Abstract
Aim
This long-term study aimed to evaluate recurrence and evolution of primary biliary cirrhosis (PBC) after orthotopic liver transplantation (OLT).
Methods
We reviewed “blindly” allograft biopsy specimens of women who underwent transplantation for PBC (n = 84), and women who received a transplant for chronic hepatitis C virus infection (CHCV) (n = 108). All needle liver biopsy specimens obtained more than 6 months post-OLT were examined, including 83 specimens from 44 PBC patients and 152 specimens from 58 CHCV patients.
Results
Granulomatous destructive cholangitis was found in five biopsies from four PBC patients (P = 0.0048). Non-necrotizing epithelioid cell granulomas were present in four biopsies from four PBC patients, and in two biopsies from one CHCV patient. Piecemeal necrosis (P = 0.0002), lobular necroinflammatory activity (P < 0.0001), steatosis (P < 0.0001) and fibrosis (P < 0.0001) were more prevalent in CHCV patients than PBC patients. Four PBC patients developed histologic evidence of autoimmune hepatitis (AIH), at a mean time of 3.66 years post-OLT. One of these patients had histologic features of AIH/PBC overlap syndrome. All four patients developed bridging fibrosis (n = 2) or cirrhosis (n = 2). No other PBC patient had evidence of cirrhosis after OLT.
Conclusions
Histologic findings indicative of recurrent PBC were present in 15.9% of the PBC patients undergoing biopsy in this series. However, this group of patients did not suffer significant bile duct loss or fibrosis, as compared to the control group, suggesting that recurrent PBC is a mild or slowly progressive disease. Histologic evidence of AIH was observed in allograft biopsies of some PBC patients.
Keywords: autoimmune hepatitis, hepatitis C virus, liver transplantation, primary biliary cirrhosis, recurrence
INTRODUCTION
End-stage primary biliary cirrhosis (PBC) is a common indication for orthotopic liver transplantation (OLT) in women. Following OLT, the majority of PBC patients have a favorable outcome, with over two-thirds surviving more than 10 years.1,2 Whether PBC recurs after OLT originally had been a controversial issue, but many liver transplant centers have subsequently reported evidence of recurrent primary biliary cirrhosis (RPBC) following OLT from either cadaveric,3–7 or living-related donors.8–10 The incidence of recurrence and the natural history of the disease after OLT are not well established, owing to differences in study designs, lengths of follow-up, immunosuppression regimens and diagnostic criteria of recurrence in the literature. Therefore, there is a need for long-term studies.6,11
Granulomatous destructive cholangitis (“florid duct lesion”) is the diagnostic histologic feature of both PBC and RPBC,12,13 but is often absent from the biopsy material undergoing microscopic examination. Other histologic features may be suggestive but not diagnostic of RPBC, and differential diagnosis from other conditions, such as allograft rejection, hepatitis, or large bile duct problems, may be difficult.13,14 Because of the importance of histologic examination in this setting, we performed a “blinded” study of needle liver biopsy specimens from PBC patients, as compared to a control group of patients transplanted for chronic hepatitis C virus (CHCV), with a double aim: (i) to seek unbiased evidence of characteristic histologic features of RPBC; and (ii) to correlate the histologic features with clinical outcome on long-term follow-up.
PATIENTS AND METHODS
The study population consisted of all consecutive female patients with antimitochondrial antibody (AMA) positive PBC (titers equal to or greater than 1:40) undergoing OLT from 1989 to 1999 (n = 84) at The Mount Sinai Medical Center in New York City. Patients with concomitant liver disease were not included in the study. The control group of patients consisted of all consecutive HCV-antibody positive women, who were in the same age range as the PBC patients, and underwent OLT from 1990 to 1999 (n = 108) at the same institution. CHCV patients with concomitant liver disease were also excluded from the study. In all patients of both groups, the preoperative diagnosis was confirmed by histologic examination of the liver explanted at OLT. All liver allografts were taken from cadaveric donors. Post-OLT biopsies were performed when clinically indicated. In the setting of abnormal liver function tests, biopsies were performed to delineate their etiology, as well as the severity of the histologic changes.
Forty-five of the 84 PBC patients (53.5%) originally were on a cyclosporine-based immunosuppression regimen, while the remaining 39 (46.5%) were on a tacrolimus-based regimen. Forty-eight of the 108 CHCV patients (44%) originally were on a cyclosporine-based regimen, and the remaining 60 (56%) were on a tacrolimus-based regimen. Fifty-two PBC patients (61.9%) and 58 CHCV patients (53.7%) received their livers from male donors. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, and was approved by the Mount Sinai Institutional Review Board.
Histologic slides (including hematoxylin-eosin and Masson trichrome stains) from all needle liver biopsy specimens obtained more than 6 months after OLT were reviewed, and those with sufficient material were examined in a “blinded” fashion by two pathologists in conference. Biopsies performed within 10 days of a previous one were excluded from the study. The specimens were considered to be adequate for evaluation when they contained a minimum number of six portal tracts. A set of histologic features known to be characteristic of PBC or CHCV were assessed in a semiquantitative fashion (Table 1). A total number of 83 allograft biopsies from 44 PBC patients and 152 allograft biopsies from 58 CHCV patients were examined.
Table 1.
Histologic features assessed semiquantitatively in liver biopsy specimens
| Feature | Assessment | |||
|---|---|---|---|---|
| Florid duct lesion | Absent | Present | ||
| Granuloma | Absent | Present | ||
| Bile duct loss | Absent | Present in < 50% of p.t. | Present in > 50% of p.t. | |
| Bile duct damage | Absent | Present in < 1/3 of p.t. | Present in 1/3–2/3 of p.t. | Present in almost all p.t. |
| Ductular reaction | Absent | Mild | Moderate | Severe |
| Chronic portal inflammation | Absent | Mild | Moderate | Severe |
| Piecemeal necrosis | Absent | Mild | Moderate | Severe |
| Lobular necro-inflammatory activity | Absent | Mild | Moderate | Severe |
| Steatosis | Absent | Mild | Moderate | Severe |
| Fibrosis | Absent (0) | Portal (I) | Septal (II) | Bridging (III) |
| Cirrhosis | Absent | Present (IV) | ||
| Cholestasis | Absent | Present |
p.t., portal tracts. Roman numerals equate descriptive designations to numerical fibrosis scores.
Statistical analysis was performed using a SAS statistical package (JMP, Cary, NC). Non-parametric data were analyzed using a χ2-test, or a Fisher’s exact test, when indicated. The Shapiro–Wilk test was used to determine whether or not parametric data were normally distributed. Data with a normal distribution were analyzed using t-tests, while those that were not normally distributed were analyzed using Wilcoxon–Kruskal–Wallis (rank sums). A P-value of less than 0.05 was considered to be significant.
RESULTS
The median age at OLT was 55.6 years (range, 37–71 years) in the PBC group and 55.6 years (range, 39–70 years) in the CHCV group (P = 0.94, NS). The median time until the first biopsy (excluding the biopsies of the first 6 months post-OLT) was 450 days for the PBC patients, and 374 days for the CHCV patients (P = 0.0084). The median histologic follow-up time was 2.8 years (range, 0.6–12.7 years) in the PBC group and 2.2 years (range, 0.5–11.5 years) in the CHCV group (P = 0.3, NS). Statistically significant differences were found between the two groups in terms of semi-quantitative assessment of several histologic features, as shown in detail in Table 2.
Table 2.
Statistically significant differences in histologic features between the biopsy specimens of the primary biliary cirrhosis (PBC) group and those of the chronic hepatitis C virus (CHCV) group (corresponding numbers of patients in parentheses)
| PBC | CHCV | P-value | |
|---|---|---|---|
| Total number of biopsies | 83 | 152 | |
| Florid duct lesion | 5 (4) | 0 | 0.0048† |
| Piecemeal necrosis | 0.0002† | ||
| Absent | 50 (32) | 52 (33) | 0.0014* |
| Mild | 23 (16) | 69 (40) | |
| Moderate | 6 (4) | 23 (16) | |
| Severe | 4 (2) | 8 (7) | |
| Lobular necroinflammatory activity | < 0.0001† | ||
| Absent | 23 (17) | 10 (7) | < 0.0001* |
| Mild | 46 (33) | 95 (49) | |
| Moderate | 9 (8) | 35 (26) | |
| Severe | 5 (3) | 12 (10) | |
| Stage of fibrosis/cirrhosis | < 0.0001† | ||
| Absent | 38 (26) | 23 (18) | < 0.0001* |
| Portal (I) | 14 (11) | 55 (31) | |
| Septal (II) | 17 (13) | 33 (22) | |
| Bridging (III) | 7 (6) | 24 (17) | |
| Cirrhosis (IV) | 7 (2) | 17 (11) | |
| Steatosis | < 0.0001† | ||
| Absent | 62 (38) | 68 (33) | < 0.0001* |
| Mild | 19 (15) | 73 (41) | |
| Moderate | 1 (1) | 10 (9) | |
| Severe | 1 (1) | 1 (1) |
Comparison of how commonly a feature was found (presence vs. absence);
the scoring distribution (absent, mild, moderate, severe).
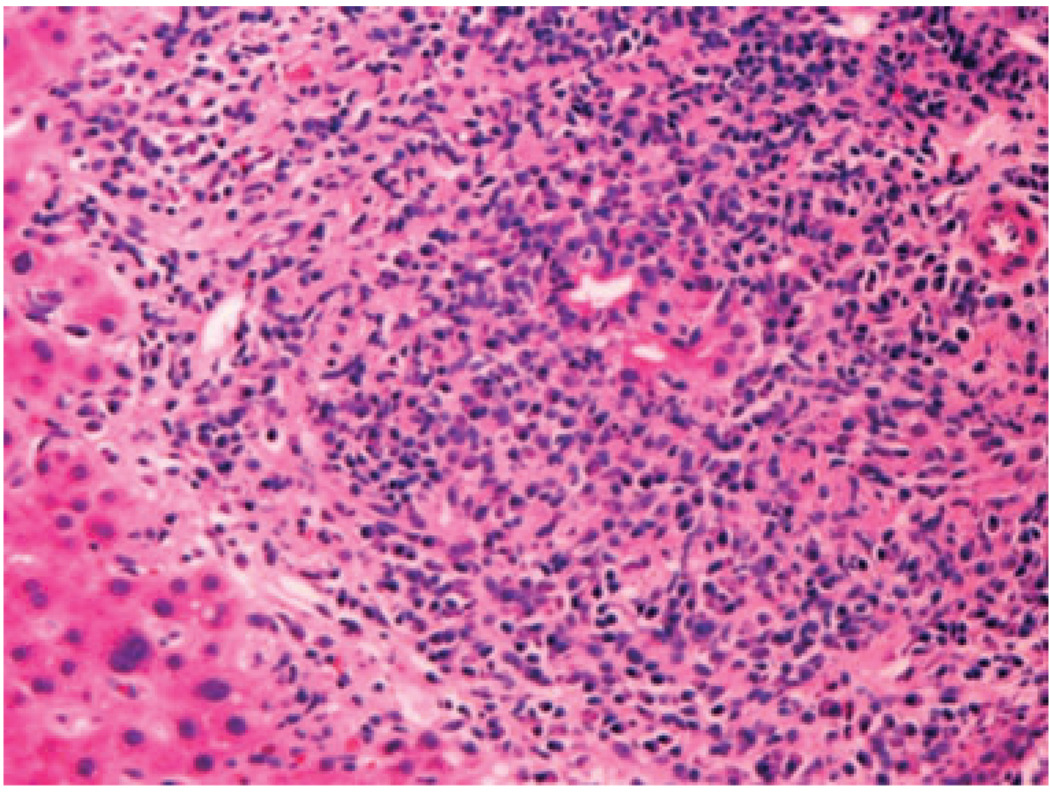
Granulomatous destructive cholangitis was present in five biopsy specimens from four patients in the PBC group, and in none of those in the CHCV group (P = 0.0048) (Fig. 1). The florid duct lesions were observed in biopsies taken at a median time of 2.75 years post-OLT (range, 2.6–8.7 years). Nonnecrotizing epithelioid cell granulomas without identifiable florid duct lesions were seen in one more biopsy specimen from one of these four patients, and in three more specimens from three additional PBC patients. The granulomas involved portal tracts in all four patients, and lobules in one of the four patients. However, there was also a patient in the CHCV group, who had 2 biopsies with small granulomas of undetermined cause in the lobules (P = 0.18, NS). Histochemical stains for microorganisms (acid fast, Gomori methenamine silver, Periodic acid Schiff) were performed in all cases with granulomas, and were negative. Careful review of the charts of these patients did not disclose any medication that could be considered responsible for granuloma formation.
Figure 1.
Granulomatous destructive cholangitis (florid duct lesion) in a liver allograft biopsy specimen with recurrent primary biliary cirrhosis (hematoxylin-eosin, 200 ×).
Overall, florid duct lesions or epithelioid cell granulomas were found in seven women of the PBC group, representing 15.9% of the patients of this group undergoing biopsy (corresponding to 8.33% of the total number of PBC patients), at a median time of 2.78 years post-OLT (range, 1.14–8.77 years). Four of these seven patients were on the cyclosporine-based immunosuppression regimen at the time of recurrence, while the other three were on the tacrolimus-based regimen. Six of these seven women had received their livers from male donors.
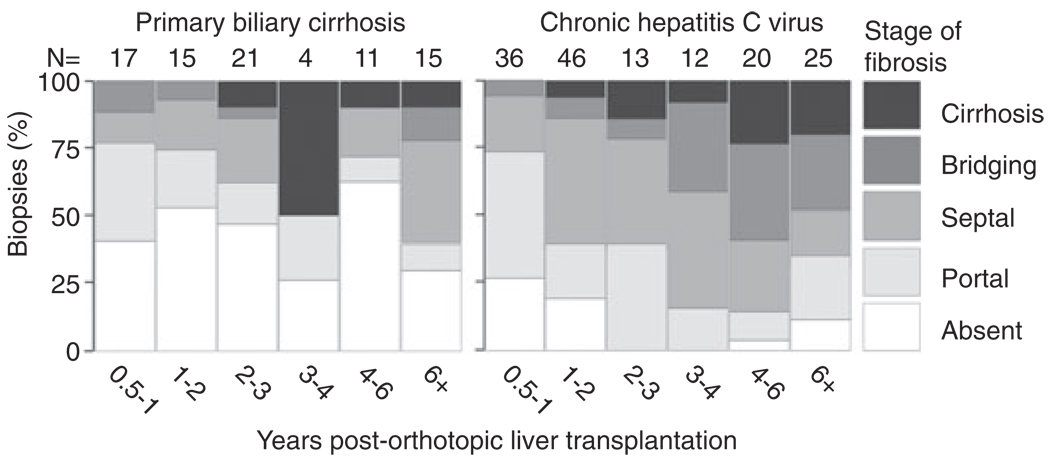
Piecemeal necrosis (interface hepatitis) and lobular necroinflammatory activity were significantly more common in the CHCV group than the PBC group (P = 0.0002, and P < 0.0001, respectively). Steatosis was also more common in the CHCV group, and was usually mild (P < 0.0001). Significant differences in the scoring distribution (absent, mild, moderate, severe) were seen in piecemeal necrosis (P = 0.0014), lobular necroinflammatory activity (P < 0.0001), and steatosis (P < 0.0001) between the groups. Fibrosis was also more prevalent and of higher stage in the CHCV group (P < 0.0001). The mosaic plot of fibrosis over time (Fig. 2) clearly illustrates the tendency for progressive fibrosis in the CHCV group, as opposed to the PBC group. There were no significant differences between the two groups in terms of various other histologic features, including bile duct damage or loss, ductular reaction, chronic portal inflammation and cholestasis.
Figure 2.
Mosaic plot of fibrosis over time. The number of liver allograft biopsies during each time period is indicated at the top of the columns.
No evidence of fibrosis was found in the biopsy specimens of five out of the seven patients in the PBC group with either florid duct lesions or epithelioid cell granulomas. These biopsies were taken at a median time of 2.95 years post-OLT (range: 1.14–7.37 years). In the sixth patient, no fibrosis was found in a biopsy specimen taken 2.7 years post-OLT, but moderate (septal) fibrosis was seen in a specimen taken 8.7 years post-OLT. In the seventh patient, severe (bridging) fibrosis was found in 2 biopsy specimens, taken at 1.8 and 2.6 years post-OLT. The biopsies of this patient were also characterized by marked necroinflammatory activity suggesting an overlap syndrome of autoimmune hepatitis (AIH) and PBC and are presented in more detail below. Cirrhosis was present in 17 biopsies from 11 patients of the CHCV group, and in seven biopsies from two patients of the PBC group.
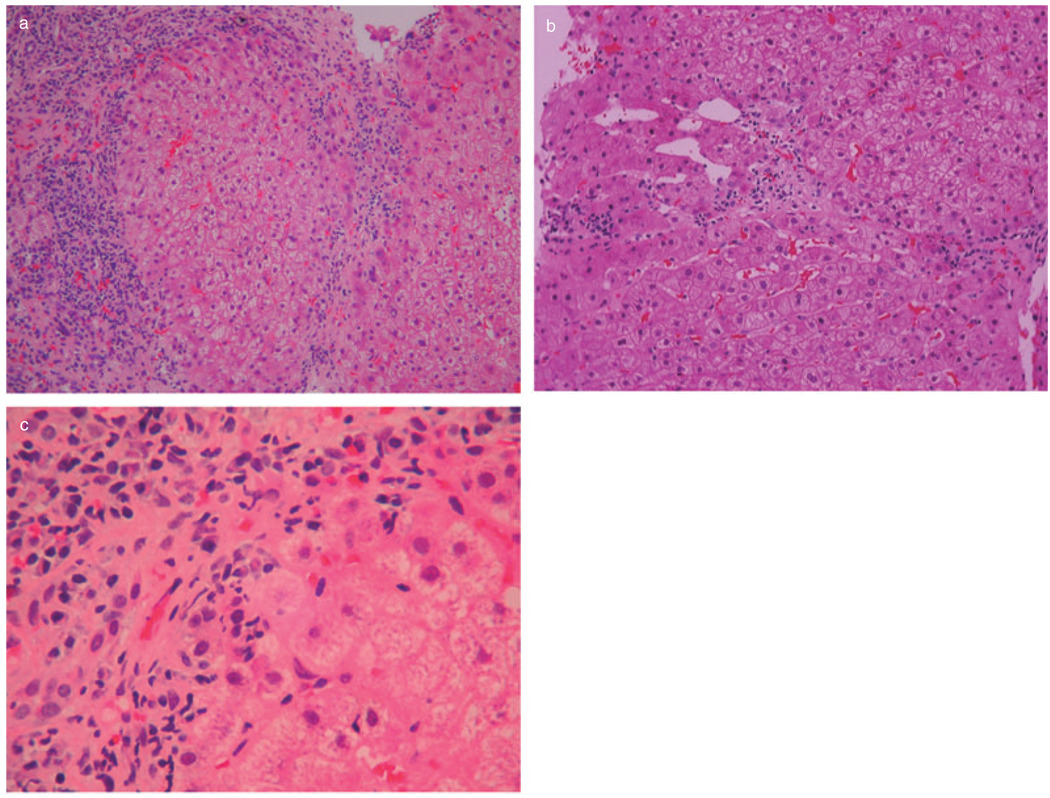
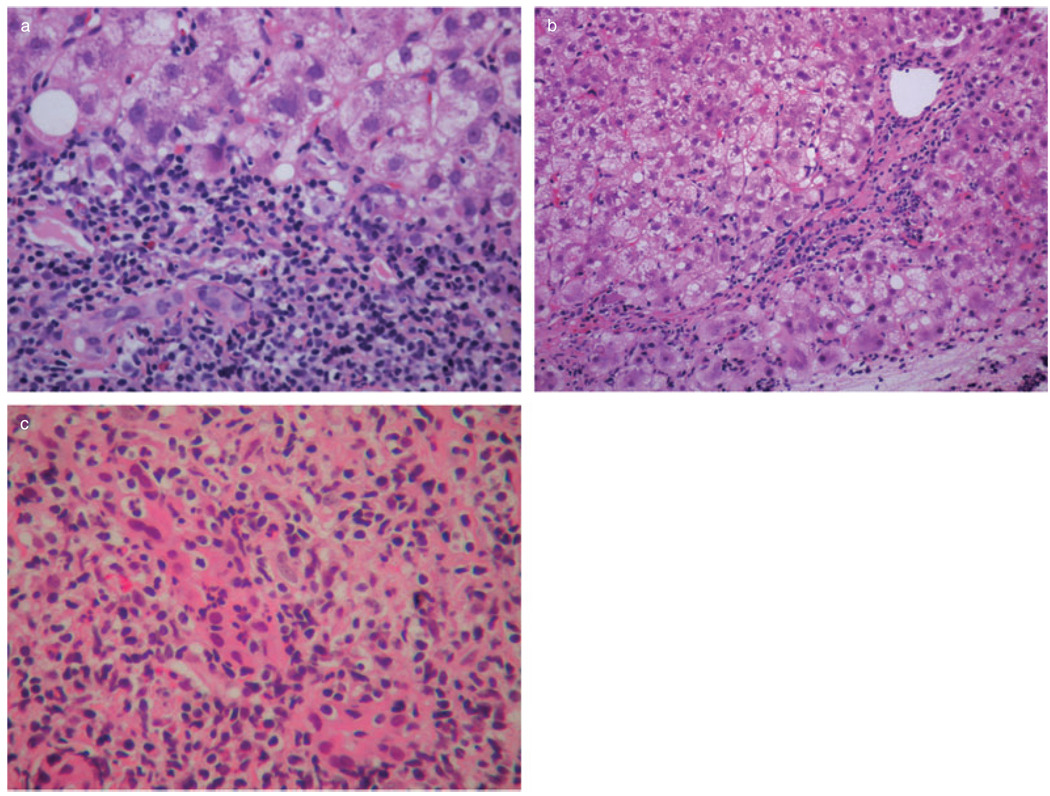
The only two cirrhotic patients of the PBC group, as well as one additional patient with bridging fibrosis, had histologic evidence of AIH, including severe necroinflammatory activity with areas of bridging necrosis and parenchymal collapse, and presence of abundant plasma cells in the inflammatory infiltrates (Fig. 3). One of the two cirrhotic patients died of sepsis, shortly after re-transplantation, while the other one was alive at the time of last follow-up. Two biopsies of yet another patient of the PBC group had a combination of prominent necroinflammatory activity and bridging fibrosis with granulomas and florid duct lesions, suggesting an overlap syndrome of AIH and PBC (Fig. 4).
Figure 3.
Liver allograft biopsy specimen with features of autoimmune hepatitis and cirrhosis (patient 1 of Table 3): (a) there is parenchymal collapse and nodularity (hematoxylin-eosin; 100 ×); (b) an area of bridging necrosis is seen (hematoxylin-eosin; 200 ×); (c) interface hepatitis with prominent plasma cells is present (hematoxylin-eosin; 400 ×).
Figure 4.
Prominent interface hepatitis (a) (hematoxylin-eosin; 400 ×), bridging fibrosis, (b) (hematoxylin-eosin; 200 ×), and florid duct lesion and (c) (hematoxylin-eosin; 400 ×) from a liver allograft biopsy specimen with features suggesting an overlap syndrome of autoimmune hepatitis and primary biliary cirrhosis (patient 4 of Table 3).
The demographic, clinical and pathologic data pertaining to these four patients with AIH are summarized in Table 3. All four were on the tacrolimus-based immunosuppression regimen. Serologic studies performed at the time of biopsy diagnosis of AIH in two patients were negative for antinuclear and smooth muscle autoantibodies, while anti-liver kidney microsome type 1 autoantibodies were detected in one of them. Serologic testing for hepatitis B virus, HCV, cytomegalovirus and Epstein–Barr virus was negative in all four patients.
Table 3.
Demographic and clinicopathologic data of four patients with histologic evidence of autoimmune hepatitis (AIH) following orthotopic liver transplantation for primary biliary cirrhosis (PBC)
| Case | Age | HLA matching | Time of biopsy (days post-OLT) |
Histologic diagnosis |
|---|---|---|---|---|
| 1 | 40 | matched: 0 loci | 1. 756 | AIH and cirrhosis (all 4 biopsies) |
| mismatched: 6 loci | 2. 901 | |||
| 3. 1177 | ||||
| 4. 1478 | ||||
| 2† | 57 | matched: 1 locus | 1. 309 | AIH and cirrhosis in last 3 biopsies |
| mismatched: 5 loci | 2. 343 | |||
| 3. 952 | ||||
| 4. 2144 | ||||
| 5. 2710 | ||||
| 3 | 55 | matched: 1 locus | 1. 2963 | AIH with bridging fibrosis (both biopsies) |
| mismatched: 5 loci | 2. 4314 | |||
| 4 | 57 | matched: 0 loci | 1. 673 | AIH/PBC overlap syndrome with bridging fibrosis (both biopsies) |
| mismatched: 6 loci | 2. 976 |
HLA, human leukocyte antigen.
Patient 2 died soon after re-transplantation. The biopsy diagnosis was further supported by the histologic findings in the explanted allograft. The other three patients were alive as of the time of last follow-up.
DISCUSSION
In this series of 84 patients undergoing OLT for PBC over a period of 10 years, definite histologic evidence of recurrent disease (i.e. presence of granulomatous destructive cholangitis) was identified in four patients undergoing biopsy at a median time of 2.75 years post-OLT. In addition, non-necrotizing epithelioid cell granulomas in portal tracts, which strongly suggest RPBC, were present in biopsy specimens of three more patients, bringing the overall incidence of definite or possible RPBC to 8.33% for the entire group, corresponding to 15.9% of the patients undergoing biopsy. The median time of RPBC diagnosis was 2.78 years post-OLT.
Similar to PBC occurring in the native liver, RPBC can be diagnosed with confidence only by histologic examination of biopsy material. “Sampling error” is an important issue, because the characteristic histologic changes may have a patchy distribution within the hepatic parenchyma. In the absence of florid duct lesions and epithelioid cell granulomas, various combinations of other histologic changes, including bile duct damage and loss, chronic portal inflammation, bile ductular reaction, and fibrosis, may be suggestive of RPBC in the appropriate clinical setting. Taking into account that transplanted livers are often simultaneously affected by more than one pathologic condition, and that such conditions may have overlapping histologic features, it is understandable that a confident diagnosis of RPBC may sometimes be difficult or impossible. Because of these reasons, our study approach was based on a detailed analysis of histologic features, as shown in Table 1.
In this “blinded” histologic study, features that generally characterize chronic hepatitis, such as piecemeal necrosis, lobular necroinflammatory activity and fibrosis, were seen in biopsy specimens from patients of both the PBC and the CHCV groups. Although the incidence and degree of these changes were greater in the CHCV group than in the PBC group, these findings further emphasize the difficulties that may be encountered in the diagnosis of any given case, especially when clinical information is inadequate.
As the number of transplanted PBC patients and the length of follow-up are increasing, it is becoming evident that RPBC is not uncommon.3–10,15,16 The incidence of this condition may have been underestimated because of sampling error, stringency of histologic criteria and short follow-up. In a recent study of 100 PBC patients with a mean follow-up of 4.7 years post-OLT, florid duct lesions indicative of RPBC were identified in 14%.16 However, when the histologic criteria for RPBC were expanded to include moderate lymphocytic cholangitis with lymphoplasmacytic portal infiltrate, the incidence of RPBC was estimated to be 26%.16 In a recent systematic review of 16 original studies of PBC patients post-OLT, Gautam et al. calculated the weighted RPBC rate to be 18%.17 Nevertheless, the findings of our study, as well as those of other recent reports,5–10,16,18,19 indicate that RPBC is generally a mild or slowly progressive disease, only occasionally leading to re-transplantation.
In terms of disease etiopathogenesis, there is evidence suggesting that certain factors associated with PBC persist after OLT. For example, aberrant, extra-mitochondrial staining by antibodies against the E2 component of pyruvate dehydrogenase, the major PBC autoantigen, has been found to occur in the bile duct epithelial cells of both native livers and allografts of PBC patients.20
It has been suggested that early steroid withdrawal post-OLT is associated with a relatively high incidence of RPBC.4,21 It has also been suggested that PBC recurs earlier and with greater severity in patients on tacrolimus-rather than cyclosporine-based immunosuppression regimens.11,18 However, a recent comprehensive review17 did not find a definite association between type of immunosuppression and disease recurrence. In our series, four of the seven patients with histologic features of RPBC were on a cyclosporine-based regimen, and the remaining three were on a tacrolimus-based one. It is of note, however, that all four patients with features of AIH post-OLT (including one with features of AIH/PBC overlap syndrome) were on tacrolimus-based immunosuppression.
Four PBC patients in our study developed AIH (also called “de novo AIH” or “immune mediated hepatitis”), documented with allograft biopsies at a mean time of 3.66 years post-OLT. One of these patients had features of AIH/PBC overlap syndrome, evidenced by granulomas and florid duct lesions in biopsy specimens. There are 11 previous reports of AIH in patients transplanted for PBC,6,22–26 including 1 with features of possible overlap syndrome.25 The exact nature and incidence of AIH in the post-transplant setting are under investigation; at present, it is not clear whether this condition represents a genuine autoimmune attack, versus a form of rejection.12,13,27,28 Reports of additional cases will help to define the incidence, clinicopathologic features and pathogenetic mechanisms of AIH in liver transplant patients.
In conclusion, our “blinded” histologic analysis identified changes indicative of RPBC in 15.9% of the PBC patients undergoing biopsy more than 6 months post-OLT. However, this group of patients did not suffer significant bile duct loss or fibrosis, as compared to the control group of CHCV patients. In this population of immunosuppressed patients, RPBC appears to be a mild or slowly progressive disease. Our findings also suggest that AIH is not rare in this patient population and warrants further investigation. When features of AIH are present in post-transplant biopsy specimens, the patients are likely to develop severe hepatic fibrosis or cirrhosis.
ACKNOWLEDGMENTS
This study was supported in part by grants from the Artz Foundation and NIH grants NIDDK 066939 and NIDA 016156.
REFERENCES
- 1.Maheshwari A, Yoo HY, Thuluvath PJ. Long-term outcome of liver transplantation in patients with PSC: a comparative analysis with PBC. Am J Gastroenterol. 2004;99:538–542. doi: 10.1111/j.1572-0241.2004.04050.x. [DOI] [PubMed] [Google Scholar]
- 2.Jacob DA, Neumann UP, Bahra M, Langrehr JM, Neuhaus R. Liver transplantation for primary biliary cirrhosis: influence of primary immunosuppression on survival. Transplant Proc. 2005;37:1691–1692. doi: 10.1016/j.transproceed.2005.03.130. [DOI] [PubMed] [Google Scholar]
- 3.Balan V, Batts KP, Porayko MK, Krom RA, Ludwig J, Wiesner RH. Histological evidence for recurrent primary biliary cirrhosis after liver transplantation. Hepatology. 1993;18:1392–1398. [PubMed] [Google Scholar]
- 4.Huebscher SG, Elias E, Buckels JAC, Mayer AD, McMaster P, Neuberger JM. Primary biliary cirrhosis: histological evidence of disease recurrence after liver transplantation. J Hepatol. 1993;18:173–184. doi: 10.1016/s0168-8278(05)80244-2. [DOI] [PubMed] [Google Scholar]
- 5.Jacob DA, Neumann UP, Bahra M, et al. Long-term follow-up after recurrence of primary biliary cirrhosis after liver transplantation in 100 patients. Clin Transpl. 2006;20:211–220. doi: 10.1111/j.1399-0012.2005.00471.x. [DOI] [PubMed] [Google Scholar]
- 6.Khettry U, Anand N, Faul RN, et al. Liver transplantation for primary biliary cirrhosis: a long-term pathologic study. Liver Transpl. 2003;9:87–96. doi: 10.1053/jlts.2003.36392. [DOI] [PubMed] [Google Scholar]
- 7.Charatcharoenwitthaya P, Pimentel S, Talwalkar JA, et al. Long-term survival and impact of ursodeoxycholic acid treatment for recurrent primary biliary cirrhosis after liver transplantation. Liver Transpl. 2007;13:1236–1245. doi: 10.1002/lt.21124. [DOI] [PubMed] [Google Scholar]
- 8.Yamagiwa S, Ichida T. Recurrence of primary biliary cirrhosis and primary sclerosing cholangitis after liver transplantation in Japan. Hepatol Res. 2007;37 Suppl 3:S449–S454. doi: 10.1111/j.1872-034X.2007.00250.x. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto E, Taniai M, Yatsuji S, et al. Long-term clinical outcome of living-donor liver transplantation for primary biliary cirrhosis. Hepatol Res. 2007;37 Suppl 3:S455–S461. doi: 10.1111/j.1872-034X.2007.00246.x. [DOI] [PubMed] [Google Scholar]
- 10.Haga H, Miyagawa-Hayashino A, Taira K, et al. Histological recurrence of autoimmune liver diseases after living-donor liver transplantation. Hepatol Res. 2007;37 Suppl 3:S463–S469. doi: 10.1111/j.1872-034X.2007.00245.x. [DOI] [PubMed] [Google Scholar]
- 11.Neuberger J, Gunson B, Huebscher S, Nightingale P. Immunosuppression affects the rate of recurrent primary biliary cirrhosis after liver transplantation. Liver Transpl. 2004;10:488–491. doi: 10.1002/lt.20123. [DOI] [PubMed] [Google Scholar]
- 12.Banff Working Group. Liver biopsy interpretation for causes of late liver allograft dysfunction. Hepatology. 2006;44:489–501. doi: 10.1002/hep.21280. [DOI] [PubMed] [Google Scholar]
- 13.Huebscher SG, Portmann BC. Transplantation pathology. In: Burt AD, Portmann BC, Ferrell LD, editors. MacSween’s Pathology of the Liver. 5th edn. London: Churchill Livingstone; 2007. pp. 815–879. [Google Scholar]
- 14.Portmann BC. Recurrence of primary biliary cirrhosis after transplantation. The pathologist’s view. Eur J Gastroenterol Hepatol. 1999;11:633–637. doi: 10.1097/00042737-199906000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Kotlyar DS, Campbell MS, Reddy KR. Recurrence of diseases following orthotopic liver transplantation. Am J Gastroenterol. 2006;101:1370–1378. doi: 10.1111/j.1572-0241.2006.00586.x. [DOI] [PubMed] [Google Scholar]
- 16.Sylvestre PB, Batts KP, Burgart LJ, Poterucha JJ, Wiesner RH. Recurrence of primary biliary cirrhosis after liver transplantation: histologic estimate of incidence and natural history. Liver Transpl. 2003;9:1086–1093. doi: 10.1053/jlts.2003.50213. [DOI] [PubMed] [Google Scholar]
- 17.Gautam M, Cheruvattath R, Balan V. Recurrence of autoimmune liver disease after liver transplantation: a systematic review. Liver Transpl. 2006;12:1813–1824. doi: 10.1002/lt.20910. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez EQ, Levy MF, Goldstein RM, et al. The changing clinical presentation of recurrent primary biliary cirrhosis after liver transplantation. Transplantation. 2003;76:1583–1588. doi: 10.1097/01.TP.0000090867.83666.F7. [DOI] [PubMed] [Google Scholar]
- 19.Neuberger J. Liver transplantation for primary biliary cirrhosis: indications and risk of recurrence. J Hepatol. 2003;39:142–148. doi: 10.1016/s0168-8278(03)00283-6. [DOI] [PubMed] [Google Scholar]
- 20.Van de Water J, Gerson LB, Ferrell LD, et al. Immunohistochemical evidence of disease recurrence after liver transplantation for primary biliary cirrhosis. Hepatology. 1996;24:1079–1084. doi: 10.1002/hep.510240517. [DOI] [PubMed] [Google Scholar]
- 21.Padbury RT, Gunson BK, Dousset B, et al. Steroid withdrawal from long-term immunosuppression in liver allograft recipients. Transplantation. 1993;55:789–794. doi: 10.1097/00007890-199304000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Heneghan MA, Portmann BC, Norris SM, et al. Graft dysfunction mimicking autoimmune hepatitis following liver transplantation in adults. Hepatology. 2001;34:464–470. doi: 10.1053/jhep.2001.26756. [DOI] [PubMed] [Google Scholar]
- 23.Jones DE, James OF, Portmann B, Burt AD, Williams R, Hudson M. Development of autoimmune hepatitis following liver transplantation for primary biliary cirrhosis. Hepatology. 1999;30:53–57. doi: 10.1002/hep.510300103. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Diaz Y, Reyes-Rodriguez R, Dorta-Francisco MC, et al. De novo autoimmune hepatitis following liver transplantation for primary biliary cirrhosis. Tranplant Proc. 2006;38:1467–1470. doi: 10.1016/j.transproceed.2006.03.071. [DOI] [PubMed] [Google Scholar]
- 25.Tan CK, Ho JMS. Concurrent de novo autoimmune hepatitis and recurrence of primary biliary cirrhosis post-liver transplantation. Liver Transpl. 2001;7:461–465. doi: 10.1053/jlts.2001.23792. [DOI] [PubMed] [Google Scholar]
- 26.Yoshizawa K, Shirakawa H, Ichijo T, et al. De novo autoimmune hepatitis following living-donor liver transplantation for primary biliary cirrhosis. Clin Transpl. 2008;22:385–390. doi: 10.1111/j.1399-0012.2007.00787.x. [DOI] [PubMed] [Google Scholar]
- 27.Vergani D, Mieli-Vergani G. Mechanisms of autoimmune hepatitis. Pediatr Transplant. 2004;8:589–593. doi: 10.1111/j.1399-3046.2004.00288.x. [DOI] [PubMed] [Google Scholar]
- 28.Schreuder TCMA, Huebscher SG, Neuberger J. Autoimmune liver diseases and recurrence after orthotopic liver transplantation: what have we learned so far? Transpl Int. 2008 doi: 10.1111/j.1432-2277.2008.00729.x. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]