Abstract
African trypanosomiasis is a disease of humans and livestock in many areas south of the Sahara. Resistance to the few existing drugs is a major impediment to the control of these diseases, and we investigated how resistance to the main veterinary drug diminazene aceturate correlates with changes in drug transport in resistant strains. The strain tbat1(−/−), lacking the TbAT1/P2 aminopurine transporter implicated previously in diminazene transport, was adapted to higher levels of diminazene resistance. The resulting cell line was designated ABR and was highly cross-resistant to other diamidines and moderately resistant to cymelarsan. Procyclic trypanosomes were shown to be a convenient model to study diamidine uptake in Trypanosoma brucei brucei given the lack of TbAT1/P2 and a 10-fold higher activity of the high-affinity pentamidine transporter (HAPT1). Diminazene could be transported by HAPT1 in procyclic trypanosomes. This drug transport activity was lacking in the ABR line, as reported previously for the pentamidine-adapted line B48. The Km for diminazene transport in bloodstream tbat1(−/−) trypanosomes was consistent with uptake by HAPT1. Diminazene transport in ABR and B48 cells was reduced compared with tbat1(−/−), but their resistance phenotype was different: B48 displayed higher levels of resistance to pentamidine and the melaminophenyl arsenicals, whereas ABR displayed higher resistance to diminazene. These results establish a loss of HAPT1 function as a contributing factor to diminazene resistance but equally demonstrate for the first time that adaptations other than those determining the initial rates of drug uptake contribute to diamidine and arsenical resistance in African trypanosomes.
Introduction
One of the many diseases that plague sub-Saharan Africa is trypanosomiasis, a disease complex formed by several species infecting domestic and wild animals (mostly Trypanosoma congolense, Trypanosoma vivax, and Trypanosoma brucei brucei) and humans (Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense) and transmitted by tsetse flies. In addition, T. vivax and the closely related animal parasites Trypanosoma evansi and Trypanosoma equiperdum can also be transmitted sexually or by other biting insects, which has spread the disease to large regions of Southern Asia and South America. The infection is known as sleeping sickness in humans, nagana in cattle, dourine in horses, and as surra in camels and other high-value livestock such as buffalo. The human disease is invariably fatal if left untreated, and the veterinary condition causes enormous damage to economies and food security. The only option for control of trypanosomiasis is chemotherapy because insect control on the necessary scale is prohibitively expensive, and vaccine development seems practically impossible because of the process of antigenic variation of African trypanosomes.
However, the choice of chemotherapeutic agents is very limited, and those available suffer from many shortcomings, such as high levels of host toxicity, parenteral administration, and perhaps most importantly increasing levels of treatment failure due to drug resistance. For treatment of the late or cerebral of stage sleeping sickness, this has led to the replacement of melarsoprol with eflornithine in many foci (Balasegaram et al., 2009) and to the recent introduction of eflornithine/nifurtimox combination therapy (Priotto et al., 2009; Yun et al., 2010). The early or hemolymphatic stage is still treated with the aromatic diamidine compound pentamidine, introduced in the 1930s (Delespaux and de Koning, 2007). For the veterinary condition, the situation is even more serious, with many reports of resistance to the two principal drugs, isometamidium (a phenanthridine) and diminazene aceturate (aromatic diamidine) (Geerts et al., 2001; Delespaux and de Koning, 2007), and no alternative treatments in development. The paucity of new drug development for both the human and veterinary diseases makes understanding of the spreading resistance phenotypes a priority. Resistance markers are required for epidemiological studies to assess the real spread of resistance, and insight into the causes of resistance and the patterns of cross-resistance must underpin rational strategies to limit the impact and further spread of the problem.
It has been known for a considerable time that trypanosomal resistance to melaminophenyl arsenicals such as cymelarsan (used against surra in camels) and melarsoprol is associated with cross-resistance to at least some of the aromatic diamidines (Williamson and Rollo, 1959; de Koning, 2008). We now know that pentamidine is salvaged by three distinct transport entities in bloodstream trypanosomes, the P2 aminopurine transporter, the high-affinity pentamidine transporter (HAPT1) and the low-affinity pentamidine transporter (LAPT1) (de Koning and Jarvis, 2001; de Koning, 2001; Matovu et al., 2003; Bridges et al., 2007). P2 is encoded by the ENT family gene TbAT1 (Mäser et al., 1999), but the genes encoding HAPT1 and LAPT1 are still unknown and the identification of these transporters has been difficult given the complexity of the three influx routes for the drug under study, the relatively low rate of uptake by HAPT1 in bloodstream trypanosomes, and the lack of specific inhibitors for LAPT1. We thus decided to investigate [3H]pentamidine transport in procyclic T. brucei brucei, which lacks the expression of P2/TbAT1 (de Koning et al., 1998), in the hope to obtain a simpler model. We found that procyclic cells lack adenosine-sensitive P2-mediated pentamidine transport but that two pentamidine uptake systems, biochemically indistinguishable from HAPT1 and LAPT1 in bloodstream forms, were expressed. This allowed us to characterize P2-independent diminazene uptake in detail and determine that this is mediated by the procyclic high-affinity pentamidine transporter, designated previously as PPT1 (de Koning, 2001), which we here demonstrate to be equivalent to HAPT1. HAPT1 was shown subsequently to be a secondary route of diminazene uptake into bloodstream forms. This gives a rationale for the continued sensitivity of P2-deficient trypanosomes to diminazene at concentrations of approximately 1 μM and significantly increases our understanding of cross-resistance between arsenical and diamidine trypanocides.
Materials and Methods
Trypanosome Strains and Cultures.
The standard drug-sensitive strain T. brucei brucei s427 (MiTat 1.2/BS221) was used throughout to obtain both bloodstream and procyclic trypanosomes. All other strains were derived from this line, and all cell lines were clonal (i.e., derived from a single cell). Identities of all strains are checked regularly using polymerase chain reaction amplification of molecular markers. The tbat1(−/−) was derived from s427 by replacement of both TbAT1 alleles with antibiotic resistance cassettes and is moderately resistant to diminazene and displays also a minor loss of sensitivity to pentamidine and melaminophenyl arsenicals (Matovu et al., 2003). The B48 line was derived in turn from tbat1(−/−) by incrementally increased exposure to pentamidine (Bridges et al., 2007). All bloodstream form cultures were maintained in HMI-9 medium (Invitrogen, Carlsbad, CA) supplemented with 2 mM β-mercaptoethanol and 10% fetal calf serum (FCS; PAA Laboratories Inc., Etobicoke, ON, Canada) at 37°C under a 5% CO2 atmosphere (Hirumi and Hirumi, 1989). Procyclic cells were grown at 25°C in SDM79 medium (Invitrogen) supplemented with 10% FCS as described previously (Gudin et al., 2006). Cells for experiments were taken at late log-phase growth.
Generation of a New Clonal Line Resistant to High Levels of Diminazene Aceturate.
In vitro drug selection in the presence of diminazene, in stepwise small increments, was carried out in duplicate on the tbat1(−/−) T. brucei cell line (Matovu et al., 2003). The selection process was initiated using the doubling dilution method on a 96-well plate. Diminazene aceturate, prepared as stock solution in sterile water, was used at a start concentration of 1.6 μM (equivalent to its IC50 value) in 200 μl of HMI-9/FCS and added to the first well in the first column of a 96-well culture plate (Corning Life Sciences, Lowell, MA) in duplicate. The T. brucei tbat1(−/−) cells, at a start cell density of 0.5 × 106 cells/ml, were dispensed into each of these wells. The plates were incubated at standard conditions for 4 days and assessed by light microscopy. Motile cells with a normal morphology were seeded again in fresh HMI-9 medium with 10% FCS at the same drug concentration into a fresh well and reincubated under the same conditions. The same cells were also seeded at twice, one half, and one tenth of that drug concentration in fresh HMI-9 medium with 10% FCS. Cells established as growing stably in drug were transferred and seeded into 1-ml volume cultures in 24-well plates, and the selection continued with gradual increase in drug concentration over a period of 5 months. The tbat1(−/−) diminazene-selected line was growing normally in the continuous presence of 0.1 μM diminazene at that point.
Alamar Blue Drug Sensitivity Assays.
Drug sensitivities were determined with the Alamar blue assay exactly as described previously (Gould et al., 2008), measuring fluorescence in 96-well plates with a FLUOstar Optima (BMG Labtech, Durham, NC) at wavelengths of 544 nm for excitation and 620 nm for emission. IC50 values were calculated by nonlinear fitting of the data to a sigmoidal dose-response curve with variable slope (Prism 5.0; GraphPad Software Inc., San Diego, CA).
Transport Assays.
Transport assays with procyclic and bloodstream form trypanosomes were performed as described previously (Wallace et al., 2002; Natto et al., 2005; Al-Salabi et al., 2007). In brief, cell cultures were harvested at late-log stage of growth, washed in assay buffer (AB; 33 mM HEPES, 98 mM NaCl, 4.6 mM KCl, 0.55 mM CaCl2, 0.07 mM MgSO4, 5.8 mM NaH2PO4, 0.3 mM MgCl2, 23 mM NaHCO3, and 14 mM glucose, pH 7.3), and resuspended at a concentration of ∼108 cells/ml. Transport was initiated by addition of 100 μl cells to 100 μl of radiolabel in AB layered over oil [7:1 dibutylphthalate/mineral oil (v/v); Sigma-Aldrich, St. Louis, MO] and terminated by the addition of an ice-cold solution of 1 ml of unlabeled permeant and immediate centrifugation through the oil layer. Radioactivity in the cell pellet was determined by liquid scintillation counting and corrected for nonspecific association of radiolabel with the cells as described previously (Wallace et al., 2002). Kinetic parameters were calculated using the appropriate linear and nonlinear regression equations in Prism 5.0. All experiments were performed in triplicate on at least three independent occasions.
Results
Pentamidine Transport in Procyclic T. brucei brucei as a Model for Bloodstream Forms.
Uptake of 1 μM [3H]pentamidine was linear for at least 450 s (linear regression, six points over 450 s; r2 = 0.98) with a rate of 0.037 ± 0.002 pmol · 107 cells−1 · s−1and fully inhibited by 1 mM unlabeled pentamidine (slope not significantly different from 0; F test) (see Supplemental Figure S1). All subsequent [3H]pentamidine transport experiments were performed using 60-s incubations, well within the linear phase of uptake.
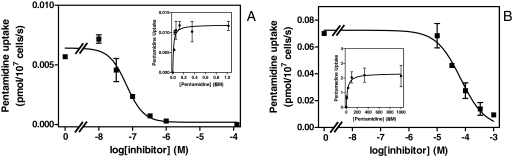
Procyclic s427, like bloodstream forms, displayed separate high- and low-affinity transport entities for [3H]pentamidine. When transport was measured at 25 nM radiolabel, unlabeled pentamidine inhibited the flux at concentrations greater than 10 nM and fully saturated the transporter at 1 μM (Fig. 1A), whereas uptake of 1 μM [3H]pentamidine was only starting to be inhibited by concentrations greater than 10 μM (Fig. 1B). Michaelis-Menten constants (Km) were determined at 0.030 ± 0.003 and 33 ± 10 μM, respectively, consistent with the HAPT1/LAPT1 system observed in bloodstream forms of the same strain. The maximum uptake rate at saturation (Vmax) for low-affinity pentamidine transport was virtually identical in both life cycle forms, but interestingly, the Vmax for the high-affinity component was 10-fold higher in procyclic forms (Table 1).
Fig. 1.
[3H]Pentamidine uptake in procyclic s427 trypanosomes at a permeant concentration of 30 nM (A) and 1 μM (B). Cells were incubated with [3H]pentamidine for 60 s in the presence or absence of various concentrations of unlabeled pentamidine as indicated. The insets depict the conversion of the inhibition plots to Michaelis-Menten saturation plots. Both graphs are representative of at least six independent experiments, each performed in triplicate. The data shown represent the average of these triplicate determinations ± S.E.M. When not shown, error bars fall within the symbols.
TABLE 1.
Comparison of [3H]pentamidine transport parameters in bloodstream and procyclic T. brucei brucei
High-affinity transport of [3H]pentamidine was measured at concentrations of 25 to 40 nM radiolabel or 12.5 nM for the determination of Km values; low-affinity transport was assayed at 1 μM [3H]pentamidine. Data given are the average of at least three independent experiments, each performed in triplicate ± S.E. Bloodstream forms were isolated from the blood of infected rats, whereas procyclics were cultured in standard SDM79 medium. Some of the values have been reported previously (de Koning, 2001).
| Bloodstream Forms | Procyclic Forms | |
|---|---|---|
| High-Affinity Transport | ||
| Pentamidine Km (μM) | 0.035 ± 0.005 | 0.030 ± 0.003 |
| Pentamidine Vmax (pmol · 107 cells−1 · s−1) | 0.0028 ± 0.0006 | 0.031 ± 0.07 |
| Propamidine Ki (μM) | 4.6 ± 0.7 | 3.7 ± 0.4 |
| Diminazene Ki (μM) | 63 ± 3 | 54 ± 16 |
| DB820 Ki (μM) | 43 ± 10 | 45 ± 18 |
| CPD0801 Ki (μM) | 40 ± 8 | 16 ± 4 |
| Low-Affinity Transport | ||
| Pentamidine Km (μM) | 56 ± 8 | 33 ± 10 |
| Pentamidine Vmax(pmol · 107 cells−1 · s−1) | 0.75 ± 0.15 | 0.78 ± 0.12 |
| Propamidine Ki (μM) | 316 ± 3 | 429 ± 180 |
| Diminazene Ki (μM) | 160 ± 50 | 180 ± 20 |
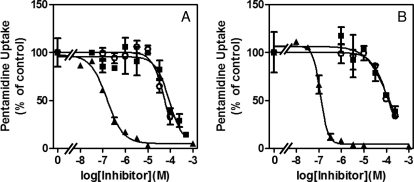
The inhibitor profile of high-affinity pentamidine uptake was also highly similar in both stages. In addition to the inhibition constants (Ki) reported previously for propamidine and diminazene aceturate (de Koning, 2001), we further compared inhibition by the furamidine analogs 6-(5-(4-amidinophenyl)furan-2-yl)nicotinamidine (DB820) and CPD0801 (formerly known as DB829) (Mathis et al., 2007). Again, we found very similar activities on high-affinity [3H]pentamidine transport in procyclic and bloodstream forms (Fig. 2 and Table 1). No reliable Ki values for DB820 and CPD0801 could be determined in relation to low-affinity pentamidine transport because of lack of inhibition in the soluble range of these compounds. Indeed, no substrate or inhibitor with higher affinity than pentamidine has been identified yet for this transport activity. However, we report here LAPT1 Ki values for propamidine and diminazene, which were similar in both trypanosomal stages (Table 1).
Fig. 2.
High-affinity pentamidine transport in bloodstream (A) and procyclic (B) forms of T. brucei brucei in the presence of various concentrations of unlabeled inhibitors:▴, pentamidine; ○, DB820; ■, CPD0801. [3H]Pentamidine concentration was 25 nM, and incubation time was 60 s. The experiments shown are representative of at least three independent experiments, each performed in triplicate. Error bars are standard errors.
Transport of Diminazene Aceturate in Procyclic Trypanosomes.
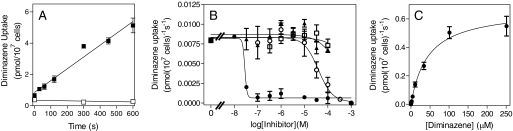
The absence of a P2 transporter in procyclic cells and the much higher activity of a high-affinity transporter, shown above to be HAPT1, allowed us to study how the diamidines are accumulated in the absence of P2. Unlike in tbat1(−/−) bloodstream trypanosomes (de Koning et al., 2004), [3H]diminazene uptake was readily measured in procyclics of the same strain (s427). Uptake was linear over 10 min with a rate of 0.0075 ± 0.0006 pmol · 107 cells−1 · s−1 and was fully saturated by 1 mM unlabeled diminazene (Fig. 3A). Figure 3B shows that this flux, although sensitive to very low concentrations of pentamidine, was not inhibited by the purines inosine or adenine, inhibitors of the P1 and P2 purine transporters, respectively (Carter and Fairlamb, 1993). Likewise, up to 1 mM adenosine had no effect on diminazene uptake (data not shown). Transport of [3H]diminazene aceturate displayed simple Michaelis-Menten kinetics consistent with a one-transporter model and a moderate affinity with a Km value of 28 ± 5 μM and a Vmax of 0.59 ± 0.11 pmol · 107 cells−1 · s−1 (n = 4) (Fig. 3C). Interestingly, this transport phenomenon was very potently inhibited by pentamidine (Fig. 3B), with a Ki value of 0.033 ± 0.004 μM (n = 3), which led us to hypothesize that, at least in procyclic trypanosomes, diminazene aceturate is transported by HAPT1.
Fig. 3.
Transport of 1 μM [3H]diminazene in procyclic trypanosomes. A, procyclic s427 were incubated for the indicated times with radiolabel in the presence (□) or absence (■) of 1 mM unlabeled diminazene aceturate. The correlation coefficient for the 1 μM line was 0.97 and it was highly significantly different from 0 (P < 0.00001; F test), whereas the 1 mM line was not significantly different from 0 (P = 0.50; F test). B, inhibition of [3H]diminazene transport by various concentrations of pentamidine (●), unlabeled diminazene (○), adenine (▴), and inosine (□). C, Michaelis-Menten saturation curve for [3H]diminazene in procyclic cells, representative of four independent experiments. All experiments were performed in triplicate, and error bars indicate S.E.M.
Resistance Profile of a Highly Diminazene-Resistant Cell Line, ABR.
The hypothesis that non–P2-dependent diminazene is mediated by HAPT in procyclics and thus presumably by HAPT1 in bloodstream forms was investigated using a strain adapted from the tbat1(−/−) clonal line by in vitro exposure to increasing diminazene concentrations; the identity of the tbat1(−/−) line was verified before adaptation (Supplemental Fig. S2). Adaptation in the presence of incremental diminazene concentrations was performed over 6 months as depicted in Supplemental Fig. S3.
The tbat1(−/−) cell culture selected against high-level resistance to diminazene was cloned by limiting dilution, and the resulting clonal line (designated ABR) was tested for its susceptibility to diminazene aceturate using the Alamar Blue assay, after a total of 59 passages. At this stage, immediately after the adaptation to diminazene, the ABR line was compared with wild-type strain 427 and to the tbat1(−/−) parent strain and found to be less susceptible than either. A full resistance profile was made after a further 27 passages in drug-free medium over a period of 3 months to ensure stability of the resistance phenotype (Table 2), which was shown to be stable.
TABLE 2.
EC550 values for the four different trypanosomes strains used in this study
| s427WT |
tbat1(−/−) |
B48 |
ABR |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EC50 | n | EC50 | n | RF | EC50 | n | RF | EC50 | n | RF | |
| nM | nM | nM | nM | ||||||||
| Pentamidine | 6.8 ± 1.1 | 12 | 15 ± 6 | 11 | 2.2 | 570 ± 200 | 6 | 83 | 340 ± 97 | 5 | 50 |
| Diminazene | 629 ± 132 | 12 | 5780 ± 1560 | 12 | 9.2 | 2670 ± 510 | 4 | 4.2 | 14,600 ± 3900 | 5 | 23 |
| DB75 | 212 ± 60 | 12 | 1670 ± 460 | 10 | 7.9 | 360 ± 110 | 5 | 1.7 | 4560 ± 1560 | 5 | 22 |
| Cymelarsan | 4.1 ± 0.4 | 12 | 13 ± 2 | 12 | 3.2 | 62 ± 15 | 6 | 15 | 27 ± 4 | 6 | 6.6 |
| PAO | 0.69 ± 0.13 | 8 | 0.76 ± 0.09 | 8 | 1.1 | 0.54 ± 0.14 | 4 | 0.8 | 0.82 ± 0.14 | 4 | 1.2 |
RF, resistance factor relative to wild-type (s427WT).
Table 2 lists EC50 values for the diamidines pentamidine, diminazene, and DB75, and for the arsenical compounds cymelarsan and phenylarsine oxide (PAO). Cymelarsan is a water-soluble member of the melaminophenyl arsenical class of trypanocides, used against veterinary trypanosomiasis, and a close homolog of the lipophilic human sleeping sickness drug melarsoprol; PAO is included as a known trypanocide that rapidly crosses the plasma membrane by passive diffusion (Carter and Fairlamb, 1993; Bridges et al., 2007). All of the drug sensitivity experiments were performed with four different cell lines in parallel: wild-type s427, the tbat1(−/−) line derived from s427, B48 derived from tbat1(−/−) by adaptation to pentamidine in vitro (Bridges et al., 2007), and the ABR line derived from tbat1(−/−) by adaptation to diminazene. Resistance factors (EC50 of derived line divided by EC50 of wild-type line) are indicated in Table 2. The EC50 values and resistance levels of the first three lines closely follow patterns reported previously: tbat1(−/−) displays a minor loss of sensitivity to pentamidine and melaminophenyl arsenicals and a significant level of resistance to DB75 and diminazene (Matovu et al., 2003; Lanteri et al., 2006); in B48, resistance to cymelarsan and pentamidine is greatly increased due to loss of HAPT1 activity (Bridges et al., 2007). Of interest is that in many assays, we observe a certain reversal of DB75 and diminazene resistance levels in B48, relative to tbat1(−/−) (although this did not reach statistical significance). In contrast to B48, the ABR line did display a significant increase in diminazene and DB75 resistance relative to the parental tbat1(−/−) line (P < 0.02 and <0.05, respectively; Student's t test). Whereas also displaying increased resistance to pentamidine and cymelarsan, this increase was less pronounced than in B48 (although this reached statistical significance only for cymelarsan; P = 0.05, Student's t test). These results indicate that the adaptation to diminazene either uses a different mechanism than adaptation to pentamidine or that the adaptation to such high levels of resistance is multifactorial. Because the adaptation of B48 was attributed to the loss of HAPT1 activity (Bridges et al., 2007), it was next investigated whether activity of the pentamidine transporters had changed in the ABR line.
Diamidine Transport in Drug-Resistant Trypanosomes.
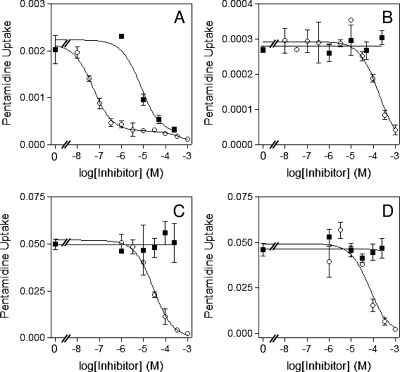
Assessment of [3H]pentamidine transport activity in the ABR line found no evidence of a high-affinity uptake component (assessed at 30 nM radiolabel). Transport of pentamidine at this high-affinity discriminatory concentration was readily inhibited by submicromolar pentamidine concentrations in wild-type cells (Fig. 4A), whereas in ABR cells, this flux was of a much lower level and sensitive only to concentrations greater than 10 μM pentamidine (Fig. 4B). Likewise, the high-affinity component in wild-type cells was sensitive to propamidine as described for HAPT1 (de Koning, 2001), which had no effect on uptake in ABR cells (Fig. 4, A and B). The most straightforward interpretation of these data is an absence of HAPT1 activity in ABR cells, with the 30 nM [3H]pentamidine taken up by the one remaining pentamidine transporter, LAPT1. Assessment of LAPT1 function at 1 μM [3H]pentamidine did reveal a wild-type pattern of low-affinity uptake that was insensitive to propamidine in ABR cells (Fig. 4, C and D). A Km value for [3H]pentamidine uptake plotted to a hyperbolic curve indicative of monophasic transport in the ABR strain was measured at 59 ± 11 μM (n = 3), indistinguishable from the published value for LAPT1 of 56 ± 8 μM; the Vmax value of 1.2 ± 0.4 pmol · 107 cells−1 · s−1 (n = 3) was also highly similar to the published value of 0.85 ± 0.15 pmol · 107 cells−1 · s−1 for LAPT1 (de Koning, 2001). The above results strongly suggest that the pentamidine cross-resistance in ABR strain adapted to high diminazene concentration was due to a loss of HAPT1 transport. Yet, the differences in resistance pattern between the pentamidine-adapted B48 and diminazene-adapted ABR lines suggest that additional adaptations may be responsible for the further increase in diminazene resistance in the latter cells. Using 1 μM [3H]diminazene, we investigated whether this is attributable to differences in diminazene uptake rates. Because the uptake rates in the resistant lines were very low, we used time courses with 7 points over 10 min in an effort to measure transport as accurately as possible (Supplemental Fig. S4) and in this way measured the rate in each cell line three to four times. Identical experiments were then performed with 1 μM [3H]pentamidine for comparison. The results are summarized in Fig. 5.
Fig. 4.
Transport of [3H]pentamidine in wild-type and ABR cell line lines. Transport of 30 nM (A and B) or 1 μM [3H]pentamidine (C and D) was assessed in bloodstream forms of the s427WT (A and C) and ABR (B and D) cell lines over 60 s in the presence or absence of various concentrations of propamidine (■) or unlabeled pentamidine (○). Pentamidine uptake was expressed as picomoles per 107 cells per second. The experiments were performed in triplicate; error bars represent S.E. of internal replicates. Data shown are representative of at least three identical and independent experiments.
Fig. 5.
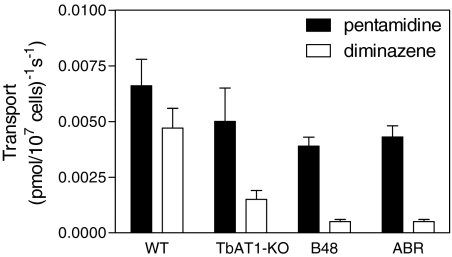
Transport of 1 μM [3H]pentamidine (■) or 1 μM [3H]diminazene (□) in four different cell lines. Transport rates were derived by linear regression from time courses with points (in triplicate) at 0, 30, 60, 120, 300, 450, and 600 s. Zero uptake levels and saturability were verified in the presence of 1 mM unlabeled permeant. Bars show the average transport of three to four experiments (each performed in triplicate) and S.E. Representative experiments are shown in Supplemental Fig. S4.
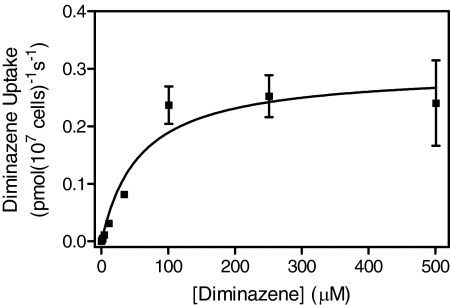
Transport of 1 μM [3H]diminazene was significantly reduced in the tbat1(−/−) line compared with s427WT (P < 0.05). The rate of [3H]diminazene transport was further reduced in the B48 line (P < 0.05), which additionally lacks HAPT1 (Bridges et al., 2007), and similarly in the ABR (P < 0.05), which also lacks HAPT1 activity (this article). In contrast, reductions in 1 μM [3H]pentamidine transport rates were much less dramatic in the resistant lines (Fig. 5), because, at this relatively high concentration of pentamidine, much of the flux is through LAPT1, with HAPT1 and P2 saturating at a lower concentration (Bray et al., 2003). Transport of 1 μM [3H]diminazene, measured over 5 min in tbat1(−/−) cells, was still saturable and displayed an average Km value of 67 ± 13 μM (n = 3) (Fig. 6), fully consistent with uptake through HAPT1 (diminazene Ki for [3H]pentamidine through HAPT1 is 63 ± 3 μM; Table 1). These results are consistent with the retained presence of LAPT1 in all four cell lines and little or no role for LAPT1 in diminazene uptake.
Fig. 6.
Saturation plot of [3H]diminazene transport in bloodstream forms of the tbat1(−/−) cell line. Transport of 1 μM label was determined in triplicate at 5 min of incubation; error bars represent S.E. The experiment shown is representative of three identical experiments.
Discussion
Although pentamidine is transported efficiently by bloodstream forms in the absence of P2 and the deletion of the P2-encoding gene, TbAT1 alone does not confer more than marginal pentamidine resistance (Matovu et al., 2003). Other therapeutically important diamidines such as diminazene aceturate (de Koning et al., 2004), DB75 (furamidine; Lanteri et al., 2006) and its aza analogs DB820 and CPD0801 (Ward et al., 2010, 2011) rely principally on P2, and tbat1(−/−) trypanosomes display significant resistance to these drugs in vitro. Notwithstanding these observations, it is evident that those diamidines are also taken up by a non-P2 mechanism, because tbat1(−/−) trypanosomes remain sensitive to approximately 1 μM concentration of these diamidines in vitro (Matovu et al., 2003; Lanteri et al., 2006), and experimental infections with tbat1(−/−) T. brucei brucei can be cured using increased doses of DB75 (Lanteri et al., 2006). To understand drug uptake and resistance mechanisms for these therapeutically important diamidines, it is thus critical to identify and characterize the non–P2-mediated uptake systems. We investigate here whether the high-affinity and low-affinity pentamidine transporters, HAPT1 and LAPT1, contribute to the uptake of these diamidines.
We have reported previously that procyclic T. brucei brucei express a high-affinity pentamidine transport system, which we designated procyclic pentamidine transporter 1 (PPT1) and which displayed similar properties to the HAPT1 transporter in bloodstream forms (de Koning, 2001). However, no conclusion was reached as to whether PPT1 might be identical with HAPT1, and the presence or absence of a low-affinity component in procyclics, equivalent to LAPT1 in bloodstream forms, was not investigated. We report here additional data on the high-affinity transport of diamidines in both life cycle stages and the first characterization of low-affinity pentamidine transport in procyclic trypanosomes. The data show that procyclic cells express a high-affinity and a low-affinity pentamidine transport system, which is indistinguishable from the HAPT1 and LAPT1 transporters characterized previously in bloodstream forms (de Koning, 2001; Matovu et al., 2003; Bridges et al., 2007), both by Km values, using pentamidine as substrate, and by inhibitor profile.
The kinetic data available are thus consistent with the HAPT1 and LAPT1 transporters of bloodstream forms also being expressed in procyclic forms, although definitive proof will require molecular studies (in progress). Although we designated previously the high-affinity pentamidine transporter of procyclics PPT1 and the low-affinity component PPT2 (de Koning, 2001), for the sake of clarity, we now refer to them as HAPT1 and LAPT1, respectively. The procyclic forms make a convenient model to study diamidine transport because of the nonexpression of the P2 aminopurine transporter (de Koning et al., 1998), which also transports pentamidine (Carter et al., 1995; de Koning and Jarvis, 2001), diminazene (de Koning et al., 2004), and other diamidines (Lanteri et al., 2006; Collar et al., 2009; Ward et al., 2010, 2011). In addition, the much higher rate of uptake through HAPT1 in procyclic forms facilitates the study of diamidine transport at the low label concentrations required.
We thus proceeded to study the uptake of [3H]diminazene in procyclic cells in detail and found that the rate of uptake was indeed much higher than in bloodstream forms lacking P2 [tbat1(−/−)] (de Koning et al., 2004). This uptake was not mediated by either the P1 or P2 adenosine transporters, because neither adenine nor inosine (inhibitors of P2 and P1, respectively) (Carter and Fairlamb, 1993; de Koning and Jarvis, 1999; Al-Salabi et al., 2007) nor adenosine had any effect on diminazene accumulation. However, diminazene transport was highly sensitive to pentamidine, with an IC50 value close to the Km value for pentamidine uptake by HAPT1. In addition, the diminazene Km was identical to its inhibition constant for HAPT1-mediated pentamidine uptake. This reciprocal inhibition clearly indicates that diminazene is a substrate for HAPT1, albeit with an affinity 3 orders of magnitude less than pentamidine. The measurements of [3H]diminazene uptake in tbat1(−/−) bloodstream forms, including the determination of the Km value, were all consistent with the same mechanism occurring in both life cycle stages.
We showed previously that adaptation of tbat1(−/−) trypanosomes to either pentamidine or melaminophenyl arsenicals leads to loss of the HAPT1 activity (Bridges et al., 2007), because this is the second-most important transporter for these trypanocides. We now show that the same happens after adaptation of bloodstream form tbat1(−/−) to increasing levels of diminazene in vitro, correlating high levels of resistance to diminazene for the first time to the sequential loss of P2/TbAT1 and HAPT1. Yet, the lines did not show exactly the same drug resistance profile, indicating that changes unrelated to these transporters may play a role in achieving such a high level of resistance.
The transport data in bloodstream forms thus conform to predictions of the procyclic model, above, that HAPT1 is responsible for most of the P2-independent diminazene uptake in bloodstream trypanosomes, and its absence seems generally to correlate with high levels of diamidine resistance: B48 and ABR both seem to have lost HAPT1 activity. We conclude that adaptation of trypanosomes without a functional P2/TbAT1 transporter may lose the HAPT1 transporter under drug pressure with diminazene (ABR) (this article), pentamidine (B48), or cymelarsan (B48; Bridges et al., 2007), leading to cross-resistance to new classes of drugs such as the furamidines (ABR/DB75). Nevertheless, it is equally clear that high-level drug resistance as achieved by prolonged drug exposure in a laboratory is indeed multifactorial. The sequential loss of the P2 and HAPT1 transporters still does not fully explain the observed resistance patterns: B48 and ABR display identical residual rates of diminazene uptake (Fig. 5) and LAPT1-mediated pentamidine transport but they differ in their level of diminazene resistance. Several possibilities for this paradox come to mind, but if the initial rates of drug transport are identical in both strains, as we established, differential drug sensitivity can theoretically be explained by 1) expression of a catabolic enzyme or pathway for diminazene in ABR, 2) introduction of an efflux pump or other sequestration/extrusion mechanism in one strain, or 3) additional non–transport-related adaptations that alter drug sensitivity of the parasite, such as at target level. The latter would most likely involve changes in mitochondrial function, because this organelle is believed to be the main target of diamidines in kinetoplastids (Basselin et al., 2002; Lanteri et al., 2008). Other dications, including bisphosphonium salts and choline-derived compounds similarly target mitochondria in Leishmania and Trypanosoma species (Ibrahim et al., 2011; Luque-Ortega et al., 2010). The possibility of specific mitochondrial changes being involved in diamidine resistance in African trypanosomes is the subject of ongoing investigations in our laboratories.
Supplementary Material
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the Consortium for Parasitic Drug Development; a Commonwealth Scholarship; and the UK Medical Research Council [Grant 84733].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.111.071555.
- HAPT1
- high-affinity pentamidine transporter 1
- LAPT1
- low-affinity pentamidine transporter 1
- FCS
- fetal calf serum
- PPT
- procyclic pentamidine transporter
- PAO
- phenylarsine oxide
- AB
- assay buffer
- DB820
- 6-(5-(4-amidinophenyl)furan-2-yl)nicotinamidine
- CPD0801
- 2,5-bis(5-amidino-2-pyridyl)furan.
Authorship Contributions
Participated in research design: Mäser, Matovu, Barrett, and de Koning.
Conducted experiments: Teka, Kazibwe, El-Sabbagh, Al-Salabi, Ward, Eze, and Munday.
Performed data analysis: Teka, Kazibwe, Barrett, and de Koning.
Wrote or contributed to the writing of the manuscript: Barrett and de Koning.
References
- Al-Salabi MI, Wallace LJ, Lüscher A, Mäser P, Candlish D, Rodenko B, Gould MK, Jabeen I, Ajith SN, de Koning HP. (2007) Molecular interactions underlying the unusually high adenosine affinity of a novel Trypanosoma brucei nucleoside transporter. Mol Pharmacol 71:921–929 [DOI] [PubMed] [Google Scholar]
- Balasegaram M, Young H, Chappuis F, Priotto G, Raguenaud ME, Checchi F. (2009) Effectiveness of melarsoprol and eflornithine as first-line regimens for gambiense sleeping sickness in nine Médecins Sans Frontières programmes. Trans R Soc Trop Med Hyg 103:280–290 [DOI] [PubMed] [Google Scholar]
- Basselin M, Denise H, Coombs GH, Barrett MP. (2002) Resistance to pentamidine in Leishmania mexicana involves exclusion of the drug from the mitochondrion. Antimicrob Agents Chemother 46:3731–3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges DJ, Gould MK, Nerima B, Mäser P, Burchmore RJ, de Koning HP. (2007) Loss of the high-affinity pentamidine transporter is responsible for high levels of cross-resistance between arsenical and diamidine drugs in African trypanosomes. Mol Pharmacol 71:1098–1108 [DOI] [PubMed] [Google Scholar]
- Carter NS, Berger BJ, Fairlamb AH.(1995) Uptake of diamidine drugs by the P2 nucleoside transporter in melarsen-sensitive and -resistant Trypanosoma brucei brucei. Trypanosoma brucei brucei. J Biol Chem 270:28153–28157 [DOI] [PubMed] [Google Scholar]
- Carter NS, Fairlamb AH. (1993) Arsenical-resistant trypanosomes lack an unusual adenosine transporter. Nature 361:173–176 [DOI] [PubMed] [Google Scholar]
- Collar CJ, Al-Salabi MI, Stewart ML, Barrett MP, Wilson WD, de Koning HP. (2009) Predictive computational models of substrate binding by a nucleoside transporter. J Biol Chem 284:34028–34035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning HP. (2001) Uptake of pentamidine in Trypanosoma brucei brucei is mediated by three distinct transporters: implications for cross-resistance with arsenicals. Mol Pharmacol 59:586–592 [DOI] [PubMed] [Google Scholar]
- de Koning HP. (2008) Ever-increasing complexities of diamidine and arsenical crossresistance in African trypanosomes. Trends Parasitol 24:345–349 [DOI] [PubMed] [Google Scholar]
- de Koning HP, Anderson LF, Stewart M, Burchmore RJ, Wallace LJ, Barrett MP. (2004) The trypanocide diminazene aceturate is accumulated predominantly through the TbAT1 purine transporter: additional insights on diamidine resistance in African trypanosomes. Antimicrob Agents Chemother 48:1515–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning HP, Jarvis SM. (2001) Uptake of pentamidine in Trypanosoma brucei brucei is mediated by the P2 adenosine transporter and at least one novel, unrelated transporter. Acta Trop 80:245–250 [DOI] [PubMed] [Google Scholar]
- de Koning HP, Watson CJ, Jarvis SM. (1998) Characterization of a nucleoside/proton symporter in procyclic Trypanosoma brucei brucei. J Biol Chem 273:9486–9494 [DOI] [PubMed] [Google Scholar]
- Delespaux V, de Koning HP. (2007) Drugs and drug resistance in African trypanosomiasis. Drug Resist Updat 10:30–50 [DOI] [PubMed] [Google Scholar]
- Geerts S, Holmes PH, Eisler MC, Diall O. (2001) African bovine trypanosomiasis: the problem of drug resistance. Trends Parasitol 17:25–28 [DOI] [PubMed] [Google Scholar]
- Gould MK, Vu XL, Seebeck T, de Koning HP. (2008) Propidium iodide-based methods for monitoring drug action in the kinetoplastidae: comparison with the Alamar Blue assay. Anal Biochem 382:87–93 [DOI] [PubMed] [Google Scholar]
- Gudin S, Quashie NB, Candlish D, Al-Salabi MI, Jarvis SM, Ranford-Cartwright LC, de Koning HP. (2006) Trypanosoma brucei: a survey of pyrimidine transport activities. Exp Parasitol 114:118–125 [DOI] [PubMed] [Google Scholar]
- Hirumi H, Hirumi K. (1989) Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J Parasitol 75:985–989 [PubMed] [Google Scholar]
- Ibrahim HM, Al-Salabi MI, El Sabbagh N, Quashie NB, Alkhaldi AA, Escale R, Smith TK, Vial HJ, de Koning HP. (2011) Symmetrical choline-derived dications display strong anti-kinetoplastid activity. J Antimicrob Chemother 66:111–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanteri CA, Stewart ML, Brock JM, Alibu VP, Meshnick SR, Tidwell RR, Barrett MP. (2006) Roles for the Trypanosoma brucei P2 transporter in DB75 uptake and resistance. Mol Pharmacol 70:1585–1592 [DOI] [PubMed] [Google Scholar]
- Lanteri CA, Tidwell RR, Meshnick SR. (2008) The mitochondrion is a site of trypanocidal action of the aromatic diamidine DB75 in bloodstream forms of Trypanosoma brucei. Antimicrob Agents Chemother 52:875–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque-Ortega JR, Reuther P, Rivas L, Dardonville C. (2010) New benzophenone-derived bisphosphonium salts as leishmanicidal leads targeting mitochondria through inhibition of respiratory complex II. J Med Chem 53:1788–1798 [DOI] [PubMed] [Google Scholar]
- Mäser P, Sütterlin C, Kralli A, Kaminsky R. (1999) A nucleoside transporter from Trypanosoma brucei involved in drug resistance. Science 285:242–244 [DOI] [PubMed] [Google Scholar]
- Mathis AM, Bridges AS, Ismail MA, Kumar A, Francesconi I, Anbazhagan M, Hu Q, Tanious FA, Wenzler T, Saulter J, et al. (2007) Diphenyl furans and aza analogs: effects of structural modification on in vitro activity, DNA binding, and accumulation and distribution in trypanosomes. Antimicrob Agents Chemother 51:2801–2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matovu E, Stewart ML, Geiser F, Brun R, Mäser P, Wallace LJ, Burchmore RJ, Enyaru JC, Barrett MP, Kaminsky R, Seebeck T, de Koning HP. (2003) Mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryot Cell 2:1003–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natto MJ, Wallace LJ, Candlish D, Al-Salabi MI, Coutts SE, de Koning HP. (2005) Trypanosoma brucei: expression of multiple purine transporters prevents the development of allopurinol resistance. Exp Parasitol 109:80–86 [DOI] [PubMed] [Google Scholar]
- Priotto G, Kasparian S, Mutombo W, Ngouama D, Ghorashian S, Arnold U, Ghabri S, Baudin E, Buard V, Kazadi-Kyanza S, et al. (2009) Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: a multicentre, randomised, phase III, non-inferiority trial. Lancet 374:56–64 [DOI] [PubMed] [Google Scholar]
- Wallace LJ, Candlish D, De Koning HP. (2002) Different substrate recognition motifs of human and trypanosome nucleobase transporters. Selective uptake of purine antimetabolites. J Biol Chem 277:26149–26156 [DOI] [PubMed] [Google Scholar]
- Ward CP, Burchmore RJ, De Koning HP, Barrett MP. (2011) Trypanocidal furamidine analogues: influence of pyridine nitrogens on trypanocidal activity, transport kinetics and resistance patterns. Antimicrob Agents Chemother doi:10.1128/AAC.01551-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward CP, Burgess KE, Burchmore RJ, Barrett MP, de Koning HP. (2010) A fluorescence-based assay for the uptake of CPD0801 (DB829) by African trypanosomes. Mol Biochem Parasitol 174:145–149 [DOI] [PubMed] [Google Scholar]
- Williamson J, Rollo IM. (1959) Drug resistance in trypanosomes: cross-resistance analyses. Br Pharm Chemother 14:423–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun O, Priotto G, Tong J, Flevaud L, Chappuis F. (2010) NECT is next: implementing the new drug combination therapy for Trypanosoma brucei gambiense sleeping sickness. PLoS Negl Trop Dis 4:e720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.