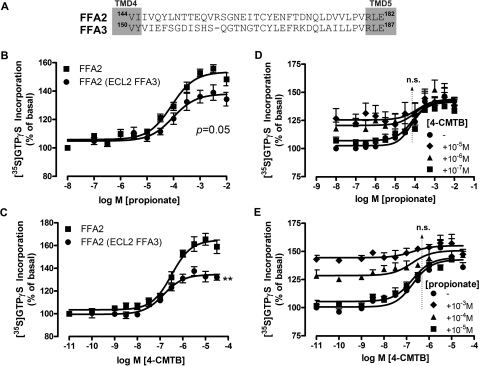
Fig. 8.
4-CMTB requires the second extracellular loop of hFFA2 for allosteric modulation of propionate. The 2nd extracellular loop of hFFA2 (Val144–Glu182) was replaced with ECL2 from the closely related hFFA3 receptor (Val150–Glu187) to generate an hFFA2(ECL2 FFA3)-eYFP chimeric protein and stable Flp-In TREx 293 cells generated as before. A, extracellular loop 2 alignment between hFFA2 and hFFA3 using ClustalX (accession numbers: hFFA2, NP005297; hFFA3, NP005295). B, propionate activates hFFA2(ECL2 FFA3)-eYFP with equivalent potency to hFFA2-eYFP WT (pEC50 = 3.96 ± 0.09 and 4.03 ± 0.14, respectively; n = 4) but with marginally reduced efficacy (p = 0.0501 according to an unpaired t test). C, 4-CMTB has significantly reduced efficacy at hFFA2(ECL2 FFA3) compared with hFFA2-eYFP WT (p < 0.001 according to an unpaired t test). Potency is equivalent between WT and chimeric receptors according to a t test (pEC50 = 6.50 ± 0.15 and 6.75 ± 0.11, respectively; n = 4). D, allosterism between 4-CMTB and propionate is lost at the hFFA2(ECL2 FFA3)-eYFP chimera. The arrow indicates potency; n.s. represents no significant difference in potency values according to one-way ANOVA (n = 3). E, reciprocal loss of allosterism at hFFA2(ECL2 FFA3)-eYFP according to one-way ANOVA and indicated by the arrow (n = 3).
Cell Culture and Generation of Stable Flp-In T-REx 293 Cells.
Cells were maintained in Dulbecco's modified Eagle's medium without sodium pyruvate (Invitrogen) supplemented with 10% (v/v) dialyzed fetal bovine serum, 1% penicillin/streptomycin mixture, and 10 μg/ml blasticidin at 37°C in a humidified atmosphere of air/CO2 (19:1). Inducible Flp-In T-REx 293 cells were generated for each of hFFA2-eYFP (Stoddart et al., 2008a), and the various receptor mutants and hFFA3-eYFP, as described previously (Stoddart et al., 2008a; Smith et al., 2009). Antibiotic-resistant clones were screened for receptor expression by fluorescence imaging and eYFP measurement in membranes using a PHERAStar FS (BMG Labtech, UK). Cells were treated with 0.5 μg/ml doxycycline 24 h before harvesting or imaging to induce receptor expression.
[35S]GTPγS Incorporation Assays.
Membranes were prepared from induced stable cell lines as described elsewhere (Stoddart et al., 2007). [35S]GTPγS binding experiments were performed in duplicate according to the method of Liu et al. (2009). In brief, 5 μg of cell membranes were added to assay buffer (50 mM Tris-HCl, pH 7.4, 10 mM MgCl2, 100 mM NaCl, 1 mM EDTA, 1 μM GDP, and 0.5% fatty acid-free bovine serum albumin) containing the indicated concentrations of ligands and preincubated for 15 min at 25°C. To initiate the assay, 50 nCi of [35S]GTPγS was added to each tube, and the reaction was terminated by rapid filtration through GF/C glass filters using a 24-well Brandel cell harvester (Alpha Biotech, Glasgow, UK) after 1-h incubation. Unbound radioligand was washed from filters by three washes with ice-cold wash buffer (50 mM Tris-HCl, pH 7.4, and 10 mM MgCl2), and [35S]GTPγS binding was determined by liquid scintillation spectrometry. Mutant FFA2 data were normalized to eYFP fluorescence to account for differences in receptor expression.
Data Analysis.
All data were quantified, grouped, and analyzed using Prism 5.02 (GraphPad Software, San Diego, CA) and are expressed as mean ± S.E.M. Data were fit to both three-parameter (fixed Hill slope) and four-parameter nonlinear regression isotherms, and in all cases, the three-parameter curve was statistically appropriate. Experimental data from [35S]GTPγS binding studies investigating the interaction of 4-CMTB with propionate in wild-type and mutant FFA2 receptors were analyzed according to the operational model of allosteric modulation according to Keov et al., (2011) using the equation
in which E indicates the effect and A and B denote the orthosteric and allosteric ligands, respectively. KA, and KB are the corresponding equilibrium dissociation constants of ligand binding to otherwise unliganded receptors. The cooperativity factor α denotes the allosteric modulation of binding affinity, whereas the empirical parameter β quantifies the allosteric modulation of orthosteric ligand efficacy; its values may range between zero and infinity, and it describes the extent to which the allosteric agent changes the efficacy of the orthosteric agonist on the ARB (agonist/receptor/modulator) ternary complex. The ability of the orthosteric and allosteric ligands to favor receptor activation is described by the parameters τA and τB, respectively; furthermore, they incorporate the intrinsic efficacy of each ligand, the total density of receptors, and the efficiency of stimulus-response coupling. Em denotes the maximal possible system response, and n indicates the slope factor of the transducer function by which occupancy is linked to response. Limitations of the operational model of allosteric modulation are related to the correlation between parameters and the dependence of some of these parameters on system properties (Keov et al., 2011). Therefore, in the current study, its main use was the determination of semiquantitative estimates of modulator affinity and overall cooperativity (αβ).
Global nonlinear curve fitting of E as the dependent variable with A and B as the independent variables yielded estimates for KA, KB, α, β, τA, and τB, except for the data shown in Figs. 7B and 8D, in which KA and KB were constrained to numerical estimates that had been obtained from the corresponding global fits of the data sets illustrated in Figs. 7C and 8E, respectively, because they were ambiguously resolved if left unconstrained. The results of global nonlinear curve fitting were compared between reciprocally performed sets of curves by subjecting the numerical values of selected parameters to a t test; p < 0.05 was considered as the level of significance. All other statistical analyses were performed as detailed in the text.
Fig. 7.
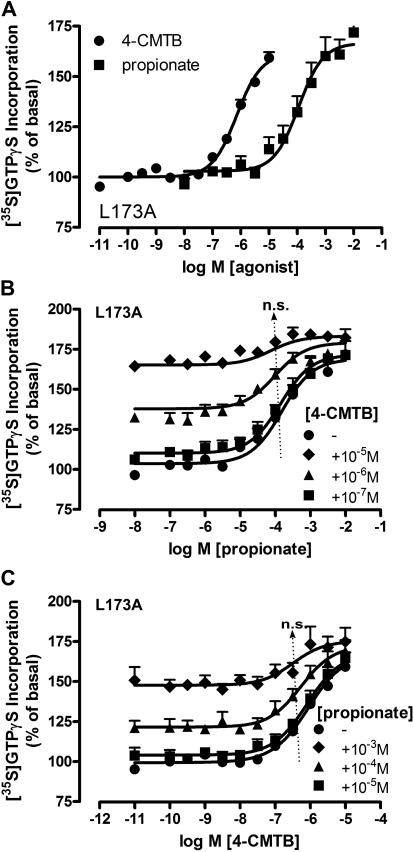
Leu173 in hFFA2-eYFP is required for 4-CMTB allosterism but not agonism. A Leu173Ala mutation was introduced into the 2nd extracellular loop of hFFA2-eYFP, and this inducible construct was expressed stably in Flp-In TREx 293 cells. A, [35S]GTPγS incorporation in response to either propionate (pEC50 = 3.95 ± 0.15) or 4-CMTB (pEC50 = 6.14 ± 0.06) is unaffected by L173A mutation within hFFA2-eYFP. B, coincubation of L173A hFFA2-eYFP with fixed concentrations of 4-CMTB and varying amounts of propionate no longer resulted in a significant shift in the potency of propionate, indicated by the arrow. n.s., not significant according to one-way ANOVA. C, the loss of allosterism was reciprocal, with no significant difference in pEC50 range indicated by the arrow. n.s., not significant according to one-way ANOVA. Data are mean ± S.E.M (n = 3) in each panel.
Results
4-CMTB Is a Selective Ago-Allosteric Modulator at FFA2.
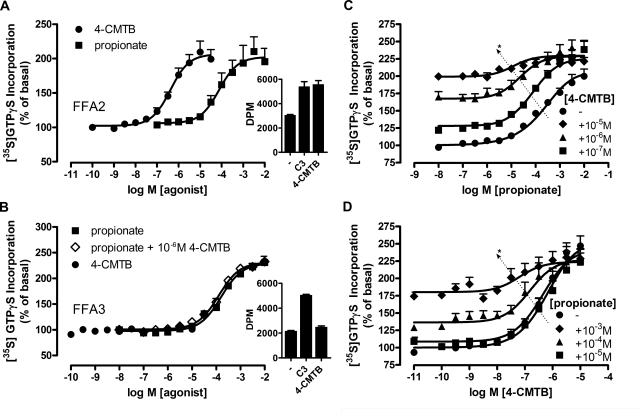
The phenylacetamide S-4-CMTB has previously been described as an ago-allosteric ligand at FFA2, acting as both a direct agonist and a positive allosteric modulator of the action of SCFAs that are the endogenous activators of this receptor (Lee et al., 2008). To confirm these findings, we employed a filtration-based [35S]GTPγS binding assay to examine the ability of racemic 4-CMTB to activate members of the pertussis toxin-sensitive Gαi/o family of G proteins, because we and others have demonstrated previously that FFA2 is both a Gαi/o and Gαq-coupled GPCR (Kimura et al., 2001; Brown et al., 2003; Le Poul et al., 2003; Stoddart et al., 2008a). In membranes from Flp-In T-REx 293 cells induced to express wild-type hFFA2 linked to eYFP (hFFA2-eYFP)(Stoddart et al., 2008a), 4-CMTB was a relatively potent agonist (pEC50 = 6.38 ± 0.12, mean ± S.E.M.) that produced maximal responses similar to those of the endogenous, orthosteric FFA2 agonist propionate (pEC50 = 4.12 ± 0.22) for stimulating [35S]GTPγS binding and, therefore, G protein activation (Fig. 1A). 4-CMTB seemed to be specific for hFFA2, because it failed to stimulate [35S]GTPγS binding via the closely related Gαi/o-coupled hFFA3 receptor (Fig. 1B).
Fig. 1.
4-CMTB is a selective ago-allosteric modulator at FFA2. Flp-In TREx 293 cells stably expressing inducible hFFA2-eYFP (A, C, and D) or hFFA3-eYFP (B) were induced with 0.5 μg/ml doxycycline for 24 h before harvesting and membrane preparation for subsequent [35S]GTPγS incorporation assays. A, both 4-CMTB and propionate are full agonists in this assay. Inset, representative example of absolute values [disintegrations per minute (DPM)] for basal (−) and propionate (C3; 10 mM) and 4-CMTB (10 μM) from a single hFFA2-eYFP membrane preparation. B, 4-CMTB is not an agonist, antagonist, or allosteric modulator of propionate at the closely related hFFA3-eYFP receptor. Inset, representative experiment for hFFA3-eYFP, as per A inset. C, 4-CMTB and propionate interact allosterically, as increasing fixed concentrations of 4-CMTB result in enhanced potency of propionate. The diagonal arrow indicates the shift in potency. *, p < 0.05 according to one-way ANOVA with Dunnett's post hoc analysis. D, the allosteric relationship is reciprocal. Again, the diagonal arrow indicates the shift in potency. *, p < 0.05 according to one-way ANOVA with Dunnett's post hoc test. Data are mean ± S.E.M (n = 3).
A hallmark of allosterism is the ability of a modulator to alter the potency and/or efficacy of an orthosteric ligand, and such effects should occur in a reciprocal fashion (Christopoulos and Kenakin, 2002; Smith et al., 2011). 4-CMTB was also a positive allosteric modulator of the effects of propionate at hFFA2 (Fig. 1C). The potency of propionate was increased in the presence of increasing concentrations of 4-CMTB (pEC50 range, 3.90 ± 0.09 in the absence of 4-CMTB to 5.16 ± 0.33 in the presence of 10−5 M 4-CMTB, p < 0.05 according to one-way ANOVA). It is noteworthy that this effect was reciprocal, because the potency of 4-CMTB was also increased in the presence of increasing concentrations of propionate (Fig. 1D; pEC50 range: 6.31 ± 0.08 in the absence of propionate to 7.20 ± 0.31 in the presence of 10−3 M propionate, p < 0.05 according to one-way ANOVA). Global analyses of the data were performed using the operational model of allosteric modulation as described previously (Keov et al., 2011), which led to the estimation of strongly positive overall cooperativity (αβ) of 4-CMTB and propionate at wild-type FFA2. Such analyses also yielded estimates of the affinity of propionate (pKA = 3.20 ± 0.29) and 4-CMTB (pKB = 5.26 ± 0.43). Furthermore, the αβ values obtained for curves in Fig. 1, C and D were not significantly different from each other, supporting reciprocity of effect (average αβ = 194). 4-CMTB was also selective for hFFA2 with respect to allosterism, in that it was not able to positively or negatively allosterically modulate the effect of propionate at hFFA3-eYFP (Fig. 1B).
4-CMTB Is a Partial Agonist at ERK1/2 and Is Specific for FFA2.
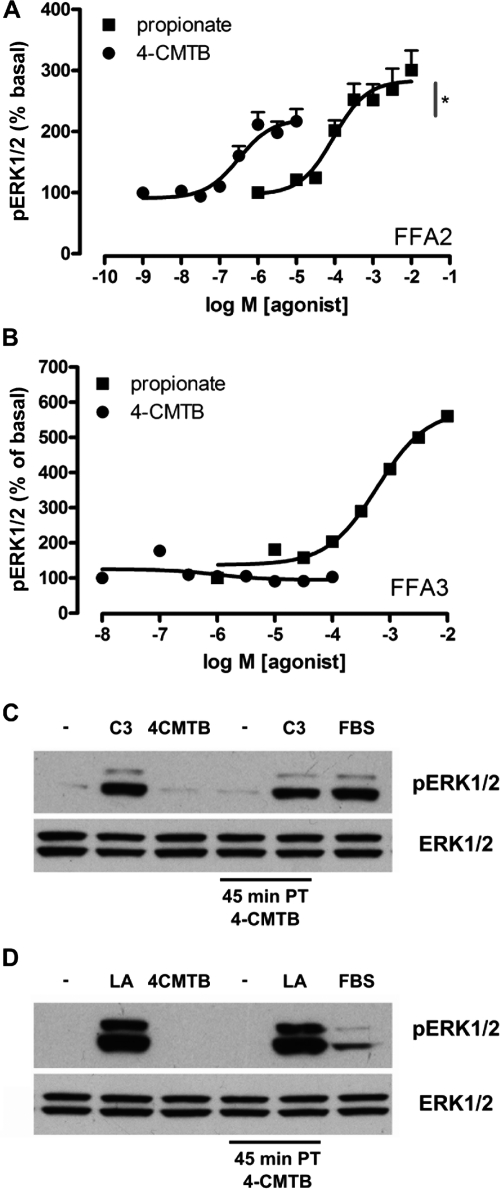
We also examined the actions of 4-CMTB at the mitogen-activated protein kinase ERK1/2 pathway and found it to be a reasonably potent (pEC50 = 6.59 ± 0.23) but partial (p < 0.05) agonist with respect to propionate (pEC50 = 4.03 ± 0.21) (Fig. 2A). As for the [35S]GTPγS assay, 4-CMTB was neither an agonist nor an antagonist at hFFA3-eYFP or hFFA1-eYFP (Fig. 2, B and C) when ERK1/2 phosphorylation was recorded as the signal, further supporting 4-CMTB selectivity.
Fig. 2.
4-CMTB is a partial agonist at ERK1/2 and is selective for FFA2 compared with FFA3 and FFA1. A, Flp-In TREx 293 cells stably expressing FFA2-eYFP were stimulated for 10 min with varying concentrations of propionate (C3) or 4-CMTB and processed for ERK1/2 activation using the AlphaScreen SureFire ERK1/2 assay (PerkinElmer Life and Analytical Sciences). *, p < 0.05 for 4-CMTB Emax being less than that for propionate according to Student's t test. Data are mean ± S.E.M (n = 3). B, equivalent experiments were performed using hFFA3-eYFP cells (n = 3), where only propionate was an agonist in the ERK1/2 pathway. C, Western blots of hFFA3-eYFP cell lysates treated as for B. Fetal bovine serum [10% (v/v)] was included as a positive control for ERK1/2 activation. D, Western blot as described for C, except that cells expressing FFA1-eYFP were stimulated with 100 μM lauric acid. PT, pretreatment.
4-CMTB Does Not Bind within the Orthosteric Binding Site of hFFA2.
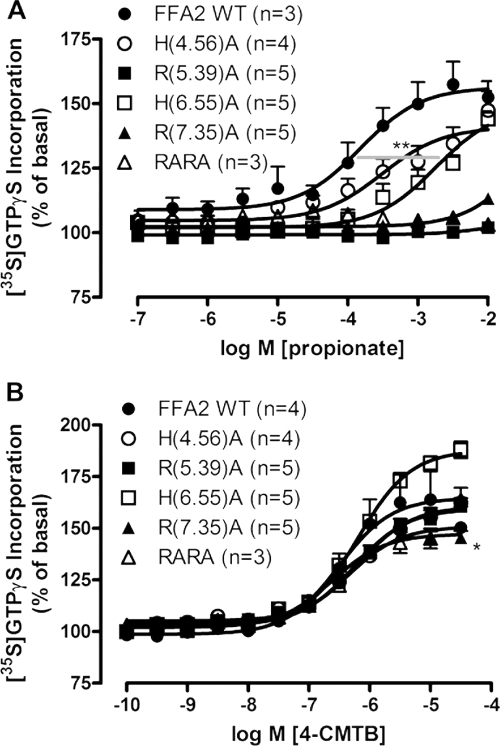
Allosteric modulators, by definition, bind at a site(s) distinct from orthosteric ligands. We have previously used site-directed mutagenesis, in concert with SCFA-mediated phosphorylation of ERK1/2 and the elevation of [Ca2+]i, to define critical orthosteric residues of hFFA2 (Stoddart et al., 2008a). Herein, membranes produced from Flp-In T-REx 293 cells induced to express R(5.39)A (residue 180) or R(7.35)A (residue 255) hFFA2-eYFP [numbered according to the system of Ballesteros and Weinstein (1995)] did not promote [35S]GTPγS binding in response to propionate, presumably because the arginines act to coordinate the carboxylate head group of the SCFA, as they do for FFA1 and FFA3 (Sum et al., 2007; Smith et al., 2009). For H(4.56)A (residue 140) there was a trend toward reduced potency, whereas for H(6.55)A (residue 242) hFFA2-eYFP, propionate potency was reduced (p < 0.01 according to a one-way ANOVA), although function was not eliminated (Fig. 3A). In contrast, 4-CMTB stimulated [35S]GTPγS binding, in each case with unaltered potency (p > 0.05) and with limited effects on maximal signal (Fig. 3B), and this was also the case in membranes containing a double R(5.39)A + R(7.35)A mutation of hFFA2-eYFP (Fig. 3B), indicating that these key residues of the orthosteric binding pocket of hFFA2 are not involved directly in 4-CMTB binding and receptor activation. It is noteworthy that radioligands are still not available for FFA2; thus, intensity of eYFP was measured as a surrogate for hFFA2 expression, even though this approach cannot clearly define cell-surface expression (Table 1). Although we found that expression was lower than wild type for each of these five mutants, in no case was the difference greater than 2-fold.
Fig. 3.
4-CMTB does not require a functional orthosteric ligand binding site for signaling. Flp-In TREx 293 cells stably expressing inducible wild-type or orthosteric binding site mutants of hFFA2-eYFP (Stoddart et al., 2008a) were induced with 0.5 μg/ml doxycycline for 24 h before harvesting and membrane preparation for subsequent [35S]GTPγS incorporation assays. A, mutation of either R(5.39)A, R(7.35)A, or both residues (RARA) completely abolishes propionate activity, whereas propionate potency is markedly impaired at the H(6.55)A mutation. **, p < 0.01 according to a one-way ANOVA with Dunnett's post hoc test. B, 4-CMTB potency is unaltered at any of the orthosteric binding site mutants of hFFA2-eYFP. Efficacy was only significantly impaired for R(7.35)A. *, p < 0.05 according to one-way ANOVA with Dunnett's post hoc analysis. Data are mean ± S.E.M with experiment repeats indicated in parentheses.
TABLE 1.
FFA2 mutant receptor expression per 5 μg of protein as determined by eYFP fluorescence
Values are presented as mean ± S.E.M.
| FFA2 Receptor | eYFP | n |
|---|---|---|
| RLU | ||
| HEK293 no receptor | 556 ± 25.9 | |
| Wild type | 4193 ± 405 | 4 |
| H140A (4.56) | 2110 ± 320 | 4 |
| L173A (ECL2) | 2041 ± 431 | 3 |
| R180A (5.39) | 1798 ± 187 | 6 |
| H242A (6.55) | 2655 ± 101 | 5 |
| R255A (7.35) | 3024 ± 201 | 5 |
| R180A/R255A (5.39/7.35) | 2072 ± 101 | 3 |
| FFA2 (ECL2 FFA3) | 1886 ± 112 | 3 |
RLU, relative light unit; HEK, human embryonic kidney.
4-CMTB Structure-Activity Relationships at FFA2.
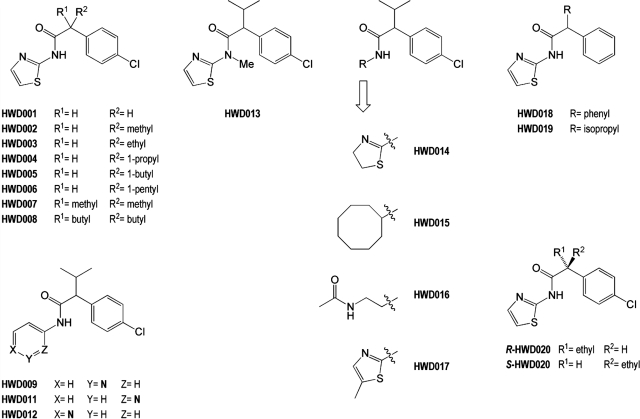
To elucidate the structural features of 4-CMTB that contribute to its binding and activity, we undertook a concise structure-activity relationship (SAR) survey with a series of 4-CMTB analogs (Fig. 4) using hFFA2-eYFP in the [35S]GTPγS assay. The [35S]GTPγS assay was deemed ideal for our SAR studies because it is the most receptor-proximal assay available, and we found propionate and 4-CMTB to have equal efficacy in this assay (unlike for ERK1/2 in Fig. 2A and [Ca2+]I; data not shown). For our targeted SAR, we focused primarily on isopropyl replacement variants (compounds HWD001–HWD008, HWD018, and HWD020) and amide N-substituent variants (HWD009, HWD011–HWD017).
Fig. 4.
Analogs used for 4-CMTB SAR survey.
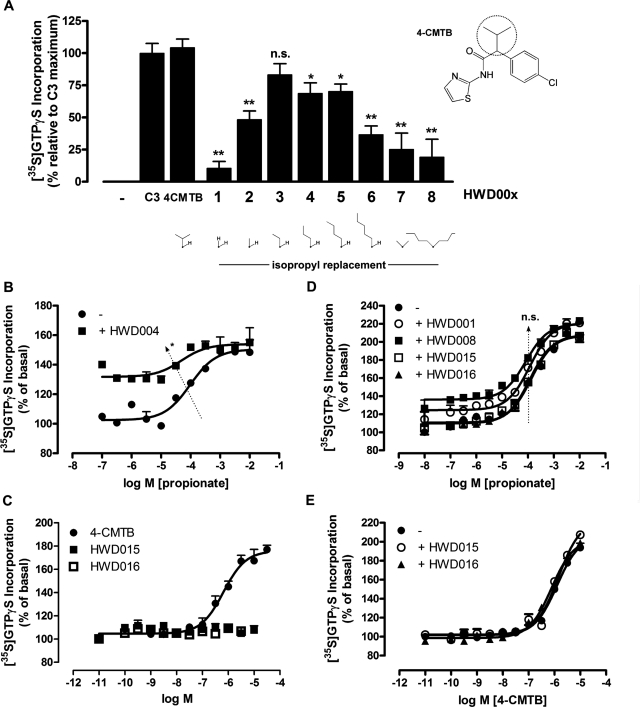
Isopropyl replacement variants displayed a chain length dependence for stimulating [35S]GTPγS binding at a maximally effective concentration (10−5 M) (Fig. 5A). The greatest maximal effect with n-alkyl chains from C1 to C5 was seen with ethyl (HWD003), a chain length equivalent to the isopropyl group of the parent ligand, although all of the compounds exhibited enhanced activity over the α-unsubstituted analog (HWD001). Disubstitution at the α-carbon, whether by methyl or bulkier butyl groups, compromised activity (HWD007 and HWD008). Although none of the ligands displayed enhanced (or even equivalent) potency compared with the parent 4-CMTB, potency values were generally within a log unit of 4-CMTB (Table 2). A compound (HWD018) with a phenyl replacement for the 4-CMTB isopropyl group was commercially available but lacked the parent ligand's chlorine atom. We therefore extended the SAR survey to include this compound and 3-methyl-2-phenyl-N-(thiazol-2-yl)butanamide (HWD019), also commercially available, to allow incremental analysis of the effects of removing the 4-CMTB chlorine and replacing the isopropyl group by phenyl. HWD018 was active as an agonist at FFA2, albeit with significantly reduced potency compared with 4-CMTB (Table 2). This reduced potency and efficacy is directly attributable to the phenyl replacement of the isopropyl group rather than removal of the chlorine, however, because HWD019 largely retained the efficacy and potency of 4-CMTB (Table 2). As 4-CMTB is an ago-allosteric modulator of hFFA2 (Fig. 1), we also examined whether the relatively conservative replacement of the isopropyl group by butyl in HWD004 affected allosterism. As shown in Fig. 5B, addition of a fixed concentration of HWD004 also produced a significant increase in potency of propionate, consistent with positive allosteric modulation. Thus, a single moderately bulky alkyl group at the α-carbon favors agonist activity, with optimal activity conferred on 4-CMTB itself by the β-branched isopropyl group. An ethyl replacement had little impact on efficacy but caused a 6-fold reduction in potency, whereas longer/larger groups and smaller groups compromise both potency and efficacy. The allosteric action of the ligands seems to be tolerant of isopropyl replacement, at least in the case of a butyl group.
Fig. 5.
Function of selected 4-CMTB SAR ligands at hFFA2-eYFP. A, 4-CMTB isopropyl replacement variants (replacement area on 4-CMTB indicated on the structure by a circle) in the [35S]GTPγS incorporation assay demonstrate chain length and bulk dependence for maximal efficacy. Significant differences from 4-CMTB maximum are indicated. *, p < 0.05, **, p < 0.01; n.s., not significant, according to one-way ANOVA with Dunnett's post hoc analysis (n = 4). B, HWD004 is also a positive allosteric modulator at 10 μM in the presence of increasing propionate concentrations. Arrow indicates shift in potency. *, p < 0.05 according to Student's t test. C, not all 4-CMTB analogs displayed agonist activity at hFFA2-eYFP. D, inactive (HWD015 and HWD016) or weakly efficacious (HWD001, HWD008) ligands were tested for allosterism (10 μM fixed concentration of HWD ligands) but failed to alter the potency of propionate. n.s., no significant difference in potencies according to one-way ANOVA. E, neither HWD015 nor HWD016 could compete at 10 μM with 4-CMTB at hFFA2-eYFP. Data represent mean ± S.E.M. of three independent experiments.
TABLE 2.
Structure-activity relationship of 4-CMTB-based ligands at [35S]GTPγS assay of G protein activation
Values are presented as mean ± S.E.M. Emax is expressed as a percentage of propionate signal. Inactive is defined as <10% propionate maximum signal at the highest concentration tested (10 μM).
| Ligand | FFA2-eYFP |
n | |
|---|---|---|---|
| pEC50 | Emax | ||
| % | |||
| Propionate | 4.39 ± 0.26 | 100.0 ± 4.0 | 3 |
| 4-CMTB | 6.18 ± 0.18 | 105.2 ± 3.8 | 3 |
| S-4-CMTB | 6.52 ± 0.12 | 118.9 ± 5.2 | 3 |
| R-4-CMTB | 5.74 ± 0.09 | 77.7 ± 4.0 | 3 |
| HWD001 | 5.24 ± 0.56 | 69.6 ± 3.0 | 3 |
| HWD002 | 5.50 ± 0.30 | 81.3 ± 3.5 | 3 |
| HWD003 | 5.38 ± 0.13 | 103.4 ± 3.5 | 3 |
| HWD004 | 5.64 ± 0.17 | 90.0 ± 2.6 | 3 |
| HWD005 | 5.50 ± 0.19 | 92.6 ± 3.3 | 3 |
| HWD006 | 5.67 ± 0.38 | 80.1 ± 4.4 | 3 |
| HWD007 | 4.99 ± 0.25 | 88.8 ± 6.0 | 3 |
| HWD008 | 5.04 ± 0.48 | 80.9 ± 9.0 | 3 |
| HWD009 | 5.05 ± 0.30 | 37.1 ± 7.3 | 3 |
| HWD011 | 6.58 ± 0.11 | 128.7 ± 6.4 | 3 |
| HWD012 | Inactive | Inactive | 3 |
| HWD013 | Inactive | Inactive | 3 |
| HWD014 | 5.39 ± 0.26 | 90.0 ± 4.4 | 3 |
| HWD015 | Inactive | Inactive | 3 |
| HWD016 | Inactive | Inactive | 3 |
| HWD017 | 6.11 ± 0.20 | 100.7 ± 3.2 | 3 |
| HWD018 | 4.72 ± 0.33 | 77.5 ± 6.6 | 3 |
| HWD019 | 5.84 ± 0.25 | 96.6 ± 3.4 | 3 |
| S-HWD020 | 5.27 ± 0.16 | 95.2 ± 13.1 | 3 |
| R-HWD020 | Inactive | Inactive | 3 |
The effect of amide N-substituent alteration was more variable. Introduction of a methyl group at the 5-position of the thiazole (HWD017) or replacement of the thiazole by 4,5-dihydrothiazole (HWD014) were well tolerated (Table 2), the latter substitution indicating that a (hetero)aromatic N-substituent is not absolutely required for activity. In contrast, more radical changes to the amide N-substituent profoundly influenced activity, and replacement of the thiazolyl ring by cyclooctyl (HWD015) or acetylaminoethyl (HWD016) completely abolished agonism (Fig. 5C). Because the N-thiazolyl amide might provide key hydrogen bonding features in binding to FFA2, we examined the impact of N-methylation on 4-CMTB and replacement of the thiazole by pyridyl groups. Significantly, N-methylation (HWD013) ablated activity, potentially consistent with the amide NH binding to FFA2 as a hydrogen bond donor (HDON). The analog (HWD011) with an N-(2-pyridyl)- group, a thiazole replacement of slightly increased size but preserving the endocyclic nitrogen as a potential hydrogen bond acceptor (HACC) site adjacent to the amide NH, possessed activity similar to that of 4-CMTB itself. In contrast, the isosteric N-(3-pyridyl)- and N-(4-pyridyl)-substituted ligands (HWD009 and HWD012), in which the endocyclic nitrogen is moved progressively around the ring, showed substantially reduced activity (HWD009) or no activity (HWD012) (Table 2). These results are consistent with a hydrogen bond acceptor role for the endocyclic nitrogen of 4-CMTB that is maintained in HWD011. In principle, this type of HACC/HDON combination might form a complementary binding motif for amide functionality in the protein (i.e., Asn/Gln side chains or the protein backbone, as seen in cocrystal structures of other proteins with N-(thiazol-2-yl)amide-containing ligands (Jadhav et al., 1997; Kamata et al., 2004)).
We next examined HWD015, HWD016, and the lower efficacy isopropyl replacement analogs HWD001 and HWD008 for evidence of allosteric activity in the presence of propionate (Fig. 5D). Consistent with the previous findings, single concentrations (10 μM) of HWD015 and HWD016 displayed no direct agonism, whereas HWD001 and HWD008 caused small increases in G protein activation. However, none of these compounds altered propionate potency at hFFA2-eYFP, indicating either an absence of allosterism or, as is most likely to be the case for the low efficacy compounds HWD001 and HWD008, an effect on propionate binding and function that is beyond the sensitivity of this assay. Finally, to determine whether HWD015 and HWD016 were in fact binding to hFFA2 and acting therefore as simple antagonists of 4-CMTB, each of the ligands was coincubated with increasing concentrations of 4-CMTB. As shown in Fig. 5E, neither amide N-substituent variant was able to modulate 4-CMTB agonism, indicating that these ligands are not able to bind to the receptor with significant affinity. As anticipated, counterscreening of the SAR ligands at hFFA3-eYFP revealed all but one compound to be inactive; only HWD017 was able to partially stimulate [35S]GTPγS at the very highest concentration used (3 × 10−5 M) (not shown).
The S-Isomer Is Required for Maximal Biological Activity of 4-CMTB.
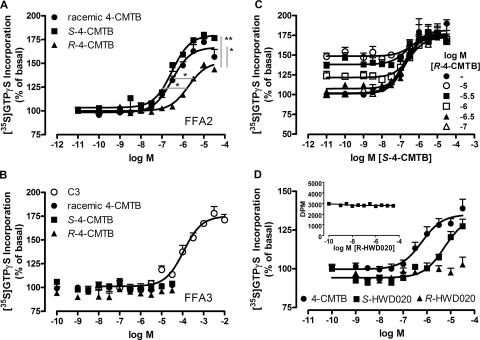
In the study that identified 4-CMTB as an agonist at FFA2, the authors described only the use of the S-stereoisomer of 4-CMTB (Lee et al., 2008). To further characterize 4-CMTB structure and function, and in light of the reasonable tolerance of racemic mixes of isopropyl group substitutions described in Fig. 5, we prepared both R- and S-4-CMTB and examined their activity alone and in combination at hFFA2-eYFP. S-4-CMTB was slightly more potent and efficacious at hFFA2 than the racemate (Fig. 6A) and produced maximal responses similar to those of the orthosteric agonist propionate. It is noteworthy that R-4-CMTB was also able to stimulate G protein activity with greater potency than propionate but significantly lower efficacy than either S- and racemic 4-CMTB or propionate. No signal was observed for racemic, S-, or R-4-CMTB at FFA3 (Fig. 6B), indicating that the stereochemistry of 4-CMTB was not responsible for differences in selectivity at hFFA2 versus hFFA3. Attempts to perform Schild analysis to determine the apparent affinity of 4-CMTB for hFFA2 using the stereoisomers were unsuccessful, because we were unable to add sufficient R-4-CMTB to compete with S-4-CMTB (Fig. 6C). Finally, given that an ethyl replacement for the bulkier isopropyl group in racemic 4-CMTB retained agonist efficacy in HWD003, we examined separate enantiomers for an α-ethyl-substituted compound. Conveniently, both antipodes of the unchlorinated α-ethyl analog HWD020 were commercially available. Only S-HWD020 promoted [35S]GTPγS binding within the concentration range that could be employed (Fig. 6D) and this was not inhibited by increasing concentrations of R-HWD020 (Fig. 6D, inset) indicating that only S-HWD020 is able to bind to the receptor within concentration ranges practical to test. Thus, although enantiopure compounds are not critical for function, 4-CMTB-related ligands with an S-configured stereogenic center at the α-position are the preferred agonists for G protein activation.
Fig. 6.
S-4-CMTB is more potent than R-4-CMTB at hFFA2-eYFP. A, racemic 4-CMTB and individual stereoisomers were examined for their ability to promote [35S]GTPγS incorporation. R-4-CMTB was significantly less potent and efficacious than both racemic 4-CMTB and S-4-CMTB, according to one-way ANOVA with Dunnett's post hoc analysis. *, p < 0.05; **, p < 0.01. B, 4-CMTB stereoisomers are not active at the closely related hFFA3-eYFP. C, R-4-CMTB and S-4-CMTB are not additive when coincubated, suggesting binding to the same site on hFFA2-eYFP. D, only the S-isomer of the structurally related ligand HWD020 is able to activate hFFA2-eYFP. Inset, R-HWD020 is unable to inhibit signaling in response to 30 μM S-HWD020. Data are mean ± S.E.M (n = 3) for all panels.
Allosterism Is Impaired at Leu173Ala hFFA2-eYFP.
One of the key residues suggested by Lee et al. (2008) to contribute to recognition of 4-CMTB by FFA2 was Leu173, located in ECL2 of hFFA2. Their preferred homology model predicted an H-bond interaction between Arg255(7.35) and the carbonyl backbone of Leu173. Given this link between the possible site of binding of the orthosteric and allosteric ligands and the requirement that there must be communication between such sites to produce reciprocal modulation of ligand function, we explored allosterism at a Leu173Ala mutant of hFFA2-eYFP. Both propionate and 4-CMTB were, on their own, effective agonists at Leu173Ala-hFFA2-eYFP with potencies similar to that of wild-type hFFA2-eYFP (Fig. 7A). Global analysis of allosteric interactions (Keov et al., 2011) in Fig. 7, B and C, as per Fig. 1, revealed that overall allosterism was both reciprocal and significantly reduced for this mutant (Table 3) compared with wild-type FFA2 (average αβ = 21, p < 0.01 according to one-way ANOVA with Newman-Keuls multiple comparison test). Thus, the overall structure of FFA2 ECL2 was considered for involvement in the transmission of allosterism between propionate and 4-CMTB.
TABLE 3.
Parameters obtained from the operational model of allosteric modulation, where A is propionate (fixed concentrations) and B is 4-CMTB (variable concentrations)
| FFA2 WT |
FFA2 L173A |
FFA2 (ECL2 FFA3) |
||||
|---|---|---|---|---|---|---|
| Mean | S.E.M. | Mean | S.E.M. | Mean | S.E.M. | |
| logKA | −3.20 | 0.29 | −3.13 | 0.30 | −3.82 | 0.27 |
| logKB | −5.26 | 0.43 | −5.27 | 0.37 | −6.43 | 0.26 |
| logα | 1.88 | 0.59 | 0.82 | 0.56 | 0.13 | 0.42 |
| logβ | 0.31 | 0.11 | 0.66 | 0.21 | 0.23 | 0.11 |
| logαβ | 2.19 | 0.71 | 1.48 | 0.78 | 0.36 | 0.53 |
| αβ | 155 | 30* | 2.3** | |||
P < 0.05 according to one-way ANOVA with Newman-Keuls multiple comparisons post hoc analysis.
P < 0.01 according to one-way ANOVA with Newman-Keuls multiple comparisons post hoc analysis.
Allosterism, but Not Agonism, Is Abolished by an ECL2 Swap Between hFFA2 and hFFA3.
Given the appreciated difficulties in trying to predict specific ECL conformations (Peeters et al., 2011), we decided to further explore the role of ECL2 in 4-CMTB allosterism and agonism by taking advantage of the observation that 4-CMTB selectively activates hFFA2 but not the closely related hFFA3 receptor (as demonstrated in Fig. 1). Thus, we generated a hFFA2(ECL2 hFFA3)-eYFP chimera in which ECL2 of hFFA3 replaced the equivalent sequence of hFFA2 (Fig. 8A). Again, a stable Flp-In T-REx 293 cell line able to induce expression of this construct was generated and tested initially for signaling to propionate as a measure of correct folding of the receptor (Fig. 8B). It is noteworthy that hFFA2(ECL2 hFFA3)-eYFP retained propionate responsiveness. Furthermore, 4-CMTB potency was equivalent to that of wild-type hFFA2-eYFP, indicating that the binding of 4-CMTB is largely unperturbed by the differences in both amino acid sequence and potentially broader conformational dissimilarities between hFFA2 and hFFA3 in this region (Fig. 8C). However, maximal efficacy was reduced for both ligands, reflecting either the significantly reduced receptor expression of this chimera or subtle structural differences affecting receptor activation. Despite retention of agonism, however, allosteric communication between propionate and 4-CMTB was now completely abolished (Fig. 8, D and E; Table 3; average αβ = 1.2). Thus, it seems that either the nonconserved residues in ECL2 or the overall conformation of this region is required for allosterism between 4-CMTB and an orthosteric agonist at hFFA2. The importance of ECL2 in receptor activation has been shown for several GPCRs (Sum et al., 2009; Bokoch et al., 2010). Because it is impossible to accurately predict the conformation of ECL2 using comparative modeling, it is difficult, therefore, to provide a mechanistic basis for the influence of ECL2 on 4-CMTB allosterism.
Discussion
The free fatty acid receptor FFA2 is attracting considerable interest because of its potential role in the regulation of inflammation and the importance of gut microflora in generating SCFAs that activate this receptor. However, selectivity between FFA2 and the closely related receptor FFA3 remains problematic (Brown et al., 2003; Le Poul et al., 2003; Stoddart et al., 2008a,b). Although selective orthosteric ligands have not yet been reported for either FFA2 or FFA3, a pair of recent studies has identified a series of ligands, based on the phenylacetamide S-4-CMTB, that are thought to circumvent this issue by binding to an allosteric site on FFA2 (Lee et al., 2008; Wang et al., 2010). Using the predominantly Gαi/o-specific [35S]GTPγS binding assay to examine both wild-type and mutated versions of hFFA2, we confirmed both the direct agonism and positive allosteric effects on the function of the SCFA propionate by 4-CMTB and related ligands. In previous studies, we had identified key basic residues in FFA2, on the basis of alignments of FFA2 with both FFA3 and FFA1 and the recognition that corresponding fatty acid amides are not agonists at these receptors (Stoddart et al., 2008a), that, after mutation, either eliminated or substantially reduced the potency of SCFAs at FFA2. This allowed us to define, in part, the mode of binding of the orthosteric SCFAs at both FFA2 and FFA3 (Stoddart et al., 2008a). It is noteworthy that mutation of these key residues, His4.56, Arg5.39, His6.55, and Arg7.35, did not alter the measured potency of 4-CMTB, confirming that 4-CMTB does not share a common binding site with the SCFAs.
These studies were combined with a targeted SAR survey of 4-CMTB analogs to investigate the possible mode of binding of the phenylacetamides. The SAR survey focused on variations in the ligand's α-substitution and N-(thiazolyl)amide substructure, the latter because we envisaged that it might constitute a key hydrogen bonding motif for binding the receptor. Our SAR data are in close agreement with those recently disclosed by Wang et al. (2010) during the course of our work. Together, the results of these two studies are consistent with a receptor-bound state for the ligand that exhibits a near coplanar conformation for the N-(thiazolyl)amide functionality and in which the amide NH and thiazole nitrogen present, respectively, HDON and HACC sites for engagement of the protein. Precisely this conformational organization and functional role is seen in the cocrystal structures of two unrelated N-(2-thiazolyl)amide-containing compounds bound to human glucokinase and HIV protease (Jadhav et al., 1997; Kamata et al., 2004). In both instances, the N-(thiazolyl)amide engages two adjacent peptide linkages in the protein backbone, and a corresponding engagement in FFA2 would therefore implicate interaction of the motif with a loop region rather than the core transmembrane helices, wherein the backbone hydrogen bonding sites are sequestered in intrahelix interactions. At the present stage, however, we cannot discount other engagement modes for the N-(thiazolyl)amide, such as hydrogen bonding to side-chain amide functionality in Asn or Gln residues. From the compounds with α-alkyl group variations, it seems that a C2 chain length is optimal, although branching at the β carbon, as in the isopropyl group of 4-CMTB itself, is preferred for ligand potency. It is noteworthy that Wang et al. (2010) reported that only the S-enantiomer of 4-CMTB possessed biological activity when measuring regulation of cAMP, although they also demonstrated that a requirement for chiral structures was not absolutely necessary for ligand function by showing that ligands having the α-carbon as part of a cycloalkyl group can retain a good measure of the activity of 4-CMTB. In our [35S]GTPγS binding assay, however, we found R-4-CMTB to be only 6-fold less potent than the racemic material or separate S-enantiomer of 4-CMTB, suggesting that the absolute configuration of the ligand may be less critical for activity than originally indicated.
A key finding of our study was that apparent allosteric communication between orthosteric and allosteric sites within hFFA2 was reduced by a single point mutation within ECL2 (L173A) and abolished by replacement of ECL2 from FFA2 with that of FFA3. This finding was confirmed both by loss of significant shifts in potency with increasing concentrations of modulating ligand and by significant reduction of global cooperativity estimates (Table 3). In both cases, this occurred without any change in propionate or 4-CMTB potency as direct agonists. Although a truly striking set of observations, such interactions remain impossible to model structurally. The available atomic level structures of GPCRs show ECL2 to adopt very different conformations (Peeters et al., 2011), and it is simply impractical to estimate this de novo with any expectation of accuracy. However, these data clearly demonstrate the importance of this region in communication between the two binding sites and are consistent with the appreciated role of ECL2 in receptor activation (Banères et al., 2005; Conner et al., 2007; Scarselli et al., 2007; Huber et al., 2008; Ahn et al., 2009; Bokoch et al., 2010; Unal et al., 2010; Peeters et al., 2011). To date, the best attempt to delineate the molecular mechanisms of allosterism, efficacy, and cooperativity have been at the M4 muscarinic acetylcholine receptor, although these required a series of well-defined and selective probes, including dualsteric ligands, and existing knowledge of allosteric and orthosteric binding sites (Nawaratne et al., 2010). Clearly, much remains to be determined with respect to the mechanism of allosteric signal propagation at FFA2 and will depend upon the development of more potent and selective ligands and greater understanding of ligand binding at both orthosteric and allosteric sites on the receptor.
Supplementary Material
Acknowledgments
We thank Laia Miret Casals and Fernando Albericio from the Universitat de Barcelona (Spain) for verifying the purity of commercially sourced ligands and Andreas P. Andersen for preparing S- and R-4-CMTB.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by The Welcome Trust [Grant 089600/Z/09/Z] (to G.M.) and an Australian C.J. Martin National Health and Medical Research Council and National Heart Foundation Overseas Research Fellowship (to N.J.S.).
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.070789.
- GPCR
- G protein-coupled receptor
- FFA
- free fatty acid
- SCFA
- short-chain fatty acid
- 4-CMTB
- 4-chloro-α-(1-methylethyl)-N-2-thiazolylbenzeneacetamide
- ECL2
- second extracellular loop
- GTPγS
- guanosine 5′-O-(3-thio)triphosphate
- eYFP
- enhanced yellow fluorescent protein
- h
- human
- ANOVA
- analysis of variance
- ERK
- extracellular signal-regulated kinase
- SAR
- structure-activity relationship
- HDON
- hydrogen bond donor
- HACC
- hydrogen bond acceptor.
Authorship Contributions
Participated in research design: Smith, Tikhonova, Adams, and Milligan.
Conducted experiments: Smith, Ward, and Hudson.
Contributed new reagents or analytic tools: Stoddart, Kostenis, Ulven, and Morris.
Performed data analysis: Smith, Hudson, Tränkle, Adams, and Milligan.
Wrote or contributed to the writing of the manuscript: Smith, Tikhonova, Adams, and Milligan.
References
- Ahn KH, Bertalovitz AC, Mierke DF, Kendall DA. (2009) Dual role of the second extracellular loop of the cannabinoid receptor 1: ligand binding and receptor localization. Mol Pharmacol 76:833–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. (2008) Guide to receptors and channels (GRAC), 3rd ed. Br J Pharmacol 153 (Suppl. 2):S1–S209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony J, Kellershohn K, Mohr-Andrä M, Kebig A, Prilla S, Muth M, Heller E, Disingrini T, Dallanoce C, Bertoni S, et al. (2009) Dualsteric GPCR targeting: a novel route to binding and signaling pathway selectivity. FASEB J 23:442–450 [DOI] [PubMed] [Google Scholar]
- Ballesteros JA, Weinstein H. (1995) Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci 25:366–428 [Google Scholar]
- Banères JL, Mesnier D, Martin A, Joubert L, Dumuis A, Bockaert J. (2005) Molecular characterization of a purified 5-HT4 receptor: a structural basis for drug efficacy. J Biol Chem 280:20253–20260 [DOI] [PubMed] [Google Scholar]
- Bokoch MP, Zou Y, Rasmussen SG, Liu CW, Nygaard R, Rosenbaum DM, Fung JJ, Choi HJ, Thian FS, Kobilka TS, et al. (2010) Ligand-specific regulation of the extracellular surface of a G-protein-coupled receptor. Nature 463:108–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, et al. (2003) The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278:11312–11319 [DOI] [PubMed] [Google Scholar]
- Christopoulos A. (2002) Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat Rev Drug Discov 1:198–210 [DOI] [PubMed] [Google Scholar]
- Christopoulos A, Kenakin T. (2002) G protein-coupled receptor allosterism and complexing. Pharmacol Rev 54:323–374 [DOI] [PubMed] [Google Scholar]
- Conn PJ, Jones CK, Lindsley CW. (2009) Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol Sci 30:148–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner M, Hawtin SR, Simms J, Wootten D, Lawson Z, Conner AC, Parslow RA, Wheatley M. (2007) Systematic analysis of the entire second extracellular loop of the V(1a) vasopressin receptor: key residues, conserved throughout a G-protein-coupled receptor family, identified. J Biol Chem 282:17405–17412 [DOI] [PubMed] [Google Scholar]
- Eglen RM. (2005) Muscarinic receptor subtype pharmacology and physiology. Prog Med Chem 43:105–136 [DOI] [PubMed] [Google Scholar]
- Huber T, Menon S, Sakmar TP. (2008) Structural basis for ligand binding and specificity in adrenergic receptors: implications for GPCR-targeted drug discovery. Biochemistry 47:11013–11023 [DOI] [PubMed] [Google Scholar]
- Jadhav PK, Ala P, Woerner FJ, Chang CH, Garber SS, Anton ED, Bacheler LT. (1997) Cyclic urea amides: HIV-1 protease inhibitors with low nanomolar potency against both wild type and protease inhibitor resistant mutants of HIV. J Med Chem 40:181–191 [DOI] [PubMed] [Google Scholar]
- Kamata K, Mitsuya M, Nishimura T, Eiki J, Nagata Y. (2004) Structural basis for allosteric regulation of the monomeric allosteric enzyme human glucokinase. Structure 12:429–438 [DOI] [PubMed] [Google Scholar]
- Kenakin TP. (2009) '7TM receptor allostery: putting numbers to shapeshifting proteins. Trends Pharmacol Sci 30:460–469 [DOI] [PubMed] [Google Scholar]
- Keov P, Sexton PM, Christopoulos A. (2011) Allosteric modulation of G protein-coupled receptors: a pharmacological perspective. Neuropharmacology 60:24–35 [DOI] [PubMed] [Google Scholar]
- Kimura M, Mizukami Y, Miura T, Fujimoto K, Kobayashi S, Matsuzaki M. (2001) Orphan G protein-coupled receptor, GPR41, induces apoptosis via a p53/Bax pathway during ischemic hypoxia and reoxygenation. J Biol Chem 276:26453–26460 [DOI] [PubMed] [Google Scholar]
- Kotarsky K, Nilsson NE, Olde B, Owman C. (2003) Progress in methodology. Improved reporter gene assays used to identify ligands acting on orphan seven-transmembrane receptors. Pharmacol Toxicol 93:249–258 [DOI] [PubMed] [Google Scholar]
- Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, et al. (2003) Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem 278:25481–25489 [DOI] [PubMed] [Google Scholar]
- Lee T, Schwandner R, Swaminath G, Weiszmann J, Cardozo M, Greenberg J, Jaeckel P, Ge H, Wang Y, Jiao X, et al. (2008) Identification and functional characterization of allosteric agonists for the G protein-coupled receptor FFA2. Mol Pharmacol 74:1599–1609 [DOI] [PubMed] [Google Scholar]
- Liu C, Wu J, Zhu J, Kuei C, Yu J, Shelton J, Sutton SW, Li X, Yun SJ, Mirzadegan T, et al. (2009) Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J Biol Chem 284:2811–2822 [DOI] [PubMed] [Google Scholar]
- Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, et al. (2009) Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461:1282–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G, Stoddart LA, Smith NJ. (2009) Agonism and allosterism: the pharmacology of the free fatty acid receptors FFA2 and FFA3. Br J Pharmacol 158:146–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaratne V, Leach K, Felder CC, Sexton PM, Christopoulos A. (2010) Structural determinants of allosteric agonism and modulation at the M4 muscarinic acetylcholine receptor: identification of ligand-specific and global activation mechanisms. J Biol Chem 285:19012–19021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson NE, Kotarsky K, Owman C, Olde B. (2003) Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem Biophys Res Commun 303:1047–1052 [DOI] [PubMed] [Google Scholar]
- Peeters MC, van Westen GJ, Li Q, Ijzerman AP. (2011) Importance of the extracellular loops in G protein-coupled receptors for ligand recognition and receptor activation. Trends Pharmacol Sci 32:35–42 [DOI] [PubMed] [Google Scholar]
- Scarselli M, Li B, Kim SK, Wess J. (2007) Multiple residues in the second extracellular loop are critical for M3 muscarinic acetylcholine receptor activation. J Biol Chem 282:7385–7396 [DOI] [PubMed] [Google Scholar]
- Sina C, Gavrilova O, Förster M, Till A, Derer S, Hildebrand F, Raabe B, Chalaris A, Scheller J, Rehmann A, et al. (2009) G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J Immunol 183:7514–7522 [DOI] [PubMed] [Google Scholar]
- Sleeth ML, Thompson EL, Ford HE, Zac-Varghese SE, Frost G. (2010) Free fatty acid receptor 2 and nutrient sensing: a proposed role for fibre, fermentable carbohydrates and short-chain fatty acids in appetite regulation. Nutr Res Rev 23:135–145 [DOI] [PubMed] [Google Scholar]
- Smith NJ, Bennett KA, Milligan G. (2011) When simple agonism is not enough: emerging modalities of GPCR ligands. Mol Cell Endocrinol 331:241–247 [DOI] [PubMed] [Google Scholar]
- Smith NJ, Milligan G. (2010) Allostery at G protein-coupled receptor homo- and heteromers: uncharted pharmacological landscapes. Pharmacol Rev 62:701–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NJ, Stoddart LA, Devine NM, Jenkins L, Milligan G. (2009) The action and mode of binding of thiazolidinedione ligands at free fatty acid receptor 1. J Biol Chem 284:17527–17539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soudijn W, Van Wijngaarden I, IJzerman AP. (2004) Allosteric modulation of G protein-coupled receptors: perspectives and recent developments. Drug Discov Today 9:752–758 [DOI] [PubMed] [Google Scholar]
- Stoddart LA, Brown AJ, Milligan G. (2007) Uncovering the pharmacology of the G protein-coupled receptor GPR40: high apparent constitutive activity in guanosine 5′-O-(3-[35S]thio)triphosphate binding studies reflects binding of an endogenous agonist. Mol Pharmacol 71:994–1005 [DOI] [PubMed] [Google Scholar]
- Stoddart LA, Smith NJ, Jenkins L, Brown AJ, Milligan G. (2008a) Conserved polar residues in transmembrane domains V, VI, and VII of free fatty acid receptor 2 and free fatty acid receptor 3 are required for the binding and function of short chain fatty acids. J Biol Chem 283:32913–32924 [DOI] [PubMed] [Google Scholar]
- Stoddart LA, Smith NJ, Milligan G. (2008b) International Union of Pharmacology. LXXI. Free fatty acid receptors FFA1, -2, and -3: pharmacology and pathophysiological functions. Pharmacol Rev 60:405–417 [DOI] [PubMed] [Google Scholar]
- Sum CS, Tikhonova IG, Costanzi S, Gershengorn MC. (2009) Two arginine-glutamate ionic locks near the extracellular surface of FFAR1 gate receptor activation. J Biol Chem 284:3529–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sum CS, Tikhonova IG, Neumann S, Engel S, Raaka BM, Costanzi S, Gershengorn MC. (2007) Identification of residues important for agonist recognition and activation in GPR40. J Biol Chem 282:29248–29255 [DOI] [PubMed] [Google Scholar]
- Unal H, Jagannathan R, Bhat MB, Karnik SS. (2010) Ligand-specific conformation of extracellular loop-2 in the angiotensin II type 1 receptor. J Biol Chem 285:16341–16350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jiao X, Kayser F, Liu J, Wang Z, Wanska M, Greenberg J, Weiszmann J, Ge H, Tian H, et al. (2010) The first synthetic agonists of FFA2: discovery and SAR of phenylacetamides as allosteric modulators. Bioorg Med Chem Lett 20:493–498 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.