Abstract
Benzodiazepines (BZDs) exert their therapeutic actions by binding to the GABAA receptor (GABAAR) and allosterically modulating GABA-induced chloride currents (IGABA). A variety of ligands with divergent structures bind to the BZD site, and the structural mechanisms that couple their binding to potentiation of IGABA are not well understood. In this study, we measured the effects of individually mutating 22 residues throughout the BZD binding pocket on the abilities of eszopiclone, zolpidem, and flurazepam to potentiate IGABA. Wild-type and mutant α1β2γ2 GABAARs were expressed in Xenopus laevis oocytes and analyzed using a two-electrode voltage clamp. GABA EC50, BZD EC50, and BZD maximal potentiation were measured. These data, combined with previous radioligand binding data describing the mutations' effects on BZD apparent binding affinities (J Neurosci 28:3490–3499, 2008; J Med Chem 51:7243–7252, 2008), were used to distinguish residues within the BZD pocket that contribute to BZD efficacy and BZD binding. We identified six residues whose mutation altered BZD maximal potentiation of IGABA (BZD efficacy) without altering BZD binding apparent affinity, three residues whose mutation altered binding but had no effect on BZD efficacy, and four residues whose mutation affected both binding and efficacy. Moreover, depending on the BZD ligand, the effects of some mutations were different, indicating that the structural mechanisms underlying the ability of BZD ligands with divergent structures to potentiate IGABA are distinct.
Introduction
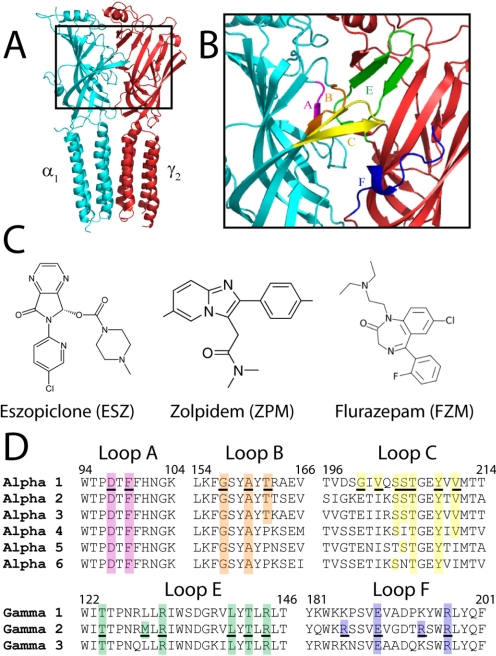
Benzodiazepines (BZDs) are commonly used in the treatment of sleep disorders, anxiety, muscle spasms, seizure disorders, and some forms of depression (Möhler et al., 2002). They exert their therapeutic actions by binding to the GABAA receptor (GABAAR) and modulating GABA-induced chloride current (IGABA). The GABAAR is a heteropentameric, ligand-gated ion channel and belongs to the cys-loop superfamily of receptors that includes the 5HT3 receptor, glycine receptor, and nicotinic acetylcholine receptor (Ortells and Lunt, 1995). The most common GABAA receptor subtype found in the brain comprises α1, β2, and γ2 subunits in a ratio of 2α:2β:γ (Chang et al., 1996; Farrar et al., 1999; Baumann et al., 2002; Sieghart and Sperk, 2002). The BZD binding site is located in the extracellular domain of the receptor at the interface of the α and γ subunits (Fig. 1A), and it is formed by six noncontiguous regions historically designated loops A to F (Fig. 1B) (Sigel and Buhr, 1997; Boileau et al., 1998; Boileau et al., 2002).
Fig. 1.
The BZD binding site at the α1/γ2 interface of the GABAAR and structures of BZD site ligands. A, homology model of the α1/γ2 interface perpendicular to the plane of the membrane. The α1 subunit is in blue and the γ2 subunit is in red. B, the region of the α1/γ2 interface that contains the BZD binding site is expanded, and BZD binding-site loop regions A to F are each highlighted in a different color. C, structures of BZD ligands ESZ, ZPM, and FZM. D, sequence alignments of the extracellular domain of α1–6 and γ1–3 rat GABAAR subunit isoforms with BZD binding site loops are shown. Loop regions are colored as in B. Residues mutated in this study are underlined and residues highlighted in color are identical. Numbering refers to α1 and γ2 residues.
Ligands that bind to the BZD site can act as negative modulators that inhibit IGABA (BZD inverse agonists), as positive modulators that potentiate IGABA (BZD agonists), or as zero modulators that bind yet have no affect on IGABA (BZD antagonists). Although multiple studies have identified residues that are involved in mediating the apparent binding affinity (Kd) of BZD-site ligands, including typical [1,4]benzodiazepines (Wieland and Lüddens, 1994; Kucken et al., 2000; Boileau et al., 2002; Derry et al., 2004), cyclopyrrolones [e.g., eszopiclone (ESZ)] (Davies et al., 2000; Hanson and Czajkowski, 2008), and imidazopyridines [e.g., zolpidem (ZPM)] (Buhr et al., 1996, 1997; Buhr and Sigel, 1997; Schaerer et al., 1998; Hanson et al., 2008), much less is known about the structural determinants that couple their binding to modulation of IGABA and govern whether a BZD-site ligand is a positive modulator, zero modulator, or negative modulator (i.e., BZD efficacy).
In general, it is believed that BZDs exert their allosteric effects by either shifting the GABAAR closed to open-state channel equilibrium (Downing et al., 2005; Rüsch and Forman, 2005; Campo-Soria et al., 2006) or altering the receptor's microscopic binding affinity for GABA (Twyman et al., 1989; Rogers et al., 1994; Lavoie and Twyman, 1996; Mellor and Randall, 1997; Thompson et al., 1999; Goldschen-Ohm et al., 2010). Regardless of the mechanism, BZD binding to the receptor is the initial perturbation that triggers structural rearrangements in the protein that result in modulation of GABAAR function. Residues that line the BZD binding site pocket probably have different roles in this process. Some residues might directly interact with the ligand and contribute to its binding affinity, and some might stabilize binding site structure, whereas others might mediate local conformational movements important for coupling BZD binding to modulation of IGABA. Identifying the residues that are involved in these actions is critical for elucidating the structural mechanisms that govern the pharmacological effects of these drugs and will help predict the therapeutic effects of new drugs.
In a previous study, we identified residues within the BZD binding site that were important for high-affinity binding of flumazenil (Ro 15-1788), ESZ, and zolpidem (ZPM) (Hanson et al., 2008; Hanson and Czajkowski, 2008). In this study, we tested the hypothesis that residues in the BZD binding site are also crucial for determining BZD efficacy. We measured the effects of 22 single cysteine mutations (Fig. 1D), which were made throughout the BZD binding site, on the abilities of flurazepam (FZM), ESZ, and ZPM to potentiate IGABA (BZD EC50 values and maximal potentiation were measured). We focused on residues that have not been extensively examined previously and for which the effects of mutating the residue on BZD apparent binding affinities were known. We identified six residues whose mutation solely altered BZD efficacy, which suggests that they are part of the allosteric pathway involved in coupling BZD binding to modulation of GABAAR function. We identified three residues that, when mutated, only altered BZD binding affinity, suggesting that they are important for ligand docking. Four additional residues in the α subunit, when mutated, decreased both the binding affinity and efficacy of the BZD ligands, which suggests that they play a role in mediating high-affinity BZD binding and the initial structural rearrangements in the sites that help couple binding to modulation of IGABA and likely contribute to the structural integrity of the binding site. Moreover, we provide evidence that the structural mechanisms underlying the ability of BZD ligands of diverse structure to modulate IGABA are distinct.
Materials and Methods
Site-Directed Mutagenesis.
Rat cDNAs encoding the GABAAR α1, β2, and γ2L subunits in the pUNIV vector (Venkatachalan et al., 2007) were used. Cysteine mutations in the α1 and γ2L subunits were made previously (Hanson et al., 2008; Hanson and Czajkowski, 2008) using recombinant polymerase chain reaction and verified by double-stranded DNA sequencing.
Expression in Xenopus laevis Oocytes.
Expression of wild-type (WT) and mutant GABAARs was performed as described previously (Hanson and Czajkowski, 2008). Capped cRNA from NotI- digested cDNA was in vitro transcribed using the mMessage mMachine T7 kit (Ambion, Austin, TX). Xenopus laevis oocytes were harvested and prepared as described previously (Boileau et al., 1998). Oocytes were injected within 24 h of treatment with 27 nl of cRNA (1–15 pg/nl per subunit) in the ratio 1:1:10 (α/β/γ) (Boileau et al., 2002) and stored at 16°C in ND96 buffer (96 mΜ NaCl, 2 mΜ KCl, 1 mΜ MgCl2, 1.8 mΜ CaCl2, and 5 mΜ HEPES, pH 7.2) supplemented with 100 μg/ml bovine serum albumin until used for electrophysiological recordings.
Two-Electrode Voltage Clamp.
Electrophysiological recordings were performed as described previously (Hanson and Czajkowski, 2008). Oocytes were held at −80 mV under a two-electrode voltage clamp while being continuously perfused with ND96 at a rate of 5 ml/min in a bath volume of 200 μl. Borosilicate glass electrodes (0.4–1.0 mΩ) (Warner Instruments, Hamden, CT) were filled with 3 Μ KCl. Electrophysiological data were collected using GeneClamp 500 (Molecular Devices, Sunnyvale, CA) interfaced to a computer with a Digidata 1200 device (Molecular Devices). Recordings were made using the Whole Cell Program, version 3.6.7 (kindly provided by J. Dempster, University of Strathclyde, Glasgow, UK). Stock solutions of FZM (Sigma/RBI, Natick, MA) were dissolved in ND96 and diluted in ND96 for working concentrations. GABA (Sigma-Aldrich, St. Louis, MO) solutions were prepared fresh daily with ND96. Stock solutions of 3-carbomethoxy-4-ethyl-6,7-dimethoxy-β-carboline (DMCM; Sigma/RBI), ZPM (Sigma-Aldrich), and ESZ (kindly provided by Sepracor, Inc., Marlborough, MA) were prepared in dimethyl sulfoxide and subsequently diluted in ND96 for working concentrations in which the final concentration of dimethyl sulfoxide (≤2%) did not affect GABAAR function.
Concentration-Response Analysis.
GABA concentration-response curves were determined as described previously (Hanson and Czajkowski, 2008). Six to 12 concentrations of GABA were used to determine each GABA EC50 value. Each current response was scaled to a low, nondesensitizing concentration of GABA applied just before the test concentration to correct for any drift in IGABA responsiveness over the course of the experiment. Concentration-response data were fit by the following equation: I = Imax/[1 + (EC50/[A]nH)], in which I is the peak response to a given drug concentration, Imax is the maximal amplitude of current, EC50 is the drug concentration that produces the half-maximal response, [A] is drug concentration, and nH is the Hill coefficient using Prism version 4.0 (GraphPad Software Inc., San Diego, CA). The EC50 values in Table 1 for four mutants (γR185C, γE189C, γR194C, and γR197C) are from Hanson and Czajkowski (2008) with the associated errors in S.E.M., rather than the S.D. reported in the 2008 publication. Two values that were significantly different from WT values in Hanson and Czajkowski (2008) (γR185C and γR194C) are no longer significant in the present study because the GABA EC50 value for WT receptors is slightly lower than in the previous report, and the full data set that was used for the analysis of variances (ANOVAs) in both studies are different.
TABLE 1.
Summary of GABA dose-response data for WT and mutant α1β2γ2 GABAARs
Data are mean ± S.E.M. for n experiments. nH values are calculated Hill coefficients. Imax range is the lowest and highest maximal GABA current amplitude measured for each of the receptors. The loop where each mutation is located is indicated. In Hanson and Czajkowski (2008), GABA EC50 values for γR185C and γR194C were decreased 2.5-fold compared to WT and were statistically different. Here, these values are no longer significant because of a slight decrease in the WT EC50 value reported here and because of differences in the data sets analyzed by ANOVA.
| Loop | Receptor | EC50 | nH | n | Imax range |
|---|---|---|---|---|---|
| μM | μA | ||||
| WT αβγ | 18.4 ± 4.4 | 1.50 ± 0.09 | 5 | 8.4–11.6 | |
| A | αD97Cβγ | 485 ± 67** | 0.66 ± 0.10** | 5 | 2.3–4.0 |
| A | αF99Cβγ | 391 ± 94* | 0.75 ± 0.06** | 7 | 2.7–3.1 |
| B | αG157Cβγ | 408 ± 93* | 0.65 ± 0.03** | 8 | 1.4–4.1 |
| B | αA160Cβγ | 560 ± 138** | 0.60 ± 0.04** | 6 | 3.3–5.0 |
| B | αT162Cβγ | 39.8 ± 13.2 | 0.89 ± 0.12** | 4 | 7.6–9.3 |
| C | αG200Cβγ | 33.0 ± 11.0 | 1.03 ± 0.02** | 3 | 4.4–8.0 |
| C | αV202Cβγ | 40.0 ± 22.5 | 0.99 ± 0.06** | 3 | 6.0–7.2 |
| C | αS204Cβγ | 22.0 ± 4.7 | 1.04 ± 0.03** | 3 | 2.7–8.8 |
| C | αS205Cβγ | 230 ± 126* | 1.08 ± 0.20* | 3 | 2.6–7.7 |
| C | αT206Cβγ | 268 ± 58.0* | 0.59 ± 0.05** | 3 | 1.9–2.8 |
| C | αY209Cβγ | 319 ± 170* | 0.68 ± 0.13** | 3 | 3.5–8.8 |
| C | αV211Cβγ | 80.3 ± 27.5 | 1.11 ± 0.19* | 3 | 3.2–11.4 |
| E | αβγT126C | 49.1 ± 7.8** | 1.13 ± 0.26 | 4 | 4.3–8.2 |
| E | αβγM130C | 45.9 ± 7.5* | 1.43 ± 0.02 | 3 | 2.6–3.5 |
| E | αβγR132C | 15.4 ± 3.1 | 1.64 ± 0.04 | 3 | 5.8–9.2 |
| E | αβγL140C | 22.2 ± 7.1 | 1.28 ± 0.21 | 3 | 4.4–11.0 |
| E | αβγT142C | 11.9 ± 0.2 | 1.61 ± 0.12 | 4 | 10.6–13.7 |
| E | αβγR144C | 3.0 ± 0.7* | 1.55 ± 0.13 | 3 | 7.6–16.4 |
| F | αβγR185Ca | 10.7 ± 2.0 | 1.52 ± 0.16 | 3 | 11.2–16.0 |
| F | αβγE189Ca | 16.6 ± 3.1 | 1.50 ± 0.03 | 4 | 5.2–11.2 |
| F | αβγR194Ca | 11.0 ± 2.4 | 1.41 ± 0.10 | 3 | 12.4–17.2 |
| F | αβγR197Ca | 17.4 ± 2.3 | 1.17 ± 0.04 | 4 | 9.7–12.0 |
Values are from Hanson and Czajkowski (2008) with errors in S.E.M. not S.D. as previously reported.
p < 0.05, significantly different from wild-type α1β2γ2.
p < 0.01, significantly different from wild-type α1β2γ2.
BZD concentration responses (six to eight different concentrations) were measured at GABA EC15. BZD modulation was defined as follows: [(IGABA+BZD/IGABA) − 1], in which IGABA+BZD is the current response in the presence of GABA and BZD, and IGABA is the current evoked by GABA alone (GABA EC15). When measuring BZD concentration responses, each application of GABA + BZD is preceded by a brief pulse of GABA EC15 alone. Wash times between application of GABA + BZD and the following application of GABA alone were increased with every increase in BZD concentration. During the experiment, the magnitude of the currents elicited by the GABA EC15 pulses alone did not change (<3%), even for high concentrations of BZD, which indicates complete washout of the BZD. BZD concentration-response curves were fit with the equation P = Pmax /(1 + (EC50/[A])nH), in which [A] is the BZD concentration, EC50 is the concentration of BZD eliciting half-maximal current potentiation, Pmax is the maximal BZD potentiation of IGABA, P is the potentiation amplitude, and nH is the Hill coefficient. The reported values for maximal potentiation were determined from curve fitting the data.
Statistical Analysis.
All data are from at least three different oocytes from at least two different frogs. Data represent mean ± S.E.M. Significant differences in EC50 values and maximal BZD modulation values were determined by one-way ANOVA, followed by a post hoc Dunnett's test using Prism (version 4.0; GraphPad Software, San Diego, CA). Log (EC50) values were used for statistical analyses.
Results
We previously made 22 single cysteine mutations throughout the BZD binding site in loops A (D97C and F99C), B (G157C, A160C, and T162C), and C (G200C, V202C, S204C, S205C, T206C, Y209C, and V211C) of the α1 subunit and loops E (T126C, M130C, R132C, L140C, T142C, and R144C) and F (R185C, E189C, R194C, and R197C) of the γ2 subunit (Fig. 1). We examined the effects of these mutations on BZD binding using competitive radioligand binding experiments (see Table 2 for Mut/WT Ki values) (Hanson et al., 2008; Hanson and Czajkowski, 2008). The mutations in the γ loop F region had no effect on BZD apparent binding affinity, whereas at least one mutation in each of the α loops A and B and γ loop D altered the affinities of all of the ligands tested (Ro 15-1788, ZPM, and ESZ), suggesting that these regions are critical for the binding of a variety of structurally diverse BZD-site ligands (Hanson et al., 2008). In contrast, several of the mutations in α loop C and γ loop E altered the binding of some BZDs but not others, suggesting that residues in these regions help define BZD selectivity (Hanson et al., 2008). Here, we tested the hypothesis that residues in the BZD binding site are not only important for BZD binding but also play a role in defining BZD efficacy. Cysteine mutant subunits were coexpressed with WT subunits in X. laevis oocytes to form α1β2γ2 GABAARs and analyzed using two-electrode voltage clamp. We examined the effects the mutations had on IGABA and on FZM, ESZ, and ZPM modulation of EC15 IGABA.
TABLE 2.
Summary of BZD concentration response data and binding data for WT and mutant α1β2γ2 GABAARs
Data are mean ± S.E.M. for n experiments. The loop where each mutation is located is indicated. Maximal potentiation is calculated as [(IGABA + BZD/IGABA) − 1]. The values for BZD binding affinities (Ki) were determined previously and the ratio of mutant to WT binding affinity is shown. Values significantly different from wild type α1β2γ2 are indicated.
| Loop | Receptor | Flurazepam |
Eszopiclone |
Zolpidem |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max Potentiation | EC50 | n | Mut/WT Kia | Max Potentiation | EC50 | n | Mut/WT Kib | Max Potentiation | EC50 | n | Mut/WT Kib | ||
| nM | nM | nM | |||||||||||
| WT αβγ | 2.3 ± 0.2 | 442 ± 34 | 3 | 1.0 | 2.8 ± 0.3 | 55 ± 8.5 | 8 | 1.0 | 2.8 ± 0.2 | 76.9 ± 27.4 | 6 | 1.0 | |
| A | αD97Cβγ | 0.8 ± 0.2** | 941 ± 140 | 3 | 1.1 ± 0.2** | 166 ± 49 | 3 | N.D. | 2.5 ±0.2 | 331 ± 18 | 3 | N.D. | |
| A | αF99Cβγ | 0.1 ± 0.0** | 3 | -0.5 ± 0.0** | 1400 ± 290** | 3 | 8.1** | 0.7 ± 0.2** | 3670 ± 1900** | 3 | 2.9* | ||
| B | αG157Cβγ | 0.9 ± 0.2** | 1620 ± 760 | 4 | 0.7 ± 0.1** | 1230 ± 140** | 3 | 42** | 1.3 ± 0.1* | 2820 ± 610** | 3 | 20** | |
| B | αA160Cβγ | 1.0 ± 0.2** | 368 ± 14 | 3 | 0.8 ± 0.1** | 140 ± 47 | 3 | 1.2 | 1.4 ± 0.1* | 118 ± 13 | 3 | 1.3 | |
| B | αT162Cβγ | 2.1 ± 0.1 | 500 ± 53 | 3 | 2.5 ± 0.5 | 131 ± 20 | 3 | 0.7 | 2.5 ± 0.3 | 410 ± 84 | 3 | 1.8 | |
| C | αG200Cβγ | 2.0 ± 0.0 | 454 ± 83 | 3 | 3.7 ± 0.5 | 230 ± 8.1 | 4 | 2.4** | 2.4 ± 0.5 | 996 ± 481** | 3 | 9.7** | |
| C | αV202Cβγ | 1.4 ± 0.4* | 922 ± 165 | 3 | 2.2 ± 0.4 | 522 ± 130** | 3 | 5.0** | 3.2 ± 0.3 | 2490 ± 250** | 3 | 9.0** | |
| C | αS204Cβγ | 1.5 ± 0.1 | 305 ± 43 | 3 | 1.7 ± 0.2 | 139 ± 39 | 3 | 1.2 | 3.6 ± 0.4 | 2100 ± 280** | 3 | 7.0** | |
| C | αS205Cβγ | 2.0 ± 0.1 | 366 ± 68 | 3 | 3.2 ± 0.2 | 75 ± 21 | 3 | 0.7 | 2.4 ± 0.2 | 143 ± 63.4 | 3 | 0.7 | |
| C | αT206Cβγ | 1.4 ± 0.3 | 946 ± 188 | 3 | 0.4 ± 0.0** | 50.9 ± 19.1 | 3 | 0.02** | 1.2 ± 0.2* | 245 ± 42 | 3 | 1.2 | |
| C | αY209Cβγ | 0.7 ± 0.1** | 2770 ± 649** | 3 | 0.8 ± 0.2* | 766 ± 250** | 3 | N.D. | 0.9 ± 0.2** | 14,300 ± 2300** | 3 | N.D. | |
| C | αV211Cβγ | 2.3 ± 0.1 | 511 ± 93 | 3 | 3.0 ± 0.4 | 115 ± 21 | 3 | 1.2 | 4.9 ± 0.1** | 356 ± 117 | 3 | 1.1 | |
| E | αβγT126C | 2.5 ± 0.3 | 280 ± 35.7 | 3 | 3.4 ± 0.2 | 14.4 ± 4.1 | 3 | 1.4 | 3.9 ± 0.2 | 9.6 ± 1.7 | 3 | 1.2 | |
| E | αβγM130C | 1.8 ± 0.2 | 305 ± 90 | 3 | 3.1 ± 0.5 | 24.5 ± 7.7 | 3 | 2.0* | 3.0 ± 0.3 | 4.4 ± 0.4 | 3 | 0.3** | |
| E | αβγR132C | 1.9 ± 0.1 | 264 ± 76.7 | 3 | 2.2 ± 0.3 | 12.5 ± 3.1 | 3 | 2.2* | 3.3 ± 0.2 | 7.03 ± 1.28 | 3 | 0.6* | |
| E | αβγL140C | 2.1 ± 0.1 | 330 ± 65 | 3 | 2.8 ± 0.2 | 12.6 ± 1.4* | 4 | 0.9 | 3.7 ± 0.2 | 12.1 ± 2.7 | 3 | 1.0 | |
| E | αβγT142C | 1.0 ± 0.0** | 561 ± 58 | 3 | 2.1 ± 0.5 | 148 ± 39** | 3 | 10* | 2.7 ± 0.3 | 980 ± 260** | 3 | 21** | |
| E | αβγR144C | 0.0 ± 0.0** | 3 | 0.2 ± 0.0** | 3 | 3.5** | 1.0 ± 0.2** | 22.5 ± 9.4 | 3 | 0.6 | |||
| F | αβγR185C | 1.7 ± 0.2 | 317 ± 82 | 3 | 2.4 ± 0.2 | 10.4 ± 1.5* | 3 | 1.4 | 2.0 ± 0.4 | 10.0 ± 2.2 | 3 | 1.1 | |
| F | αβγE189C | 1.7 ± 0.2 | 645 ± 78 | 3 | 1.3 | 3.1 ± 0.2 | 12.2 ± 3.0* | 4 | 6.3 ± 0.4** | 1838 ± 636** | 5 | 1.8a | |
| F | αβγR194C | 1.4 ± 0.1 | 332 ± 53 | 3 | 1.7 ± 0.4 | 12.8 ± 3.4 | 3 | 1.3 | 1.7 ± 0.8 | 21.2 ± 8.3 | 3 | 1.0 | |
| F | αβγR197C | 0.6 ± 0.3** | 494 ± 48 | 3 | 0.4* | 0.4 ± 0.0** | 6.0 ± 2.1* | 3 | 0.8 ± 0.2** | 13.8 ± 2.3 | 4 | 0.4a | |
Mut, mutation; N.D., not detectable.
Values from Hanson and Czajkowski (2008).
Values from Hanson et al. (2008).
p < 0.05 significantly different from wild-type α1β2γ2.
p < 0.01 significantly different from wild-type α1β2γ2.
Effects of Cysteine Substitutions on IGABA.
All of the mutant subunits assembled into functional GABAARs (Table 1). Seven of the 12 cysteine substitutions in the α1 subunit significantly increased GABA EC50 values (13–31-fold) compared with WT receptors (18.4 ± 4.4 μM) (Table 1). In general, the mutations in the γ2 subunit had smaller effects. γT126C and γM130C increased GABA EC50 approximately 3-fold, whereas γR144C decreased GABA EC50 6-fold compared with WT receptors (Table 1).
Effects of Cysteine Substitutions on FZM Modulation of IGABA.
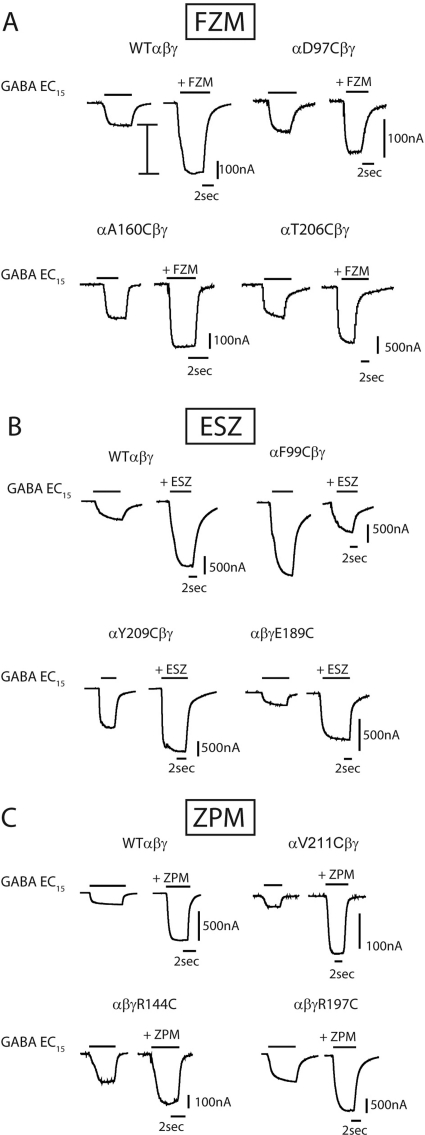
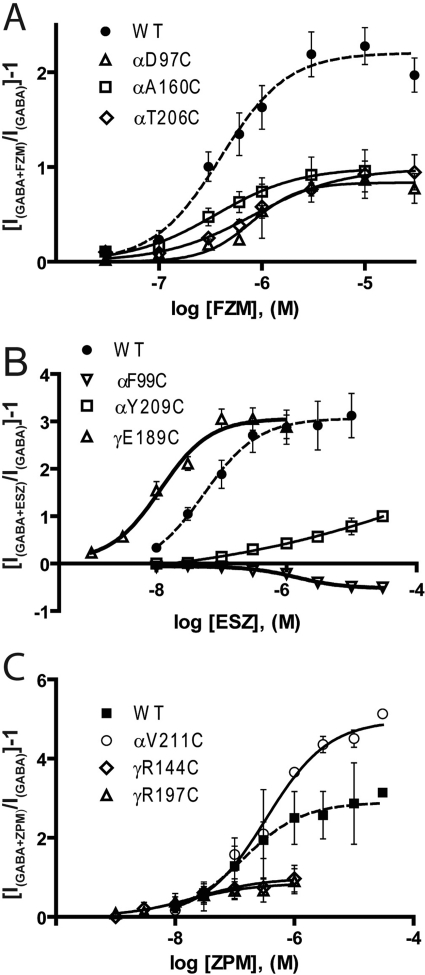
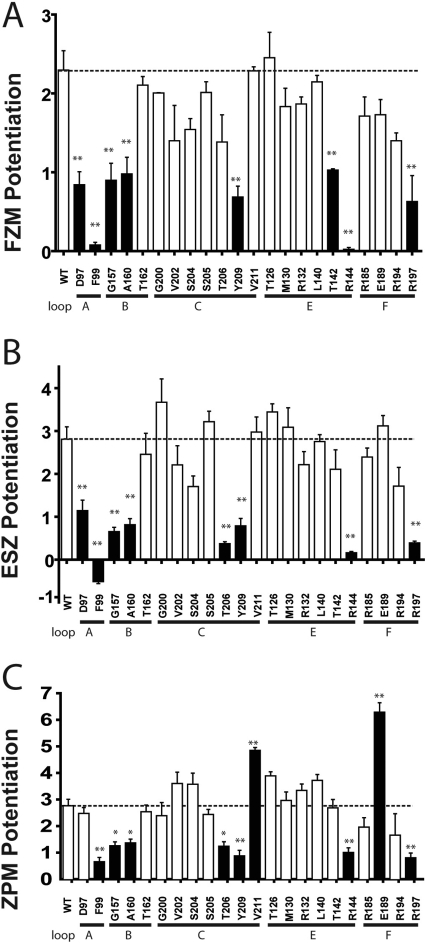
We measured the effects the mutations had on the abilities of three structurally different BZD-site positive modulators, FZM (1,4 benzodiazepine), ESZ (cyclopyrrolone), and ZPM (imidazopyridine), to potentiate GABA (EC15) currents. Current traces and dose-response curves for BZD potentiation of IGABA are depicted in Figs. 2 and 3, respectively. At saturating BZD concentrations (i.e., when the BZD binding site is fully occupied), the effects of the mutations on BZD efficacy are monitored. Eight of the 22 mutations significantly decreased FZM maximal potentiation of IGABA compared with WT receptors (potentiation = 2.3 ± 0.2) (Fig. 4; Table 2). In the α1 subunit, cysteine substitution of Asp97 and Phe99 in loop A, Gly157 and Ala160 in loop B, and Tyr209 in loop C significantly decreased FZM maximal potentiation. In the γ2 subunit, cysteine substitution of Thr142 and Arg144 in loop E and Arg197 in loop F also significantly decreased FZM maximal potentiation. Note that αF99C and γR144C almost completely eliminated FZM potentiation of IGABA, and thus FZM EC50 values could not be determined.
Fig. 2.
Effects of the mutations on BZD maximal potentiation. Representative current traces showing maximal potentiation of GABA EC15 current from oocytes expressing WT and mutant receptors by FZM (A), ESZ (B), or ZPM (C). In all cases, BZDs were at concentrations that elicited maximal responses. I bar in A indicates potentiation of IGABA. Note: in B, for αF99Cβγ receptors, ESZ inhibited IGABA.
Fig. 3.
BZD concentration-response curves from WT and mutant GABAARs for FZM (A), ESZ (B), and ZPM (C). BZD potentiation was calculated as [(IGABA+BZD/IGABA) − 1]. Data represent mean ±S.E.M. Data were fit by nonlinear regression, as described under Materials and Methods. Dashed lines are curve fits from WT receptors. BZD EC50 values and BZD maximal potentiation values are reported in Table 2.
Fig. 4.
Mutations throughout the BZD binding site affect BZD efficacy. Maximal potentiation of GABA EC15 current from WT and mutant receptors by FZM (A), ESZ (B), or ZPM (C) is plotted. BZD potentiation was calculated as [(IGABA+BZD/IGABA) − 1]. Data are mean ± S.E.M. from at least three oocytes from two or more batches. Dashed lines indicate WT levels of potentiation. Black bars indicate values that are significantly different from WT (*, p < 0.05; **, p < 0.01).
Effects of Cysteine Substitutions on ESZ Modulation of IGABA.
The effects of the mutations on ESZ were also measured. Eight of the 22 mutations altered ESZ maximal potentiation of IGABA compared with WT receptors (potentiation = 2.8 ± 0.3) (Figs. 2–4; Table 2). In the α1 subunit, D97C in loop A, G157C and A160C in loop B, and T206C and Y209C in loop C significantly decreased ESZ maximal potentiation. ESZ inhibited IGABA and became a negative modulator at αF99C-containing receptors (Figs. 2B and 3B). As reported in Hanson et al. (2008), the specific binding of [3H]Ro 15-1788, [3H]flunitrazepam, or [3H]ethyl-8-azido-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate (Ro 15-4513) to αD97C- and Y209C-mutant receptors was not detectable using a filtration-based radioligand binding assay (Table 2). The inability to detect radioligand binding is probably due to inherent limitations of filtration binding assays, which preclude measuring binding when the affinity of the radioligand is higher than 100 nM. Given that we can measure BZD modulation of IGABA for these mutant receptors, these drugs bind to the mutant receptors, probably with lower apparent affinity. The rightward shifts in the BZD concentration responses are consistent with this idea. In the γ2 subunit, mutations at Arg144 in loop E and Arg197 in loop F significantly reduced ESZ maximal potentiation. Although αA160C significantly reduced ESZ potentiation of IGABA (i.e., ESZ efficacy), this mutation had little to no effect on ESZ apparent binding affinity (Ki; Table 2).
Effects of Cysteine Substitutions on ZPM Modulation of IGABA.
The effects of the mutations on ZPM modulation of IGABA were also examined. Nine of the 22 mutations altered the ZPM maximal potentiation of IGABA (Figs. 2–4; Table 2). αF99C in loop A, αG157C and αA160C in loop B, αT206C and αY209C in loop C, γR144C in loop E, and γR197C in loop F significantly decreased ZPM potentiation compared with WT receptors (potentiation = 2.8 ± 0.3). It is interesting to note that αV211C (loop C) and γE189C (loop F) significantly increased ZPM potentiation of IGABA (1.8- and 2.3-fold, respectively; Figs. 3C and 4C). In a previous study, we reported that γE189C had no effect on ZPM potentiation (Hanson et al., 2008; Hanson and Czajkowski, 2008). The differences in results are probably due to the higher concentrations of ZPM used in this study. Although αA160C, αT206C, αV211C, γR144C, γE189C, and γR197C significantly altered ZPM potentiation of IGABA (efficacy), the mutations had little to no effect on ZPM apparent binding affinity (Ki; Table 2).
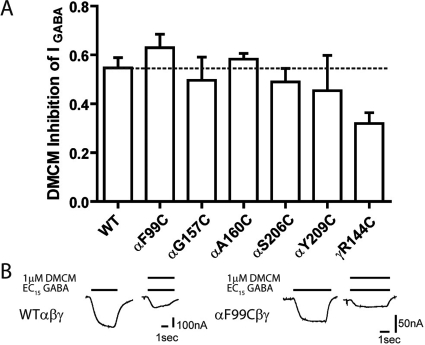
Effects of Cysteine Substitutions on DMCM Modulation of IGABA.
For a subset of mutations (αF99C, αG157C, αA160C, αT206C, αY209C, and γR144C), we also examined the ability of DMCM to inhibit GABA (EC15) currents. DMCM is a BZD site inverse agonist. None of the mutations tested significantly altered DMCM inhibition of IGABA (WT, DMCM inhibition = 0.55 ± 0.04, n = 3) (Fig. 5), indicating that the effects of the mutations on BZD-positive modulator actions are specific. DMCM inhibition of one mutant, γR144C, was decreased compared with WT, but this did not reach significance. Because only γ-containing GABAARs are modulated by DMCM, the near WT inhibition of IGABA by DMCM also indicates that the mutations do not impair subunit assembly or incorporation into functional αβγ GABAARs.
Fig. 5.
DMCM modulation of WT and mutant GABAA receptors. A, inhibition of EC15 GABA by 1 μM DMCM for WT and mutant receptors is plotted. Inhibition of GABA current was calculated as [(IGABA+DMCM/IGABA) − 1]. Data are mean ± S.E.M. from at least three oocytes from two or more batches. The dashed line indicates the level of WT inhibition. None of the mutations significantly altered DMCM inhibition of IGABA. B, representative current traces from oocytes expressing WT αβγ and αF99Cβγ receptors in response to EC15 GABA and EC15 GABA + 1 μM DMCM.
Changes in BZD Modulation of IGABA Are Not Correlated to Changes in GABA EC50.
Some mutations caused significant changes in GABA EC50, raising the possibility that the changes observed in BZD potentiation are linked to the GABA EC50 alterations. BZD-positive modulators enhance GABAAR current by decreasing GABA EC50 and shifting the GABA dose-response curve to the left. If a mutation only shifted the GABA dose-response curve to the right, one would expect that the mutation would increase FZM, ESZ, and ZPM potentiation and that inhibition by a negative modulator, such as DMCM, would decrease if a fixed GABA concentration were being used to elicit the responses. In our experiments, BZD modulation of IGABA was measured at the same effective GABA concentration (EC15) for each of the mutant and wild-type receptors, which should mitigate GABA EC50 effects on BZD modulation. Moreover, for many of the mutations, their effects on GABA EC50 and BZD potentiation were not correlated (Supplemental Fig. 1). Some mutations significantly altered BZD potentiation without affecting GABA EC50 (γThr142, γGlu189, and γArg197.), whereas others affected GABA EC50 without changing BZD potentiation (γT126C, γM130C, αS205C, and αV211C). In addition, although the αF99C, αAG157C, αA160C, αT06C, αY209C, and γR144C mutations altered GABA EC50, inhibition of IGABA by DMCM was not significantly altered (Fig. 5). Taken together, these data indicate that the observed changes in GABA EC50 are not causative for the observed alterations in the efficacies of BZD-site positive modulators (Fig. 4).
Discussion
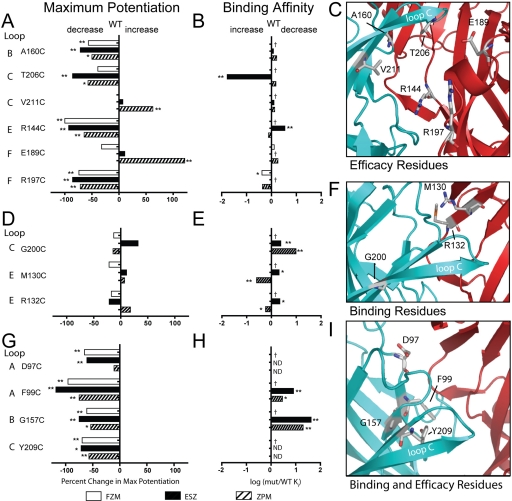
We identified four residues in the BZD binding pocket that specifically contribute to BZD-site agonist efficacy: loop B, Ala160; loop C, Thr206; loop E, Arg144; and loop F, Arg197 (Fig. 6, top row). Our data suggest that mutation of these residues significantly disrupted the abilities of ZPM, ESZ, and FZM to potentiate IGABA but had little to no effect on high-affinity binding (Table 2) (Hanson et al., 2008). Consistent with the mutations having little effect on binding, these residues are largely localized at the periphery of the binding pocket (Fig. 6C) and thus are in an ideal position to propagate local movements in the BZD binding pocket outward to more distant regions of the protein involved in modulating IGABA. We also identified two residues (αVal211 and γGlu189) that, when mutated, significantly increased ZPM potentiation of IGABA without affecting FZM or ESZ potentiation, indicating that the residues involved in coupling high-affinity BZD binding to potentiation of IGABA can be different depending on the type of BZD-site ligand bound. This result is consistent with our previous data, where we demonstrated that structural determinants for high-affinity binding of ESZ and ZPM are different (Hanson et al., 2008; Hanson and Czajkowski, 2008). We can envision that, depending on the orientation of the BZD in the binding pocket and its contact residues, some of the residues involved in the initial coupling of binding to potentiation of IGABA might differ. ZPM binding is largely dependent on shape recognition, and in silico docking has revealed that ZPM can adopt multiple orientations in the site (Hanson et al., 2008). Mutation of γGlu189 or γVal211 might cause ZPM to preferentially adopt a position that has a greater efficacy.
Fig. 6.
Summary of data highlighting residues important for BZD efficacy (A–C), BZD binding (D–F), and BZD binding and efficacy (G–I). A, D, and G plot the percentage change in maximal potentiation for FZM, ESZ, and ZPM [((mutant max potentiation − WT max potentiation)/WT max potentiation)(100)], respectively. Negative values represent a decrease in potentiation, whereas positive values indicate an increase. B, E, and H plot changes in binding affinity [log (mut Ki/WT Ki)]. Ki values for FZM, ESZ, and ZPM are from Hanson et al. (2008) and Hanson and Czajkowski (2008) and were determined by displacement of [3H]Ro 15-1788 binding. Negative values indicate increased affinity, and positive values indicate decreased affinity. C, F, and I are homology models with residues involved in BZD efficacy (C), BZD binding (F), or BZD binding and efficacy (I) shown in sticks. α subunit is blue and γ subunit is red. Loop C is labeled. Values statistically different from WT are indicated (*, p < 0.05; **, p < 0.01). ND, binding of [3H]Ro 15-1788 was not detectable, thus Ki values for FZM, ESZ, and ZPM were not determined. †, no binding data available; Max, maximal; mut, mutant.
We also identified three residues (αGly200, γMet130, and γArg132) that specifically mediate high-affinity BZD agonist binding. Mutation of these residues had no significant effects on BZD agonist efficacy but significantly altered their binding (Fig. 6, middle row). Consistent with the mutations affecting binding and not efficacy, αGly200, γMet130, and γArg132 are located on β strands (Fig. 6F) that line the core of the BZD binding pocket. Previous mutagenesis studies have demonstrated the importance of αGly200 and γMet130 in BZD binding. The glycine at position 200 is found only in the GABAAR α1-subunit isoform, α2–6 subunits have a glutamate at aligned positions (Fig. 1D). Schaerer et al. (1998) showed that replacing α1Gly200 with glutamate decreases ZPM binding affinity. Mutation of α6Glu200 to its α1 counterpart in a background of three other point mutations confers ZPM binding to the BZD-insensitive α6 subunit (Wieland and Lüddens, 1994). Mutation of γ2Met130 to a variety of different residues also alters ZPM binding (Buhr and Sigel, 1997), and replacement of the aligned lysine in the γ1 subunit (Fig. 1D) with a methionine increases the binding affinity of a variety of typical BZDs (Wingrove et al., 1997).
In this study, we also identified residues that are important for both high-affinity BZD agonist binding and BZD efficacy: αAsp97and αPhe99 in loop A, αGly157 in loop B, and αTyr209 in loop C. Introducing cysteines at these positions decreased ZPM and ESZ binding and decreased the efficacy of FZM, ZPM, and ESZ potentiation of IGABA (Fig. 6, bottom row; Table 2). The binding of ZPM and ESZ to αD97C and αY209C-containing receptors was so disrupted that their binding affinities could not be reliably measured (Hanson et al., 2008). These residues are located in the back of the BZD binding pocket in loop A (Asp97 and Phe99), the side of the pocket in loop B (Gly157), and at the base of loop C facing directly into the binding site (Tyr209) (Fig. 6I). αAsp97and αPhe99 in loop A are located near αHis101, which has been previously shown to be important for binding of ZPM (Wieland et al., 1992; Wieland and Lüddens, 1994), zopiclone (the racemate of ESZ) (Davies et al., 1998), flunitrazepam (Berezhnoy et al., 2004), and diazepam (Davies et al., 2000; Berezhnoy et al., 2004). Mutation of αHis101 to arginine has also been shown to alter BZD efficacy (Benson et al., 1998). Previous studies have also identified αGly157 in loop B and αTyr209 in loop C as important determinants for BZD binding (Amin et al., 1997; Tan et al., 2007b). All of the residues we have identified that are important for both high-affinity BZD agonist binding and BZD efficacy are located in the α subunit and are conserved in all α subunit isoforms (Fig. 1D). Residues in the α subunit are likely to play critical roles in BZD efficacy because a single α subunit contributes to forming both a GABA and BZD binding site at the β-α and α-γ interfaces, respectively. Thus, BZD-induced movements might be directly propagated through the α subunit from the BZD site to the GABA binding site. Previous studies have demonstrated that BZDs cause movements at the GABA binding site interface (Kloda and Czajkowski, 2007; Sancar and Czajkowski, 2011).
It is interesting to note that mutating αPhe99 to cysteine caused ESZ to switch from a potent positive modulator to a negative modulator (Fig. 2B) and had similar effects on the BZD agonist diazepam, making it a weak negative modulator (Tan et al., 2007a). It is not unprecedented that a single mutation can alter BZD action from enhancement to inhibition of IGABA. The γT142S mutation and mutations of αHis101 cause the inverse agonist Ro 15-4513 and the antagonist flumazenil to become BZD agonists and potentiate IGABA (Mihic et al., 1994; Benson et al., 1998). How these mutations result in switches in BZD actions is not clear. Many structurally diverse ligands bind to the BZD binding site, which indicates that the site can accommodate a variety of ligands. We speculate that the mutations might alter the positioning of the drug in the site and/or positioning of nearby residues, which then induces different downstream allosteric rearrangements.
In previous studies, we identified residues and regions in the γ2 subunit, outside of the BZD binding pocket, that were critical for coupling BZD agonist binding to potentiation of IGABA actions but were not involved in coupling DMCM binding to inhibition of IGABA (Boileau and Czajkowski, 1999; Kloda and Czajkowski, 2007; Hanson and Czajkowski, 2008). Here, none of the mutations we tested significantly altered the inhibitory abilities of DMCM (Fig. 5), which demonstrates that, even at the level of the BZD binding site, the structural mechanisms underlying the coupling of DMCM binding to inhibition of IGABA are different from those underlying BZD agonist modulation.
The BZD binding site of the GABAA receptor is pharmacologically complex. Structurally diverse ligands can bind to it and elicit a range of actions from potentiation of IGABA to inhibition. Residues that line the BZD binding site pocket probably play different roles in mediating these actions. In this study, we have identified specific residues that contribute to BZD binding affinity, other residues that contribute to BZD efficacy, and others that mediate both binding and efficacy. Moreover, we show that local structural elements important for coupling BZD binding to modulation IGABA are not only different for BZD-positive modulators versus negative modulators, but they are also different for structurally diverse BZD-positive modulators, which indicates that, at the level of the binding site, there is not a single common set of BZD-induced movements that underlies BZD-positive modulation. We envision that, depending on how a BZD occupies the site (e.g., the orientation of the BZD in the site and its interactions with the receptor), its binding elicits distinct motions within the site, which can induce different downstream allosteric rearrangements. It has been demonstrated for G protein-coupled receptors that even structurally similar agonists interacting with the same orthosteric site can bind to and activate the receptor via different structural mechanisms (Ghanouni et al., 2001; Swaminath et al., 2004, 2005). In summary, the data in this study provide substantial new insights into the structural determinants important for BZD allosteric modulation of GABAA receptor function. Our results, which identify residues within the BZD binding site that encode BZD efficacy versus affinity, will aid in the design of more efficacious and selective drugs.
Supplementary Material
Acknowledgments
We thank Dr. Ken Satyshur for homology modeling and Dr. Susan Hanson for help with mutagenesis.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant T32-GM008688]; the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grants F31-NS071995, R01-NS34727]; and in part by a research grant from Sepracor, Inc.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.069542.
- BZD
- benzodiazepine
- GABAAR
- GABAA receptor
- ESZ
- eszopiclone
- Ro 15-1788
- flumazenil
- FZM
- flurazepam
- ZPM
- zolpidem
- DMCM
- 3-carbomethoxy-4-ethyl-6,7-dimethoxy-β-carboline
- IGABA
- GABA-induced chloride current
- WT
- wild type
- ANOVA
- analysis of variance
- Ro 15-4513
- ethyl-8-azido-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylate.
Authorship Contributions
Participated in research design: Morlock and Czajkowski.
Conducted experiments: Morlock.
Performed data analysis: Morlock and Czajkowski.
Wrote or contributed to the writing of the manuscript: Morlock and Czajkowski.
References
- Amin J, Brooks-Kayal A, Weiss DS. (1997) Two tyrosine residues on the alpha subunit are crucial for benzodiazepine binding and allosteric modulation of gamma-aminobutyric acidA receptors. Mol Pharmacol 51:833–841 [DOI] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E. (2002) Forced subunit assembly in alpha1beta2gamma2 GABAA receptors. Insight into the absolute arrangement. J Biol Chem 277:46020–46025 [DOI] [PubMed] [Google Scholar]
- Benson JA, Löw K, Keist R, Mohler H, Rudolph U. (1998) Pharmacology of recombinant gamma-aminobutyric acidA receptors rendered diazepam-insensitive by point-mutated alpha-subunits. FEBS Lett 431:400–404 [DOI] [PubMed] [Google Scholar]
- Berezhnoy D, Nyfeler Y, Gonthier A, Schwob H, Goeldner M, Sigel E. (2004) On the benzodiazepine binding pocket in GABAA receptors. J Biol Chem 279:3160–3168 [DOI] [PubMed] [Google Scholar]
- Boileau AJ, Baur R, Sharkey LM, Sigel E, Czajkowski C. (2002) The relative amount of cRNA coding for gamma2 subunits affects stimulation by benzodiazepines in GABA(A) receptors expressed in Xenopus oocytes. Neuropharmacology 43:695–700 [DOI] [PubMed] [Google Scholar]
- Boileau AJ, Czajkowski C. (1999) Identification of transduction elements for benzodiazepine modulation of the GABA(A) receptor: three residues are required for allosteric coupling. J Neurosci 19:10213–10220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau AJ, Kucken AM, Evers AR, Czajkowski C. (1998) Molecular dissection of benzodiazepine binding and allosteric coupling using chimeric gamma-aminobutyric acidA receptor subunits. Mol Pharmacol 53:295–303 [DOI] [PubMed] [Google Scholar]
- Buhr A, Baur R, Malherbe P, Sigel E. (1996) Point mutations of the alpha 1 beta 2 gamma 2 gamma-aminobutyric acid(A) receptor affecting modulation of the channel by ligands of the benzodiazepine binding site. Mol Pharmacol 49:1080–1084 [PubMed] [Google Scholar]
- Buhr A, Schaerer MT, Baur R, Sigel E. (1997) Residues at positions 206 and 209 of the alpha1 subunit of gamma-aminobutyric acidA receptors influence affinities for benzodiazepine binding site ligands. Mol Pharmacol 52:676–682 [DOI] [PubMed] [Google Scholar]
- Buhr A, Sigel E. (1997) A point mutation in the gamma2 subunit of gamma-aminobutyric acid type A receptors results in altered benzodiazepine binding site specificity. Proc Natl Acad Sci USA 94:8824–8829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo-Soria C, Chang Y, Weiss DS. (2006) Mechanism of action of benzodiazepines on GABAA receptors. Br J Pharmacol 148:984–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Wang R, Barot S, Weiss DS. (1996) Stoichiometry of a recombinant GABAA receptor. J Neurosci 16:5415–5424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M, Bateson AN, Dunn SM. (1998) Structural requirements for ligand interactions at the benzodiazepine recognition site of the GABA(A) receptor. J Neurochem 70:2188–2194 [DOI] [PubMed] [Google Scholar]
- Davies M, Newell JG, Derry JM, Martin IL, Dunn SM. (2000) Characterization of the interaction of zopiclone with gamma-aminobutyric acid type A receptors. Mol Pharmacol 58:756–762 [DOI] [PubMed] [Google Scholar]
- Derry JM, Dunn SM, Davies M. (2004) Identification of a residue in the gamma-aminobutyric acid type A receptor alpha subunit that differentially affects diazepam-sensitive and -insensitive benzodiazepine site binding. J Neurochem 88:1431–1438 [DOI] [PubMed] [Google Scholar]
- Downing SS, Lee YT, Farb DH, Gibbs TT. (2005) Benzodiazepine modulation of partial agonist efficacy and spontaneously active GABA(A) receptors supports an allosteric model of modulation. Br J Pharmacol 145:894–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar SJ, Whiting PJ, Bonnert TP, McKernan RM. (1999) Stoichiometry of a ligand-gated ion channel determined by fluorescence energy transfer. J Biol Chem 274:10100–10104 [DOI] [PubMed] [Google Scholar]
- Ghanouni P, Gryczynski Z, Steenhuis JJ, Lee TW, Farrens DL, Lakowicz JR, Kobilka BK. (2001) Functionally different agonists induce distinct conformations in the G protein coupling domain of the beta 2 adrenergic receptor. J Biol Chem 276:24433–24436 [DOI] [PubMed] [Google Scholar]
- Goldschen-Ohm MP, Wagner DA, Petrou S, Jones MV. (2010) An epilepsy-related region in the GABA(A) receptor mediates long-distance effects on GABA and benzodiazepine binding sites. Mol Pharmacol 77:35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SM, Czajkowski C. (2008) Structural mechanisms underlying benzodiazepine modulation of the GABA(A) receptor. J Neurosci 28:3490–3499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SM, Morlock EV, Satyshur KA, Czajkowski C. (2008) Structural requirements for eszopiclone and zolpidem binding to the gamma-aminobutyric acid type-A (GABAA) receptor are different. J Med Chem 51:7243–7252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloda JH, Czajkowski C. (2007) Agonist-, antagonist-, and benzodiazepine-induced structural changes in the alpha1 Met113-Leu132 region of the GABAA receptor. Mol Pharmacol 71:483–493 [DOI] [PubMed] [Google Scholar]
- Kucken AM, Wagner DA, Ward PR, Teissére JA, Boileau AJ, Czajkowski C. (2000) Identification of benzodiazepine binding site residues in the gamma2 subunit of the gamma-aminobutyric acidA receptor. Mol Pharmacol 57:932–939 [PubMed] [Google Scholar]
- Lavoie AM, Twyman RE. (1996) Direct evidence for diazepam modulation of GABAA receptor microscopic affinity. Neuropharmacology 35:1383–1392 [DOI] [PubMed] [Google Scholar]
- Mellor JR, Randall AD. (1997) Frequency-dependent actions of benzodiazepines on GABAA receptors in cultured murine cerebellar granule cells. J Physiol 503:353–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihic SJ, Whiting PJ, Klein RL, Wafford KA, Harris RA. (1994) A single amino acid of the human γ-aminobutyric acid type A receptor γ2 subunit determines benzodiazepine efficacy. J Biol Chem 269:32768–32773 [PubMed] [Google Scholar]
- Möhler H, Fritschy JM, Rudolph U. (2002) A new benzodiazepine pharmacology. J Pharmacol Exp Ther 300:2–8 [DOI] [PubMed] [Google Scholar]
- Ortells MO, Lunt GG. (1995) Evolutionary history of the ligand-gated ion-channel superfamily of receptors. Trends Neurosci 18:121–127 [DOI] [PubMed] [Google Scholar]
- Rogers CJ, Twyman RE, Macdonald RL. (1994) Benzodiazepine and beta-carboline regulation of single GABAA receptor channels of mouse spinal neurones in culture. J Physiol 475:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüsch D, Forman SA. (2005) Classic benzodiazepines modulate the open-close equilibrium in alpha1beta2gamma2L gamma-aminobutyric acid type A receptors. Anesthesiology 102:783–792 [DOI] [PubMed] [Google Scholar]
- Sancar F, Czajkowski C. (2011) Allosteric modulators induce distinct movements at the GABA-binding site interface of the GABA-A receptor. Neuropharmacology 60:520–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaerer MT, Buhr A, Baur R, Sigel E. (1998) Amino acid residue 200 on the alpha1 subunit of GABA(A) receptors affects the interaction with selected benzodiazepine binding site ligands. Eur J Pharmacol 354:283–287 [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. (2002) Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem 2:795–816 [DOI] [PubMed] [Google Scholar]
- Sigel E, Buhr A. (1997) The benzodiazepine binding site of GABAA receptors. Trends Pharmacol Sci 18:425–429 [DOI] [PubMed] [Google Scholar]
- Swaminath G, Deupi X, Lee TW, Zhu W, Thian FS, Kobilka TS, Kobilka B. (2005) Probing the beta2 adrenoceptor binding site with catechol reveals differences in binding and activation by agonists and partial agonists. J Biol Chem 280:22165–22171 [DOI] [PubMed] [Google Scholar]
- Swaminath G, Xiang Y, Lee TW, Steenhuis J, Parnot C, Kobilka BK. (2004) Sequential binding of agonists to the beta2 adrenoceptor. Kinetic evidence for intermediate conformational states. J Biol Chem 279:686–691 [DOI] [PubMed] [Google Scholar]
- Tan KR, Baur R, Gonthier A, Goeldner M, Sigel E. (2007a) Two neighboring residues of loop A of the alpha1 subunit point towards the benzodiazepine binding site of GABAA receptors. FEBS Lett 581:4718–4722 [DOI] [PubMed] [Google Scholar]
- Tan KR, Gonthier A, Baur R, Ernst M, Goeldner M, Sigel E. (2007b) Proximity-accelerated chemical coupling reaction in the benzodiazepine-binding site of gamma-aminobutyric acid type A receptors: superposition of different allosteric modulators. J Biol Chem 282:26316–26325 [DOI] [PubMed] [Google Scholar]
- Thompson SA, Smith MZ, Wingrove PB, Whiting PJ, Wafford KA. (1999) Mutation at the putative GABA(A) ion-channel gate reveals changes in allosteric modulation. Br J Pharmacol 127:1349–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman RE, Rogers CJ, Macdonald RL. (1989) Differential regulation of gamma-aminobutyric acid receptor channels by diazepam and phenobarbital. Ann Neurol 25:213–220 [DOI] [PubMed] [Google Scholar]
- Venkatachalan SP, Bushman JD, Mercado JL, Sancar F, Christopherson KR, Boileau AJ. (2007) Optimized expression vector for ion channel studies in Xenopus oocytes and mammalian cells using alfalfa mosaic virus. Pflugers Arch 454:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland HA, Lüddens H. (1994) Four amino acid exchanges convert a diazepam-insensitive, inverse agonist-preferring GABAA receptor into a diazepam-preferring GABAA receptor. J Med Chem 37:4576–4580 [DOI] [PubMed] [Google Scholar]
- Wieland HA, Lüddens H, Seeburg PH. (1992) A single histidine in GABAA receptors is essential for benzodiazepine agonist binding. J Biol Chem 267:1426–1429 [PubMed] [Google Scholar]
- Wingrove PB, Thompson SA, Wafford KA, Whiting PJ. (1997) Key amino acids in the gamma subunit of the gamma-aminobutyric acidA receptor that determine ligand binding and modulation at the benzodiazepine site. Mol Pharmacol 52:874–881 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.