Abstract
2-Arachidonyl glycerol (2-AG) is an endogenous arachidonic acid derivative capable of suppressing interleukin (IL)-2 production by activated T cells. 2-AG-mediated IL-2 suppression is dependent on cyclooxygenase-2 (COX-2) metabolism and peroxisome proliferator activated receptor γ (PPARγ) activation. The objective of the present studies was to examine whether 15-deoxy-Δ12,14-PGJ2-glycerol ester (15d-PGJ2-G), a putative metabolite of 2-AG, can mimic the actions of 2-AG on IL-2 regulation through PPARγ activation. 15d-PGJ2-G bound PPARγ-ligand binding domain in a PPARγ competitive binding assay. 15d-PGJ2-G treatment activated PPARγ in a reporter assay, and PPARγ activation was attenuated when a PPARγ antagonist, 2-chloro-5-nitro-N-4-pyridinylbenzamide (T0070907), was present. 15d-PGJ2-G treatment suppressed IL-2 production by activated Jurkat cells, which was partially attenuated when pretreated with T0070907. Moreover, IL-2 suppression was pronounced when 15d-PGJ2-G was present 30 min before or after T-cell activation. Concordant with IL-2 suppression, 15d-PGJ2-G treatment decreased nuclear factor of activated T cells (NFAT) transcriptional activity in transiently transfected Jurkat cells. It is noteworthy that T0070907 alone markedly increased NFAT reporter activity, suggesting the existence of endogenous PPARγ activation and modulation of NFAT. Because COX-2 metabolism of 2-AG is important for IL-2 suppression, the effect of 2-AG on COX-2 and PPARγ mRNA expression was investigated. 2-AG treatment decreased the up-regulation of COX-2 mRNA after T-cell activation, which suggests negative feedback limiting COX-2-mediated metabolism of 2-AG. PPARγ mRNA expression was increased upon activation, and 2-AG treatment produced a modest decrease in PPARγ mRNA expression. Collectively, our findings suggest that 15d-PGJ2-G activates PPARγ to decrease NFAT transcriptional activity and IL-2 expression in activated T cells.
Introduction
2-Arachidonyl glycerol (2-AG) is an endogenous arachidonic acid derivative released on demand from membrane precursors (Piomelli, 2003). 2-AG was first isolated from canine gut (Mechoulam et al., 1995; Sugiura et al., 1995) and has subsequently been identified in several tissues and cell types, including rat microglial cells (Carrier et al., 2004), neuronal cells (Stella et al., 1997), human platelets, mouse macrophages, and human lymphocytes (Berdyshev et al., 2001). Many of the effects of 2-AG have been attributed to cannabinoid receptors CB1 and CB2, hence 2-AG was classified as an endocannabinoid (Mechoulam et al., 1995; Sugiura et al., 1995). Within the immune system, 2-AG has been demonstrated to suppress cytokine production, including tumor necrosis factor-α release from lipopolysaccharide (LPS)-treated murine macrophages, LPS-treated rat microglial cells, and IL-6 production by J774 macrophages (Berdyshev, 2005). With respect to T-cell function, 2-AG produced a concentration-dependent suppression of anti-CD3-induced T-cell proliferation and the mixed lymphocyte response (Lee et al., 1995). In addition, 2-AG suppressed IL-2 secretion through impairment of NFAT in activated murine splenocytes (Ouyang et al., 1998) and Jurkat T cells (Rockwell et al., 2006). IL-2 is a critical cytokine for T-cell growth and development and is rapidly up-regulated upon T-cell activation. Although many of the 2-AG-mediated immunomodulatory effects have been attributed to ligation of the CB2 receptor, it has also been demonstrated that 2-AG, anandamide (AEA), and arachidonic acid (AA) all suppress IL-2 at almost identical IC50 values (Rockwell and Kaminski, 2004; Rockwell et al., 2006). Further characterization of 2-AG-mediated immune modulation revealed that IL-2 suppression in activated murine splenocytes occurs independently of both CB1 and CB2 receptors and involves, at least in part, the activation of PPARγ (Rockwell et al., 2006).
PPARγ belongs to the nuclear transcription factor superfamily and usually exists as a heterodimer with retinoid X receptor. In the resting state, this complex is on the PPAR response elements that are present in the regulatory regions of various target genes, and it may or may not exist in association with corepressors depending on the promoter. Ligand binding promotes the dissociation of corepressors (if associated) and the recruitment of coactivators such as steroid receptor coactivator 1 and cAMP response element-binding protein/E1A binding protein p300. Coactivator recruitment facilitates the integration of histone acetyl transferases and thus helps in transcriptional activation of target genes (Glass and Rosenfeld, 2000). In addition to transactivation, ligand-activated PPARγ/retinoid X receptor also participates in transrepression by physical association with and sequestration of transcription factors, including NFAT, nuclear factor of the κ-enhancer in B cells, and activator protein-1, leading to subsequent inhibition of their functions in gene transcription (Ricote et al., 1998; Yang et al., 2000). Although all three PPAR subtypes have been detected in various immune cell types, PPARγ1 expression has been detected in T cells, and its activation has been correlated with IL-2 suppression (Clark et al., 2000).
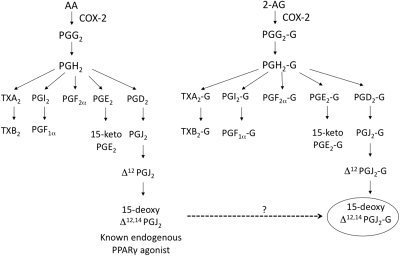
2-AG treatment activates PPARγ as evidenced by increased PPARγ-dependent adipogenesis, causing the following: 1) the differentiation of 3T3-L1 fibroblasts into adipocytes; 2) increased mRNA expression of adipocyte protein 2, a gene regulated by PPARγ; and 3) increased PPARγ-specific luciferase activity and PPAR response element binding (Rockwell et al., 2006). It is noteworthy that 2-AG-mediated IL-2 suppression is dependent on COX-2 metabolism of 2-AG, suggesting that a metabolite of 2-AG is responsible for the IL-2 suppression (Rockwell et al., 2008). A comparison of the eicosanoid pathway of AA and 2-AG (which is structurally similar to AA) predicts that the potential metabolites of 2-AG are glycerol esters of various prostaglandins and thromboxanes produced from AA (Kozak et al., 2002) (Fig. 1). One of the putative metabolites of 2-AG is a glycerol ester of a prostaglandin, 15d-PGJ2-G. Because one of the known agonists for PPARγ is 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2), the overall objective of the current studies was to investigate whether 15d-PGJ2-G could mimic the IL-2-suppressive activity of 2-AG through PPARγ activation.
Fig. 1.
Comparison of the metabolic pathway of AA and 2-AG. AA and 2-AG can be metabolized by COX-2 to form PGH2 and PGH2-G, respectively. Depending on the downstream enzymes, AA and 2-AG can be further metabolized to form various prostaglandins and their glycerol esters, respectively. 15-Deoxy-Δ12,14 PGJ2 is a known endogenous agonist for PPARγ and it is hypothesized that 15-deoxy-Δ12,14-PGJ2-G (the glycerol ester of 15-deoxy-Δ12,14-PGJ2) may act as an agonist for PPARγ. TX, thromboxane.
Materials and Methods
Reagents.
2-AG and AEA were provided by the National Institute on Drug Abuse (Bethesda, MD). Ciglitazone (CGZ), rosiglitazone (RGZ), 15d-PGJ2, 15d-PGJ2-G, and 2-chloro-5-nitro-N-4-pyridinylbenzamide (T0070907) were purchased from Cayman Chemical (Ann Arbor, MI). All other reagents were purchased from Sigma-Aldrich Co. (St. Louis, MO) unless otherwise indicated.
Cell Culture.
Jurkat cells (clone E6–1; American Type Culture Collection, Manassas, VA) and Jurkat T-Ag cells (Jurkat T cells stably transfected with large T antigen; a generous gift from Dr. Arthur Weiss, University of California, San Francisco, CA) were maintained in RPMI 1640 medium supplemented with 10% bovine calf serum (BCS), 100 units/ml penicillin, 100 μg/ml streptomycin, 1× solutions of nonessential amino acids, and sodium pyruvate (Invitrogen, Carlsbad, CA). HEK293T cells were purchased from Open Biosystems and maintained in complete DMEM, consisting of Dulbecco's modified Eagle's medium supplemented with 10% bovine calf serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 3.7 g/l sodium bicarbonate.
IL-2 ELISA.
Jurkat cells were cultured in triplicate (5 × 105 cells/ml) in 48-well culture plates (1 ml/well). The supernatants were collected 24 h after stimulation with phorbol 12-myristate 13-acetate plus calcium ionophore (40 nM PMA/0.5 μM Io), and IL-2 protein was quantified by a sandwich ELISA method as described previously (Kaplan et al., 2003). The IL-2 standard (human recombinant IL-2), mouse anti-human IL-2 antibody, and biotinylated anti-human IL-2 antibody were purchased from BD Pharmingen (San Diego, CA).
Lanthascreen TR-FRET PPARγ Competitive Binding Assays.
PPARγ binding was assessed according to the manufacturer's instructions (Invitrogen). The assay was performed in black 384-microwell plates (Matrical Bioscience, Spokane WA). In brief, a 20-μl total reaction mixture contained 0.5 nM PPARγ-LBD (GST), 5 nM terbium-tagged anti-GST antibody, 5 nM Fluormone Pan-PPAR Green, 5 mM dithiothreitol, and varying concentrations of RGZ, CGZ, 2-AG, AEA, and 15d-PGJ2-G (1 pM–100 μM). The negative control was devoid of the agonist but included everything else contained in the agonist wells. After 3-h incubation in the dark, TR-FRET measurements were made in the SPECTRAmax GEMINI XS spectrofluorometer (Molecular Devices, Sunnyvale, CA) using the following settings: optical module, LanthaScreen; delay time, 100 μs; and integration time, 200 μs. The ratiometric emission at 520 nm/490 nm (520/490) was plotted against various agonist concentrations. The data were analyzed by using Prism software (GraphPad Software, Inc., San Diego) using the sigmoidal curve equation with variable slope to obtain IC50 values. The assay quality/robustness score −Z′ was calculated and was found to be 0.68 (a value above 0.5 indicates a robust assay).
Plasmids.
Human PPARγ-LBD and pFR-luc reporter gene plasmids were provided by authors J.P.V.H. and J.T.T. The LBD of human PPARγ was fused to the DNA-binding domain of the yeast transcription factor Gal4 under the control of the simian virus 40 promoter. This plasmid was cotransfected with pFR, a luciferase reporter under the control of the Gal4 DNA response element (Vanden Heuvel et al., 2006). NFAT-luc reporter gene plasmid was purchased from Clontech (Mountain View, CA).
Transient Transfection Assays.
For PPARγ reporter assay in HEK293T cells, 2.5 × 104 cells per well were preseeded in a 96-well plate in growth medium for 16 to 20 h. The cells were then incubated with the transfection reagents [25 ng of hPPARγ-LBD, 25 ng of pFR-luc, and 0.125 μl of Lipofectamine 2000 (Invitrogen, Carlsbad, CA) for every well] for 4 h in serum-free medium. After the 4-h incubation, the medium containing the transfection reagents was removed and replaced with complete growth medium, and the cells were allowed to recover for 1 h. Hence, 5 h after transfection, the cells were cultured in the absence or presence of either vehicle (0.02% DMSO) or T0070907 for 30 min, followed by the addition of either 15d-PGJ2 or 15d-PGJ2-G (0.1–10 μM). Treatments were performed in triplicate. For Jurkat T-Ag, the cells (5 × 105 cells/ml) were incubated with transfection reagents (0.75 μg of hPPARγ-LBD, 0.75 μg of pFR-luc, and 3 μl of Lipofectamine 2000 for every 5 × 105 cells) for 4 h in RPMI 1640 medium with 2% BCS. After the 4-h incubation, the cells were treated with either CGZ (positive control), 15d-PGJ2, or 15d-PGJ2-G. Treatments were performed in triplicate. For studies with Jurkat, 5 × 105 cells/ml were incubated with transfection reagents (1.5 μg of NFAT-luc and 3 μl of Lipofectamine 2000 for every 5 × 105 cells) for 4 h in RPMI 1640 medium with 2% BCS. After the 4-h incubation, the cells were cultured in the absence or presence of either vehicle (0.02% DMSO) or T0070907 for 30 min, followed by the addition of cyclosporin A (CsA), CGZ, 15d-PGJ2, or 15d-PGJ2-G for 30 min followed by PMA/Io stimulation (40 nM/0.5 μM). Twenty-four hours after transfection (for HEK293T cells, Jurkat T-Ag cells, and Jurkat cells), luciferase activity was determined using the Luciferase Assay System and Reporter Lysis Buffer from Promega (Madison, WI). Protein determinations were performed using a bicinchoninic acid assay (BCA; Sigma-Aldrich). The luciferase activity was normalized to the determined amount of total protein.
Real-Time PCR.
Jurkat T cells (5 × 106 cells) were treated with 2-AG (20 μM) for 30 min at 37°C, followed by 40 nM PMA/0.5 μM Io stimulation for 2, 4, 8, and 12 h in complete medium containing 2% BCS in a 60- × 15-mm cell culture dish (Corning Life Sciences, Lowell, MA). Cells were harvested, and RNA isolation was performed using the Promega SV Total RNA Isolation System (Promega, Madison, WI). Total RNA was reverse-transcribed using random primers with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). cDNA was amplified with TaqMan primers and probe sets (Applied Biosystems) and analyzed using a 7900 HT Fast Real-Time PCR System (Applied Biosystems).
Statistical Analysis.
The mean ± S.E. was determined for each treatment group in the individual experiments. Homogenous data were evaluated by one- or two-way parametric analysis of variance. Dunnett's two-tailed t test was used to compare treatment groups to the VEH control when significant differences were observed using Prism software and SigmaStat software (Systat Software, Inc., San Jose, CA).
Results
15d-PGJ2-G Activates PPARγ-LBD in HEK293T and Jurkat T-Ag Cells.
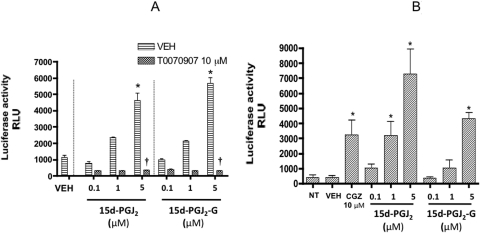
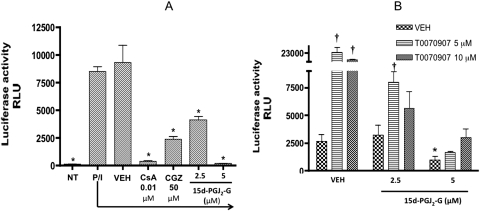
A comparison of the eicosanoid pathway of AA and 2-AG suggested that AA and 2-AG can both be metabolized by COX-2 to form PGH2 and PGH2-G (glycerol ester) and, depending on the various downstream enzymes, they can form various prostaglandins/thromboxanes and glycerol esters of prostaglandins/thromboxanes, respectively (Kozak et al., 2002) (Fig. 1). One of the putative metabolites of 2-AG is 15d-PGJ2-G, which is the glycerol ester of 15d-PGJ2, a known agonist for PPARγ. 15d-PGJ2-G treatment of HEK293T cells transfected with PPARγ reporter plasmids induced PPARγ-LBD-driven luciferase activity in a concentration-dependent manner (Fig. 2A). 15d-PGJ2 was used as a positive control and also induced PPARγ-LBD-driven luciferase activity in a concentration-dependent manner. It is noteworthy that in the presence of the PPARγ antagonist T0070907, PPARγ reporter activity was abolished, confirming that 15d-PGJ2-G activates PPARγ-LBD (Fig. 2A). Because 15d-PGJ2-G activates PPARγ-LBD in HEK293T cells and it has been demonstrated that 2-AG, other PPARγ agonists, and 15d-PGJ2 suppress IL-2 in activated T cells (Rockwell et al., 2006), we investigated whether 15d-PGJ2-G activates PPARγ-LBD in a T-cell line. We performed PPARγ reporter assays in Jurkat T-Ag cells because constitutive expression of the large T antigen is essential for the robust expression of the PPARγ-LBD plasmid (Jurkat cells—clone E6–1 lacks large T antigen). In Jurkat T-Ag cells transfected with PPARγ reporter plasmids, 15d-PGJ2-G induced PPARγ-LBD-driven luciferase activity in a concentration-dependent manner. CGZ and 15d-PGJ2, which are known PPARγ agonists (Forman et al., 1995), also activated PPARγ-LBD in Jurkat T-Ag cells (Fig. 2B).
Fig. 2.
PPARγ-LBD reporter activity in HEK293T and Jurkat T-Ag cells treated with 15d-PGJ2-G. A, HEK293T cells (2.5 × 104 cells per well) were preseeded in a 96-well plate in growth medium for 16 to 20 h. The cells were then transiently transfected with hPPARγ-LBD and pFR-luc. After transfection, the cells were cultured in the absence or presence of either vehicle (0.02% DMSO) or T0070907 for 30 min, followed by the addition of vehicle (VEH of agonist, 0.1% EtOH), 15d-PGJ2, or 15d-PGJ2-G (0.1–5 μM). Twenty-four hours after transfection, the luciferase activity was quantified in relative light units (RLU) by chemiluminescence assay. The results are the mean ± S.E. of triplicate cultures. Statistical significance is indicated by *, p < 0.05 compared with VEH of agonist alone and †, p < 0.05 compared with VEH for T0070907 within each treatment group. The results are representative of three separate experiments. B, Jurkat T-Ag cells (5 × 105 cells/ml) were transiently transfected with hPPARγ-LBD and pFR-luc using Lipofectamine 2000 for 4 h in RPMI 1640 medium with 2% BCS in a 48-well plate. After the 4-h incubation, the cells were either left untreated (NT, no treatment) or treated with CGZ, 15d-PGJ2, 15d-PGJ2-G, or vehicle (VEH of agonist, 0.1% EtOH). Twenty-four hours after transfection, the luciferase activity was quantified in relative light units (RLU) by chemiluminescence assay. The results are the mean ± S.E. of triplicate cultures. Statistical significance is indicated by *, p < 0.05 compared with VEH of agonist. The results are representative of three separate experiments.
15d-PGJ2-G Binds to PPARγ-LBD in a PPARγ Competitive Binding Assay.
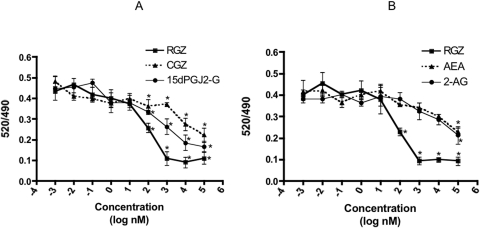
To verify direct binding of 15d-PGJ2-G to PPARγ, a Lanthascreen TR-FRET PPARγ competitive binding assay was performed. PPARγ agonists RGZ and CGZ were used as comparative controls. 15d-PGJ2-G bound PPARγ-LBD by competitively displacing the Fluormone Pan-PPAR Green, as evidenced by the decrease in the 520/490 ratio (Fig. 3A). The IC50 values were calculated from these concentration response curves and are as follows: RGZ, 74.6 nM; 15d-PGJ2-G, 367.5 nM; and CGZ, 3.45 μM. These results show that 15d-PGJ2-G binds to PPARγ-LBD with lower affinity than RGZ but with higher affinity than CGZ. In addition, the endocannabinoids 2-AG and AEA were also assayed for binding to PPARγ-LBD (Fig. 3B). The determined IC50 values were 2-AG, 13.5 μM; and AEA, 26.8 μM. The rank order potency is RGZ > 15d-PGJ2-G > CGZ > 2-AG > AEA. These results further show that 2-AG itself is a relatively low-affinity ligand for PPARγ, especially compared with 15d-PGJ2-G.
Fig. 3.
Lanthascreen TR-FRET PPARγ competitive binding assay. A, effect of 15d-PGJ2-G, CGZ, and RGZ. B, effect of 2-AG and AEA. The reaction mixture contained 0.5 nM PPARγ-LBD (GST), 5 nM Terbium-tagged anti-GST antibody, 5 nM Fluormone Pan-PPAR Green, 5 mM dithiothreitol, and varying concentrations of RGZ, CGZ, 15d-PGJ2-G, 2-AG, and AEA (1 pM–100 μM). After 3-h incubation in the dark, TR-FRET measurements were made in the SPECTRAmax GEMINI XS spectrofluorometer. The results are the mean ± S.E. of triplicate cultures. Statistical significance is indicated by *, p < 0.05 compared with the TR-FRET ratio of respective vehicle control (VEH of RGZ, 5% EtOH: 0.43063; VEH of CGZ and 15d-PGJ2-G, 1% EtOH: 0.44179; VEH of 2-AG and AEA, 1% EtOH: 0.43342). The results are representative of three separate experiments.
15d-PGJ2-G Suppresses IL-2 Secretion in a Concentration-Dependent and Time-Dependent Manner.
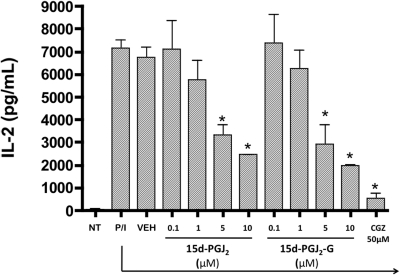
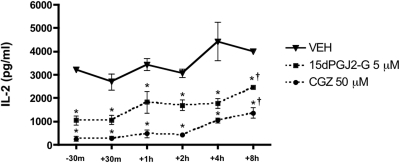
Because 15d-PGJ2-G bound to and activated PPARγ, the effect of 15d-PGJ2-G-mediated PPARγ activation on IL-2 secretion was investigated in PMA/Io-stimulated Jurkat T cells. 15d-PGJ2-G treatment produced a robust concentration-dependent suppression of IL-2 secretion compared with the vehicle control (VEH, 0.1% EtOH) (Fig. 4). Treatment with increasing concentrations of 15d-PGJ2 (comparative control) produced a concentration-dependent suppression of IL-2 secretion (Rockwell et al., 2006). CGZ (50 μM used as positive control) also suppressed IL-2 secretion (Clark et al., 2000). Furthermore, we investigated the effect of the presence of 15d-PGJ2-G at various times in relation to stimulation of Jurkat T cells. Time-of-addition studies in activated Jurkat T cells with 15d-PGJ2-G demonstrated that the presence of 15d-PGJ2-G 30 min before or after stimulation of Jurkat cells produced pronounced IL-2 suppression, whereas when 15d-PGJ2-G was added at later time points, 15d-PGJ2-G-mediated IL-2 suppression was attenuated (Fig. 5). A similar profile was obtained with the presence of CGZ at varying time points. Overall, these results suggest that 15d-PGJ2-G has to be present early during T-cell activation for robust IL-2 suppression.
Fig. 4.
Effect of 15d-PGJ2-G upon IL-2 secretion by activated Jurkat cells. Jurkat cells (5 × 105 cells/ml) were either left untreated (NT, no treatment) or treated with CGZ (50 μM), 15d-PGJ2 (0.1–10 μM), 15d-PGJ2-G (0.1–10 μM), or vehicle (VEH of agonist, 0.1% EtOH) for 30 min. Cells were then stimulated with 40 nM PMA/0.5 μM ionomycin (P/I) for 24 h. The supernatants were harvested, and IL-2 production was measured by ELISA. The results are the mean ± S.E. of triplicate cultures. *, p < 0.05, statistically significant compared with VEH of agonist. The results are representative of three separate experiments.
Fig. 5.
Time of addition studies with 15d-PGJ2-G in activated Jurkat cells. At time −30 min to 8 h after stimulation with PMA/Io (P/I), Jurkat cells (5 × 105 cells/ml) were treated with CGZ (50 μM), 15d-PGJ2-G (5 μM), or vehicle (VEH of agonist, 0.1% EtOH). 24 h after stimulation, the supernatants were harvested, and IL-2 production was measured by ELISA. The results are the mean ± S.E. of triplicate cultures. Statistical significance is indicated by *, p < 0.05 compared with the time-matched VEH control, and †, p < 0.05 compared with the respective −30 and +30 min treatment. The results are representative of three separate experiments.
A PPARγ Antagonist, T0070907, Attenuates 15d-PGJ2-G-Mediated IL-2 Suppression.
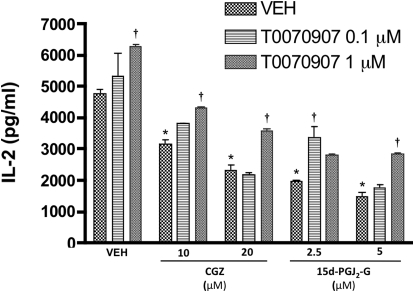
Because 15d-PGJ2-G activated PPARγ and caused IL-2 suppression in activated Jurkat cells, we investigated whether a PPARγ antagonist, T0070907, attenuated the IL-2 suppression. Pretreatment of Jurkat cells with T0070907 partially attenuated the IL-2 suppression caused by 15d-PGJ2-G and CGZ. It is noteworthy that T0070907 (1 μM), in the presence of VEH (0.1% EtOH), produced an increase in IL-2 secretion, suggesting an intrinsic endogenous activity through PPARγ (Fig. 6). It is notable that T0070907 at higher concentrations (2 and 5 μM) did not attenuate 15d-PGJ2-G-mediated IL-2 suppression and that this may be due to other off-target effects of the antagonist (data not shown).
Fig. 6.
Effect of T0070907 on 15d-PGJ2-G-mediated IL-2 secretion by activated Jurkat cells. Jurkat cells (5 × 105 cells/ml) were treated with vehicle (VEH of antagonist, 0.02% DMSO) or T0070907 (0.1 and 1 μM) for 45 min, followed by the addition of CGZ (10 or 20 μM), 15d-PGJ2-G (2.5 or 5 μM), or vehicle (VEH of agonist, 0.1% EtOH) for 30 min. Cells were then stimulated with 40 nM PMA/0.5 μM ionomycin (P/I) for 24 h. The supernatants were harvested, and IL-2 production was measured by ELISA. The results are the mean ± S.E. of triplicate cultures. Statistical significance is indicated by *, p < 0.05 compared with (VEH of agonist + VEH of antagonist) and †, p < 0.05 compared with VEH of T0070907 within each treatment group. The results are representative of three separate experiments.
15d-PGJ2-G Decreases Transcriptional Activity of NFAT and T0070907 Alone Markedly Increases NFAT Reporter Activity in Activated Jurkat Cells.
One of the most important transcription factors involved in IL-2 gene transcription is NFAT and the increased availability of nuclear NFAT after activation of T cells is responsible for increased IL-2 gene transcription (Clipstone and Crabtree, 1992). Ligation of PPARγ with 15d-PGJ2 decreased DNA binding of NFAT and caused sequestration of NFAT to PPARγ in human peripheral blood T cells (Yang et al., 2000). Because 15d-PGJ2-G activated PPARγ and decreased PMA/Io-stimulated IL-2 secretion, we evaluated the effect of 15d-PGJ2-G on NFAT transcriptional activity. 15d-PGJ2-G treatment of activated Jurkat cells transiently transfected with NFAT-luc caused a concentration-dependent decrease in PMA/Io-stimulated luciferase activity (Fig. 7A). CsA, an inhibitor of calcineurin that prevents NFAT dephosphorylation and entry into the nucleus, was used as a positive control (Clipstone and Crabtree, 1992). It is noteworthy that CGZ also decreases the transcriptional activity of NFAT, suggesting that activation of PPARγ causes a decrease in the transcriptional activity of NFAT (Fig. 7A). Because PPARγ agonists such as troglitazone and 15d-PGJ2 decreased the transcriptional activity of NFAT (Yang et al., 2000), and we also showed that 15d-PGJ2-G and CGZ decreased the transcriptional activity of NFAT, we investigated whether a PPARγ antagonist can attenuate the 15d-PGJ2-G-mediated decrease in NFAT transcriptional activity in activated Jurkat cells. It is noteworthy that NFAT reporter activity was greatly enhanced with T0070907 (5 and 10 μM) in the presence of the vehicle of agonist (Fig. 7B). The T0070907-mediated enhanced NFAT reporter activity was attenuated in the presence of 15d-PGJ2-G. It has also been demonstrated earlier that 2-AG decreases NFAT reporter activity in activated Jurkat cells (Rockwell et al., 2006). It is noteworthy that the 2-AG-mediated decrease in NFAT reporter activity was attenuated in the presence of the PPARγ antagonist T0070907 (Rockwell et al., 2006). These data suggest that the NFAT reporter activity observed after PMA/Io stimulation is a balance between the reporter activity caused by PMA/Io stimulation and the suppression of reporter activity caused by the endogenous agonist(s) for PPARγ. In the presence of T0070907, the suppression of the reporter activity caused by PPARγ activation [by the endogenous agonist(s)] may be relieved; hence, an increase in NFAT reporter activity was observed.
Fig. 7.
NFAT reporter activity in Jurkat cells treated with 15d-PGJ2-G in the absence (A) and presence (B) of the PPARγ antagonist T0070907. Jurkat cells (5 × 105 cells/ml) were transiently transfected with NFAT luciferase reporter using Lipofectamine 2000 for 4 h in RPMI 1640 medium with 2% BCS in a 48-well plate. A, after the 4-h incubation, the cells were either left untreated (NT, no treatment) or treated with vehicle (VEH of agonist, 0.1% EtOH), CsA (0.01 μM), CGZ (50 μM), or 15d-PGJ2-G (5 and 10 μM) for 30 min followed by 40 nM PMA/0.5 μM Io stimulation (P/I). B, after the 4-h incubation, the cells were treated with vehicle of the antagonist (0.02% DMSO) or T0070907 (5 and 10 μM) for 30 min. Then the cells were treated with vehicle (VEH of agonist, 0.1% EtOH), or 15d-PGJ2-G (2.5–5 μM) for 30 min followed by P/I. Twenty-four hours after transfection, the luciferase activity was quantified in relative light units (RLU) by chemiluminescence assay. The results are the mean ± S.E. of triplicate cultures. In A, statistical significance is indicated by *, p < 0.05 compared with VEH of agonist. In B, statistical significance is indicated by *, p < 0.05 compared with (VEH of agonist + VEH of T0070907) and †, p < 0.05 compared with VEH for T0070907 within each treatment group. The results are representative of three separate experiments.
2-AG Significantly Decreases PMA/Io-Mediated Increase in COX-2 mRNA Expression but Produces Only a Modest Decrease in PPARγ mRNA Expression.
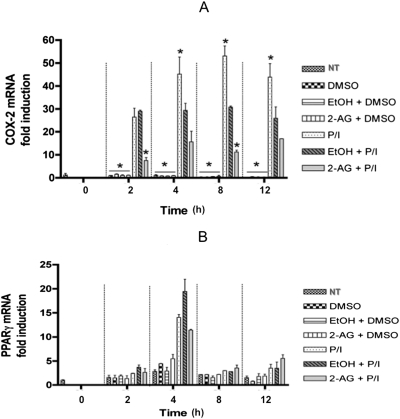
COX-2 oxygenates 2-AG as effectively as AA and may lead to the generation of glycerol esters of prostaglandins (Kozak et al., 2002). We have previously demonstrated that PMA/Io stimulation of Jurkat cells caused an increase in both COX-2 mRNA and protein expression (Rockwell et al., 2008). We now investigated the effect of the presence of 2-AG on COX-2 mRNA expression in activated Jurkat cells. PMA/Io stimulation increased COX-2 mRNA expression at 2, 4, 8, and 12 h after stimulation as demonstrated previously (Rockwell et al., 2008). It is noteworthy that 2-AG treatment (20 μM) significantly decreased PMA/Io-mediated increase in COX-2 mRNA expression at 2 and 8 h (Fig. 8A). On the other hand, PMA/Io stimulation of Jurkat cells caused an increase in PPARγ mRNA expression only at 4 h after stimulation, and the expression decreased sharply at 8 h and 12 h. 2-AG treatment modestly decreased the PMA/Io-mediated increase in PPARγ mRNA expression at 4 h, although it was not statistically significant (Fig. 8B). These results suggest the existence of a negative feedback loop to limit the generation of metabolites from 2-AG by COX-2 metabolism, and it is tempting to speculate that this feedback inhibition may serve as a checkpoint to limit IL-2 suppression by PPARγ activation.
Fig. 8.
Real-time PCR analysis of COX-2 mRNA (A) and PPARγ mRNA (B) levels in resting and activated Jurkat T cells treated with 2-AG. Jurkat cells were left untreated (NT, no treatment), treated with vehicles (VEH of 2-AG, 0.1% EtOH; VEH of PMA/Io, 0.1% DMSO) or 2-AG (20 μM) in the presence or absence of 40 nM PMA/0.5 mM ionomycin (P/I) for the indicated times (0–12 h) after which the RNA was isolated. COX-2 mRNA and PPARγ mRNA levels were detected by real-time PCR analysis and normalized to 18S mRNA levels. The results are expressed as fold induction over the NT samples at 0 h. The results are the mean ± S.E. of triplicate cultures. Statistical significance is indicated by *, p < 0.05 compared with the time-matched EtOH + P/I. The results are representative of three separate experiments.
Discussion
The major objective of the current studies was to rigorously investigate the role of 15d-PGJ2-G, a putative metabolite of 2-AG, in mediating IL-2 suppression. The activation of a PPARγ-specific Gal4-responsive reporter by 15d-PGJ2-G in HEK293T and Jurkat T-Ag cells suggests that this putative metabolite can act as a PPARγ agonist. It is noteworthy that 15d-PGJ2-G bound PPARγ-LBD with high affinity, as evidenced by the IC50 value (367.5 nM) compared with that of 2-AG (13.5 μM). This observation implicates that 15d-PGJ2-G is a higher affinity PPARγ agonist than the parent molecule, 2-AG. In addition, the rank order of IC50 for PPARγ agonists is RGZ > 15d-PGJ2-G > CGZ. These IC50 values are concordant with the reported EC50 values for RGZ (60 ± 4 nM) and CGZ (3.0 ± 0.7 μM), as evidenced by transactivation assays in CV-1 cells transfected with a chimera consisting of the PPARγ-LBD fused to the Gal4 DNA-binding domain, together with a reporter plasmid containing a GAL4-responsive promoter driving expression of chloramphenicol transferase (Willson et al., 1996). Furthermore, 15d-PGJ2-G also produced a concentration-dependent decrease in IL-2 secretion that was pronounced if 15d-PGJ2-G was present 30 min before or after T-cell activation. 15d-PGJ2-G-mediated IL-2 suppression was partially attenuated in the presence of T0070907, a PPARγ antagonist, suggesting the involvement of PPARγ in the IL-2 suppression. The present studies are the first to demonstrate that 15d-PGJ2-G, a putative metabolite of 2-AG that is downstream of COX-2 metabolism, might be responsible for the IL-2 suppression observed by 2-AG.
The expression of PPARγ1 has been established in T cells, and its activation has been correlated with IL-2 suppression (Clark et al., 2000). Many endogenous ligands of PPARγ have been identified, such as 15d-PGJ2 (Forman et al., 1995), 5-S-hydroxyeicosatetraenoic acid (Nagy et al., 1998), polyunsaturated fatty acids (Kliewer et al., 1997), 13-oxooctadecadienoic acid (Bull et al., 2003), 2,4-dienone 13-oxooctadecadienoic acid (Altmann et al., 2007), and components of oxidized low-density lipoprotein, such as 9-hydroxyoctadecadienoic acid and 13-S-hydroxyoctadecadienoic acid (Nagy et al., 1998). Of these ligands, 5-S-hydroxyeicosatetraenoic acid is produced by the action of 15-lipooxygenase on AA, whereas 15d-PGJ2 is produced by the action of COX-2 on AA. We have demonstrated previously that pretreatment with the COX-2-specific inhibitor N-[2-(cyclohexyloxyl)-4-nitrophenyl]-methane sulfonamide (NS398) attenuated the 2-AG-mediated IL-2 suppression in activated Jurkat cells (Rockwell et al., 2008). Therefore, we focused our studies on 15d-PGJ2-G, the putative COX-2 metabolite of 2-AG, which is structurally similar to the known endogenous PPARγ agonist, 15d-PGJ2.
2-AG is a substrate for COX-2, and there is considerable evidence suggesting the formation of glycerol esters of prostaglandins from 2-AG (Kozak et al., 2002). The diversity of prostaglandins (PGs) obtained from AA and 2-AG may provide a unique repertoire of mediators customized for specific responses. It has been demonstrated that the glycerol esters of PGs are in general more stable than the free acid prostaglandins, suggesting that the glycerol esters of PGs may have a longer duration of action, although it is possible for 15d-PGJ2-G to undergo hydrolysis to form 15d-PGJ2 with time. However, these studies are novel in that they suggest that a metabolite rather than the parent molecule itself is responsible for aspects of the immunomodulatory activity mediated by 2-AG.
The ability of 15d-PGJ2-G to bind and activate PPARγ-LBD and suppress IL-2 secretion in activated Jurkat cells strongly suggests that PPARγ activation is involved in IL-2 suppression. 15d-PGJ2-G must be present early during T-cell activation to robustly suppress IL-2, implying that PPARγ activation interferes with molecular events active early during IL-2 gene transcription. It has been demonstrated that NFAT can associate with PPARγ when activated with the PPARγ agonists troglitazone and 15d-PGJ2 (Yang et al., 2000). Likewise, NFAT reporter assays showed decreased reporter activity in activated Jurkat cells when treated with 15d-PGJ2-G and 2-AG (Rockwell et al., 2006). It is noteworthy that CGZ also decreased NFAT reporter activity, suggesting that PPARγ activation leads to a decrease in NFAT transcriptional activity. Together, these results show that PPARγ activation by 15d-PGJ2-G impairs NFAT function in IL-2 gene transcription. It is noteworthy that in the NFAT reporter system, T0070907 markedly enhanced reporter activity, suggesting the existence of endogenous PPARγ activity in activated T cells. It would seem that, upon treatment with T0070907, endogenous PPARγ activity is antagonized leading to increased function/release of NFAT that is reflected as increased reporter activity. In agreement with these results, the modest increase in IL-2 production in activated Jurkat cells when treated with 1 μM T0070907 alone is consistent with the observed increase in NFAT activity. In another study that is concordant with these results, activated EL4.IL2 T cells treated with the PPARγ antagonist 2-chloro-5-nitrobenzanilide (GW9662), showed increased interferon γ (IFNγ) mRNA levels and protein expression (Cunard et al., 2004). It is notable that NFAT is also critical for IFNγ gene transcription (Macian, 2005). This T0070907-mediated induction of NFAT transcriptional activity is in contrast with our previous studies in which T0070907 enhanced IL-2 production but had no significant effect on NFAT reporter activity in activated Jurkat cells (Rockwell et al., 2006). The discrepancy might be due to the fact that in the previous studies, T0070907 was present in the culture for only 12 h, whereas in these studies, T0070907 was present for 20 h.
We demonstrated previously that there is a robust increase in COX-2 mRNA and protein expression upon T-cell activation (Rockwell et al., 2008). Treatment with 2-AG decreased the increase in COX-2 mRNA caused by T-cell activation, implying the existence of a negative feedback loop that decreases COX-2 expression after activation, which leads to decreased generation of metabolites, and thus may be limiting IL-2 suppression that is mediated by PPARγ activation. The negative feedback loop may be either PPARγ-dependent or independent. There is evidence for inhibition of COX-2 by PPARγ ligands in human cervical cancer cells, and it seems to be mediated predominantly through impairment of activator protein-1 binding to the cAMP response element site in the COX-2 promoter (Han et al., 2003). In macrophage-like differentiated U937 cells treated with LPS, 15d-PGJ2 treatment caused a decrease in COX-2 mRNA expression and COX-2 promoter activity by interfering with the nuclear factor of the κ-enhancer in B cell signaling pathway, implying the existence of a negative feedback loop between PPARγ activation and COX-2 expression (Inoue et al., 2000). PPARγ ligands decreased PMA-mediated induction of COX-2 transcription in a concentration-dependent manner in human epithelial cells (Subbaramaiah et al., 2001) and down-regulated COX-2 mRNA and protein expression in human liver cancer cell line HepG2 (Li et al., 2003). In contrast, there is also evidence that pirinixic acid (WY-14,643), a prototypical peroxisome proliferator, enhanced COX-2 expression in human mammary cells and colonic epithelial cells (Meade et al., 1999). WY-14,643 is primarily an activator of PPARα, but it also activates PPARγ (Lehmann et al., 1997) and PPARβ to a lesser extent (Schmidt et al., 1992). Perhaps the increase in COX-2 expression is due to the actions on PPARα and PPARβ. These results taken together suggest that PPARγ generally seems to inhibit COX-2 expression, and this effect may be cell type and/or tissue type-specific. In our study, COX-2 mRNA was decreased by 2-AG treatment beginning at 2 h, whereas PPARγ expression was up-regulated only at 4 h. Therefore, the decrease in COX-2 mRNA expression may be independent of PPARγ at earlier time points and dependent or independent of PPARγ activation during the later time points. More studies are required to understand the molecular mechanisms involved in the negative feedback regulation of COX-2 by PPARγ.
Although the synthetic PPARγ agonist rosiglitazone (Avandia) is currently categorized for restricted use in the management of diabetes, there is mounting evidence that PPARγ activation is involved in immune regulation and in moderating inflammatory responses. PPARγ in T cells has been demonstrated to prevent gut inflammation in mice with experimental inflammatory bowel disease (Guri et al., 2010). Likewise, PPARγ selectively suppressed human and mouse Th17 differentiation (T helper cells secreting IL-17 that play a crucial role in autoimmune diseases such as multiple sclerosis) (Klotz et al., 2009). Likewise, PPARγ has been shown to negatively regulate allergic rhinitis in mice (Fukui et al., 2009). Furthermore, PPARγ also plays an essential role in the control of inflammatory responses by acting on various immune cells such as T cells (Clark et al., 2000), dendritic cells (Angeli et al., 2003), macrophages (Ricote et al., 1998), and mast cells (Sugiyama et al., 2000). Collectively, the aforementioned findings suggest that PPARγ may play an important function in maintaining immune homeostasis and in preventing autoimmune diseases. Further studies are necessary for the identification of the putative metabolite 15d-PGJ2-G within activated T cells and is the focus of ongoing studies in our laboratory. Identification and characterization of the putative metabolite 15d-PGJ2-G will provide important insights into the role of 2-AG and PPARγ in maintaining immune homeostasis.
Acknowledgments
We thank Dr. Arthur Weiss for providing Jurkat T-Ag cells. We thank Kimberly Hambleton for administrative assistance with the preparation of the manuscript.
This work was supported in part by the National Institutes of Health National Institute on Drug Abuse [Grant R01-DA12740]; and by a research fellowship from Pfizer Global Research and Development.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.070441.
- 2-AG
- 2-arachidonyl glycerol
- IL-2
- interleukin-2
- COX-2
- cyclooxygenase-2
- PPARγ
- peroxisome proliferator activated receptor γ
- 15d-PGJ2-G
- 15-deoxy-Δ12,14-PGJ2-glycerol ester
- NFAT
- nuclear factor of activated T cells
- CB1 and CB2
- cannabinoid receptor 1 and 2
- LPS
- lipopolysaccharide
- AEA
- anandamide
- AA
- arachidonic acid
- PG
- prostaglandin
- CGZ
- ciglitazone
- CsA
- cyclosporin A
- IFNγ
- interferon γ
- BSA
- bovine serum albumin.
Authorship Contributions
Participated in research design: Raman, Kaplan, Thompson, Vanden Heuvel, and Kaminski.
Conducted experiments: Raman.
Contributed new reagents or analytic tools: Thompson and Vanden Heuvel.
Performed data analysis: Raman.
Wrote or contributed to the writing of the manuscript: Raman, Kaplan, Thompson, Vanden Heuvel, and Kaminski.
References
- Altmann R, Hausmann M, Spöttl T, Gruber M, Bull AW, Menzel K, Vogl D, Herfarth H, Schölmerich J, Falk W, et al. (2007) 13-Oxo-ODE is an endogenous ligand for PPARgamma in human colonic epithelial cells. Biochem Pharmacol 74:612–622 [DOI] [PubMed] [Google Scholar]
- Angeli V, Hammad H, Staels B, Capron M, Lambrecht BN, Trottein F. (2003) Peroxisome proliferator-activated receptor gamma inhibits the migration of dendritic cells: consequences for the immune response. J Immunol 170:5295–5301 [DOI] [PubMed] [Google Scholar]
- Berdyshev EV. (2005) Endocannabinoids in Inflammation and Immune Response. CRC Press, Boca Raton, FL [Google Scholar]
- Berdyshev EV, Schmid PC, Krebsbach RJ, Schmid HH. (2001) Activation of PAF receptors results in enhanced synthesis of 2-arachidonoylglycerol (2-AG) in immune cells. FASEB J 15:2171–2178 [DOI] [PubMed] [Google Scholar]
- Bull AW, Steffensen KR, Leers J, Rafter JJ. (2003) Activation of PPAR gamma in colon tumor cell lines by oxidized metabolites of linoleic acid, endogenous ligands for PPAR gamma. Carcinogenesis 24:1717–1722 [DOI] [PubMed] [Google Scholar]
- Carrier EJ, Kearn CS, Barkmeier AJ, Breese NM, Yang W, Nithipatikom K, Pfister SL, Campbell WB, Hillard CJ. (2004) Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Mol Pharmacol 65:999–1007 [DOI] [PubMed] [Google Scholar]
- Clark RB, Bishop-Bailey D, Estrada-Hernandez T, Hla T, Puddington L, Padula SJ. (2000) The nuclear receptor PPAR gamma and immunoregulation: PPAR gamma mediates inhibition of helper T cell responses. J Immunol 164:1364–1371 [DOI] [PubMed] [Google Scholar]
- Clipstone NA, Crabtree GR. (1992) Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature 357:695–697 [DOI] [PubMed] [Google Scholar]
- Cunard R, Eto Y, Muljadi JT, Glass CK, Kelly CJ, Ricote M. (2004) Repression of IFN-gamma expression by peroxisome proliferator-activated receptor gamma. J Immunol 172:7530–7536 [DOI] [PubMed] [Google Scholar]
- Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. (1995) 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell 83:803–812 [DOI] [PubMed] [Google Scholar]
- Fukui N, Honda K, Ito E, Ishikawa K. (2009) Peroxisome proliferator-activated receptor gamma negatively regulates allergic rhinitis in mice. Allergol Int 58:247–253 [DOI] [PubMed] [Google Scholar]
- Glass CK, Rosenfeld MG. (2000) The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev 14:121–141 [PubMed] [Google Scholar]
- Guri AJ, Mohapatra SK, Horne WT, 2nd, Hontecillas R, Bassaganya-Riera J. (2010) The role of T cell PPAR gamma in mice with experimental inflammatory bowel disease. BMC Gastroenterol 10:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Inoue H, Flowers LC, Sidell N. (2003) Control of COX-2 gene expression through peroxisome proliferator-activated receptor gamma in human cervical cancer cells. Clin Cancer Res 9:4627–4635 [PubMed] [Google Scholar]
- Inoue H, Tanabe T, Umesono K. (2000) Feedback control of cyclooxygenase-2 expression through PPARgamma. J Biol Chem 275:28028–28032 [DOI] [PubMed] [Google Scholar]
- Kaplan BL, Rockwell CE, Kaminski NE. (2003) Evidence for cannabinoid receptor-dependent and -independent mechanisms of action in leukocytes. J Pharmacol Exp Ther 306:1077–1085 [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, et al. (1997) Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci USA 94:4318–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz L, Burgdorf S, Dani I, Saijo K, Flossdorf J, Hucke S, Alferink J, Nowak N, Novak N, Beyer M, et al. (2009) The nuclear receptor PPAR gamma selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. J Exp Med 206:2079–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak KR, Crews BC, Morrow JD, Wang LH, Ma YH, Weinander R, Jakobsson PJ, Marnett LJ. (2002) Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J Biol Chem 277:44877–44885 [DOI] [PubMed] [Google Scholar]
- Lee M, Yang KH, Kaminski NE. (1995) Effects of putative cannabinoid receptor ligands, anandamide and 2-arachidonyl-glycerol, on immune function in B6C3F1 mouse splenocytes. J Pharmacol Exp Ther 275:529–536 [PubMed] [Google Scholar]
- Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. (1997) Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem 272:3406–3410 [DOI] [PubMed] [Google Scholar]
- Li MY, Deng H, Zhao JM, Dai D, Tan XY. (2003) PPARgamma pathway activation results in apoptosis and COX-2 inhibition in HepG2 cells. World J Gastroenterol 9:1220–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macian F. (2005) NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol 5:472–484 [DOI] [PubMed] [Google Scholar]
- Meade EA, McIntyre TM, Zimmerman GA, Prescott SM. (1999) Peroxisome proliferators enhance cyclooxygenase-2 expression in epithelial cells. J Biol Chem 274:8328–8334 [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR. (1995) Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50:83–90 [DOI] [PubMed] [Google Scholar]
- Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. (1998) Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell 93:229–240 [DOI] [PubMed] [Google Scholar]
- Ouyang Y, Hwang SG, Han SH, Kaminski NE. (1998) Suppression of interleukin-2 by the putative endogenous cannabinoid 2-arachidonyl-glycerol is mediated through down-regulation of the nuclear factor of activated T cells. Mol Pharmacol 53:676–683 [DOI] [PubMed] [Google Scholar]
- Piomelli D. (2003) The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4:873–884 [DOI] [PubMed] [Google Scholar]
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. (1998) The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 391:79–82 [DOI] [PubMed] [Google Scholar]
- Rockwell CE, Kaminski NE. (2004) A cyclooxygenase metabolite of anandamide causes inhibition of interleukin-2 secretion in murine splenocytes. J Pharmacol Exp Ther 311:683–690 [DOI] [PubMed] [Google Scholar]
- Rockwell CE, Raman P, Kaplan BL, Kaminski NE. (2008) A COX-2 metabolite of the endogenous cannabinoid, 2-arachidonyl glycerol, mediates suppression of IL-2 secretion in activated Jurkat T cells. Biochem Pharmacol 76:353–361 [DOI] [PubMed] [Google Scholar]
- Rockwell CE, Snider NT, Thompson JT, Vanden Heuvel JP, Kaminski NE. (2006) Interleukin-2 suppression by 2-arachidonyl glycerol is mediated through peroxisome proliferator-activated receptor gamma independently of cannabinoid receptors 1 and 2. Mol Pharmacol 70:101–111 [DOI] [PubMed] [Google Scholar]
- Schmidt A, Endo N, Rutledge SJ, Vogel R, Shinar D, Rodan GA. (1992) Identification of a new member of the steroid hormone receptor superfamily that is activated by a peroxisome proliferator and fatty acids. Mol Endocrinol 6:1634–1641 [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. (1997) A second endogenous cannabinoid that modulates long-term potentiation. Nature 388:773–778 [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K, Lin DT, Hart JC, Dannenberg AJ. (2001) Peroxisome proliferator-activated receptor gamma ligands suppress the transcriptional activation of cyclooxygenase-2. Evidence for involvement of activator protein-1 and CREB-binding protein/p300. J Biol Chem 276:12440–12448 [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. (1995) 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 215:89–97 [DOI] [PubMed] [Google Scholar]
- Sugiyama H, Nonaka T, Kishimoto T, Komoriya K, Tsuji K, Nakahata T. (2000) Peroxisome proliferator-activated receptors are expressed in human cultured mast cells: a possible role of these receptors in negative regulation of mast cell activation. Eur J Immunol 30:3363–3370 [DOI] [PubMed] [Google Scholar]
- Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ. (2006) Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-alpha, -beta, and -gamma, liver X receptor-beta, and retinoid X receptor-alpha. Toxicol Sci 92:476–489 [DOI] [PubMed] [Google Scholar]
- Willson TM, Lehmann JM, Kliewer SA. (1996) Discovery of ligands for the nuclear peroxisome proliferator-activated receptors. Ann NY Acad Sci 804:276–283 [DOI] [PubMed] [Google Scholar]
- Yang XY, Wang LH, Chen T, Hodge DR, Resau JH, DaSilva L, Farrar WL. (2000) Activation of human T lymphocytes is inhibited by peroxisome proliferator-activated receptor gamma (PPARgamma) agonists. PPARgamma co-association with transcription factor NFAT. J Biol Chem 275:4541–4544 [DOI] [PubMed] [Google Scholar]