Abstract
OBJECTIVE: To determine whether duloxetine is noninferior to (as good as) pregabalin in the treatment of pain associated with diabetic peripheral neuropathy.
PATIENTS AND METHODS: We performed a 12-week, open-label study of patients with diabetic peripheral neuropathic pain who had been treated with gabapentin (≥900 mg/d) and had an inadequate response (defined as a daily pain score of ≥4 on a numerical rating scale [0-10 points]). The first patient was enrolled on September 28, 2006, and the last patient visit occurred on August 26, 2009. Patients were randomized to duloxetine monotherapy (n=138), pregabalin monotherapy (n=134), or a combination of duloxetine and gabapentin (n=135). The primary objective was a noninferiority comparison between duloxetine and pregabalin on improvement in the weekly mean of the diary-based daily pain score (0- to 10-point scale) at end point. Noninferiority would be declared if the mean improvement for duloxetine was no worse than the mean improvement for pregabalin, within statistical variability, by a margin of –0.8 unit.
RESULTS: The mean change in the pain rating at end point was –2.6 for duloxetine and –2.1 for pregabalin. The 97.5% lower confidence limit was a –0.05 difference in means, establishing noninferiority. As to adverse effects, nausea, insomnia, hyperhidrosis, and decreased appetite were more frequent with duloxetine than pregabalin; insomnia, more frequent with duloxetine than duloxetine plus gabapentin; peripheral edema, more frequent with pregabalin than with duloxetine; and nausea, hyperhidrosis, decreased appetite, and vomiting, more frequent with duloxetine plus gabapentin than with pregabalin.
CONCLUSION: Duloxetine was noninferior to pregabalin for the treatment of pain in patients with diabetic peripheral neuropathy who had an inadequate pain response to gabapentin.
Trial Registration: clinicaltrials.gov Identifier: NCT00385671
ANCOVA = analysis of covariance; BOCF = baseline observation carried forward; BPI = Brief Pain Inventory; CI = confidence interval; DPNP = diabetic peripheral neuropathic pain; HbA1c = glycated hemoglobin; ITT = intent to treat; LOCF = last observation carried forward; MMRM = mixed-models repeated-measures; MoNI = margin of noninferiority; TCA = tricyclic antidepressant; TEAE = treatment-emergent adverse event
Diabetes mellitus is commonly associated with peripheral neuropathies, which include painful sensations in the extremities that are described as aching, burning, stabbing, or tingling.1,2 The pain associated with diabetic peripheral neuropathy may be in part due to failure of the endogenous analgesic mechanisms in the descending spinal pathways that control pain transmission to the brain.3 Historically, diabetic peripheral neuropathic pain (DPNP) has been treated with tricyclic antidepressants (TCAs), opioid analgesics, and certain anticonvulsant agents. Although TCAs have been the standard treatment for DPNP, long-term use may be associated with serious adverse effects, such as orthostatic hypotension, greater risk of adverse cardiovascular effects,4 and greater relative risk of overall mortality.5 Opioid analgesics provide prompt pain relief, but their adverse effects and potential for abuse or addiction make them less desirable for long-term treatment.6 Among anticonvulsant agents, gabapentin is a commonly prescribed medication for the management of DPNP; it is considered to have a generally benign safety profile with no clinically important drug interactions6 but may take several weeks to reach an effective dosage (1800-3600 mg/d).7
Currently, 2 medications are approved by the US Food and Drug Administration for the management of DPNP: duloxetine hydrochloride and pregabalin. The proposed mechanism of action of duloxetine, an antidepressant, is reuptake inhibition of both serotonin and norepinephrine in the central nervous system, which increases the activity of these neurotransmitters and subsequently reduces the perception of pain by modulating the pain signals.8,9 In contrast, pregabalin, an anticonvulsant agent, has a proposed analgesic mechanism of action that involves binding to the α2-δ subunit of calcium channels in hyperexcited afferent neurons, which reduces the release of glutamate, norepinephrine, and substance P, thereby reducing pain signals transmitted from the periphery to the brain.10
When treatment with an analgesic does not provide satisfactory pain reduction or is not well tolerated, one option is to switch to or add another analgesic with a different mechanism of action. Selecting a medication to switch to is based on its efficacy and adverse event profile.11 This study was undertaken to determine whether switching from gabapentin to duloxetine would provide a benefit similar to that of switching to pregabalin and to determine the benefit of adding duloxetine to gabapentin. Although TCAs are first-line therapies for DPNP, none were used as comparators in the current study because the therapeutic doses are associated with substantial adverse events.
This study used a noninferiority design that specifically examines whether a medication (in this case duloxetine) is no worse than (or as good as) a reference medication (pregabalin) for which efficacy has been established. Noninferiority compares the difference in improvement between 2 treatments to a predetermined margin of noninferiority (MoNI) for the treatment effect on the primary outcome. This margin represents the maximum treatment difference that is not considered clinically better or worse. The benefit of using a noninferiority study design is that fewer patients are required than for a head-to-head comparison trial between 2 efficacious medications. A head-to-head comparison trial between duloxetine and pregabalin would require large numbers of patients to achieve significance because the difference in efficacy between these analgesics would be small.
Because patients with diabetes generally have a high prevalence of comorbid illnesses and are often prescribed multiple medications, this was an open-label study that allowed more realistic disease management and the use of a broader range of concomitant medications. Thus, the results of this trial may be more generalizable to a clinical population.
The primary objective of this open-label, noninferiority study was to determine whether treatment with duloxetine was at least as good as pregabalin in reducing pain associated with diabetic neuropathy in patients who had an inadequate pain response to treatment with gabapentin.
PATIENTS AND METHODS
We conducted a phase 4, open-label, randomized, 12-week study in 43 study centers in the United States, Germany, Canada, and Puerto Rico. The first patient was enrolled on September 28, 2006, and the last patient visit occurred on August 26, 2009. The institutional review board for each site approved the protocol, which was developed in accordance with the ethical guidelines of Good Clinical Practice and the Declaration of Helsinki. All patients provided written consent after the study was explained and their questions answered and before study procedures were initiated.
Outpatients with type 1 or type 2 diabetes mellitus and a glycated hemoglobin (HbA1c) level of 12% or lower were eligible for inclusion in the study if they were aged at least 18 years, presented with DPNP (confirmed by a score of at least 3 on section B of the Michigan Neuropathy Screening Instrument),12 and had a daily pain score of 4 or higher based on a numerical rating scale (0-10 points). Also, patients had to be taking a stable dose of gabapentin (≥900 mg/d) for at least 5 weeks before randomization, had to be at least 80% adherent to gabapentin therapy, and had to agree not to change the dose of gabapentin between visits 1 and 2. In addition, the patient or physician had to have indicated a need to change from the current gabapentin therapy for pain management. Patients were excluded if they had a history or current diagnosis of mania, bipolar disorder, obsessive-compulsive disorder, or posttraumatic stress disorder or were judged before randomization to be at risk of suicide. Other exclusion criteria included historical exposure to drugs known to cause neuropathy, treatment with a monoamine oxidase inhibitor within 14 days, or treatment with fluoxetine within 30 days of randomization. Concomitant antidepressants (except monoamine oxidase inhibitors) and analgesics were allowed, provided the patient was taking a stable dose for a minimum of 3 months before study enrollment.
The screening period for eligibility occurred between visits 1 and 2 and lasted as long as 60 days (approximately 8 weeks) to accommodate the washout period for excluded medications. When no washout was required, patients were enrolled within 3 days after they completed all screening procedures. Eligible patients were randomized in a 1:1:1 ratio to open-label oral treatment with duloxetine monotherapy, pregabalin monotherapy, or a combination of duloxetine and gabapentin. Patients began taking the study drug on the next day after visit 2. Patients in the monotherapy treatment arms were cross-tapered from their current oral dose of gabapentin to 300 mg/d during a 1- to 2-week period; gabapentin therapy was then discontinued. Duloxetine therapy was initiated at 30 mg once daily for 1 week, after which patients received 60 mg once daily. In Germany, Puerto Rico, and the United States, pregabalin was dosed according to the US prescribing information13 and initiated at 150 mg/d for the first week with 50-mg doses taken 3 times daily. The following week, the dosage was increased to 300 mg/d and administered at 100 mg 3 times daily. In Canada, pregabalin was dosed according to Canadian prescribing information,14 and therapy was initiated at 150 mg/d but administered at 75 mg twice daily. The following week, the dosage was increased to 300 mg/d and administered at 150 mg twice daily. Patients randomized to combination treatment continued to take their current dose of gabapentin (≥900 mg once daily), initiated duloxetine therapy at 30 mg once daily for the first week, and then received duloxetine, 60 mg once daily, for the remainder of the study.
Patients were allowed to use concomitant medications for the management of pain or mood during the trial, provided they were taking a stable dose of the medication at study entry. Patients were not allowed to increase the dose of these medications but were allowed to decrease the dose.
The primary efficacy measure was the reduction from baseline in the weekly mean of the daily 24-hour pain (referred to hereafter as daily pain) diary ratings at week 12. Pain was assessed using a numerical rating scale ranging from 0 for no pain to 10 for worst possible pain. Two secondary measures derived from the pain diaries that were also analyzed weekly were worst pain and night pain ratings. Other secondary measures included the Clinical Global Impression of Severity, the Brief Pain Inventory (BPI) severity and interference items,15 the Beck Depression Inventory II,16 the Patient Global Impression of Improvement, and the Sheehan Disability Scale.17
Three response criteria were analyzed on the basis of daily pain ratings: reductions of 30% or higher from baseline, reductions of 50% or higher from baseline, and a reduction of 2 or more points from baseline. Sustained response was defined as at least a 30% reduction from baseline to end point, at least a 30% reduction from baseline 2 weeks before the last week in the study period, and at least a 20% continued reduction from baseline every week in between.
Safety measures included spontaneously reported treatment-emergent adverse events (TEAEs), discontinuation due to adverse events, mean changes and categorical changes in vital signs, and mean changes and rates of abnormal laboratory analytes, including HbA1c levels.
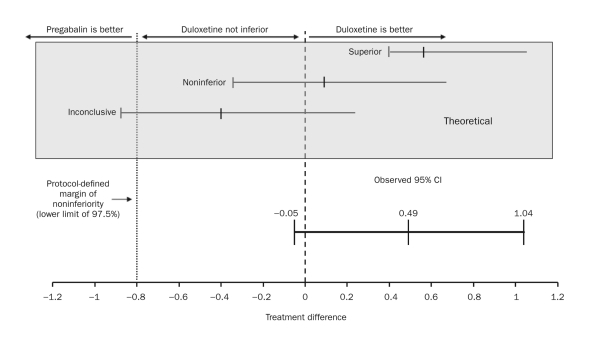
Noninferiority in this study focuses on differences in improvement in pain reduction between duloxetine and pregabalin (primary objective) and between duloxetine and duloxetine plus gabapentin (secondary objective) at week 12. The 97.5% 1-sided confidence limit, which is equivalent to the lower limit of a 2-sided 95% confidence interval (CI), for the difference between duloxetine and pregabalin was compared with the protocol-defined MoNI. The MoNI represents the maximum disadvantage of duloxetine relative to its comparator that is not considered clinically meaningful. If the lower limit of the CI exceeds the MoNI, noninferiority is established.
The MoNI was based on results from an analysis of 3 DPNP placebo-controlled duloxetine studies18-20 using only patients previously treated with gabapentin. In these patients, the estimated mean improvement (decrease) in pain severity at week 12 was –2.74 for duloxetine, 60 mg once daily, and –1.09 for placebo, representing a mean –1.65 advantage of duloxetine over placebo. Thus, the prespecified MoNI of –0.8 represents slightly less than 50% of the previously observed advantage for duloxetine over placebo in a similar population of patients.
This study was designed to randomize 400 patients to duloxetine, pregabalin, or duloxetine plus gabapentin in a 1:1:1 ratio. With the assumption that 6% of patients would discontinue the study before providing postbaseline data, approximately 125 patients per arm were included in the primary efficacy analysis. This sample size has 92% power for the noninferiority assessment of duloxetine compared with pregabalin in pain reduction. Assumptions supporting the sample size estimate include the use of a 1-tailed 97.5% CI, a mean improvement of 2.5 for pregabalin and 2.7 for duloxetine, a common SD of 2.3 for change from baseline to end point, and a MoNI of –0.8.
In accordance with International Committee for Harmonization recommendations,21 noninferiority analyses were conducted on the intent-to-treat (ITT) population and the per-protocol population. Because results from the ITT population can produce wider CIs owing to attrition than those from the per-protocol population, there may be some bias toward finding no difference between treatments in the ITT analysis. Thus, results of the per-protocol sensitivity analysis can support the results from the ITT population if the outcomes from both analyses are consistent.22 The ITT population included all randomized patients who had weekly mean assessments of baseline and postbaseline diary-based daily pain ratings. The per-protocol population included only those patients in the ITT population who adhered to a predetermined set of key protocol criteria: adherence to study drug therapy, Michigan Neuropathy Screening Instrument score less than 3 at study entry, gabapentin taper length of not more than 14 days, no use of prohibited medications, and no HbA1c level greater than 12% after randomization.
The primary analysis used a restricted, likelihood-based mixed-models repeated-measures (MMRM) model that included explanatory terms for the fixed, categorical effects of treatment, investigator, week, and treatment by week interaction, as well as the continuous, fixed covariates of baseline average pain severity ratings and the baseline by week interaction. An unstructured correlation matrix was used to model the within-patient errors. The noninferiority assessments were made using pairwise CIs comparing treatments at week 12.
Sensitivity analyses of noninferiority at end point were also characterized using baseline observation carried forward (BOCF) and last observation carried forward (LOCF) imputation techniques for the weekly means, with analysis based on an analysis of covariance (ANCOVA) model with terms for treatment, baseline, and investigator for both the ITT and per-protocol populations. Estimates from MMRM/ANCOVA models reported here are least-squares means.
Baseline characteristics were compared using the Fisher exact test (nominal outcomes) or analysis of variance (numeric outcomes) with terms for treatment and investigator. Analyses of secondary efficacy measures were conducted using the MMRM model as described for the primary analysis (if collected at multiple time points after baseline) and the ANCOVA model as already described (if collected at baseline or end point only) but without noninferiority assessment.
Other than for assessment of noninferiority, the term significant indicates statistical significance at a 2-sided level of .05 or less; a significance level of .10 or less is used for tests of interaction. All statistical analyses were performed using SAS statistical software, version 9.1.3 (SAS Institute, Cary, NC).
RESULTS
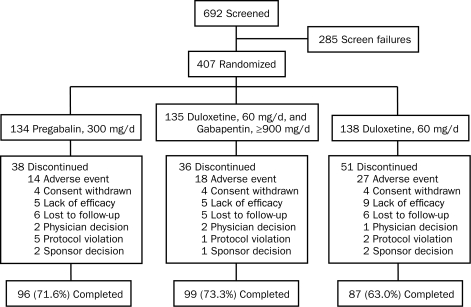
A total of 692 patients were screened, and 285 failed to meet entry criteria. The remaining 407 patients were randomized to pregabalin monotherapy, 300 mg/d (n=134); the combination of duloxetine, 60 mg/d, and gabapentin, 900 mg/d or more (n=135); or duloxetine monotherapy, 60 mg/d (n=138). Disposition of the patients is shown in Figure 1.
FIGURE 1.
Patient disposition diagram showing the flow of patients through each stage of the trial from screening through completion.
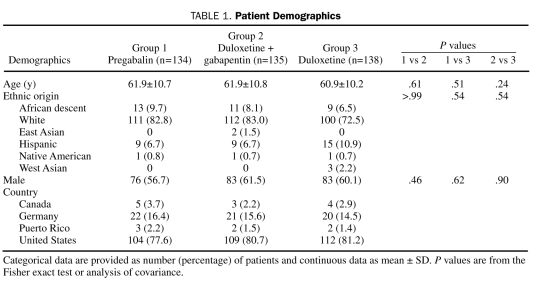
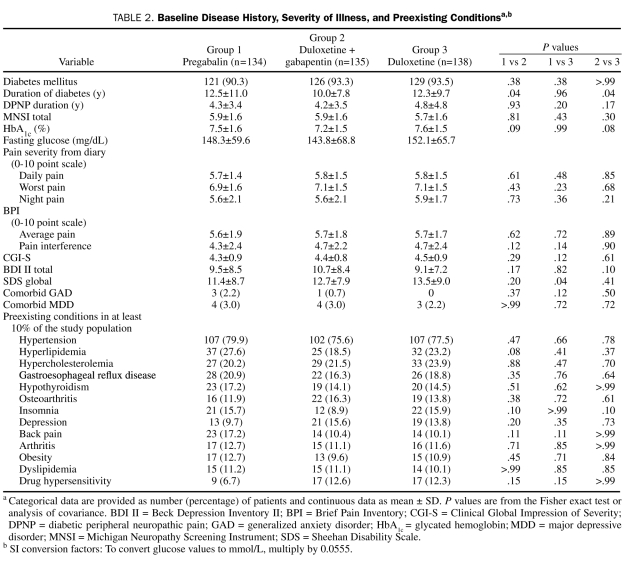
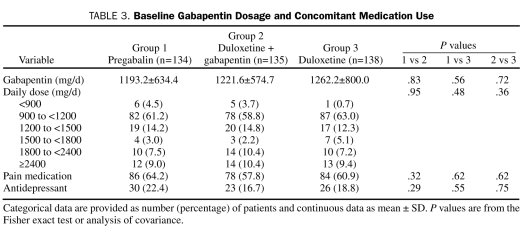
Demographic and baseline clinical characteristics of the ITT patient population, as well as preexisting conditions, were generally similar across treatment groups (Table 1 and Table 2). Most patients were white men with type 2 diabetes who were in their early 60s. The mean duration of diabetes was more than 12 years, which was significantly longer for each of the monotherapy groups compared with the combination treatment group, and the average duration of DPNP was almost 4.5 years. On average, mean HbA1c values were less than 8%. Daily pain severity as reported in patient diaries and as assessed by the BPI was moderate (rating of 4-6),23 and severity of functional disability was rated as mild (rating of 3-9) to moderate (rating of 12-18).17 Less than 5% of the patients had a comorbid major depressive disorder or generalized anxiety disorder. Preexisting conditions included arthritis, osteoarthritis, and back pain. The average gabapentin dose at baseline was more than 1200 mg/d; more than 50% of the patients reported using concomitant pain medications, and less than 20% used antidepressants (Table 3).
TABLE 1.
Patient Demographics
TABLE 2.
Baseline Disease History, Severity of Illness, and Preexisting Conditionsa,b
TABLE 3.
Baseline Gabapentin Dosage and Concomitant Medication Use
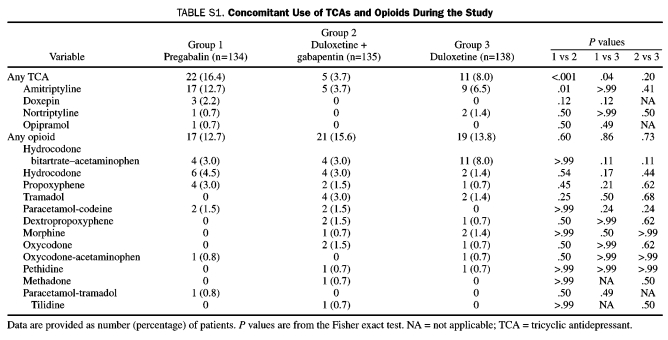
During the study, amitriptyline was the most common TCA used across treatment groups; its use was significantly greater for patients in the pregabalin group (Table S1, Supporting Online Material). Opioids were used by 57 patients, with the combination of hydrocodone bitartrate and acetaminophen being the most common. No significant between-treatment differences were found with the use of any of the opioids.
TABLE S1.
Concomitant Use of TCAs and Opioids During the Study
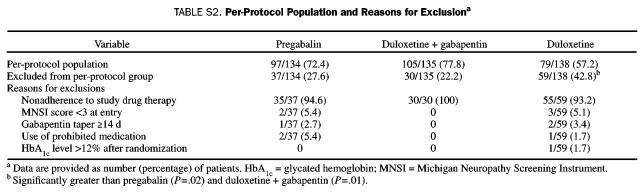
The per-protocol patient population was composed of 281 patients from the ITT population (Table S2, Supporting Online Material). More than 70% of the patients in the pregabalin and the duloxetine plus gabapentin groups were included in the per-protocol population, whereas less than 60% of the patients in the duloxetine monotherapy group were included. The most common reason for excluding patients from the per-protocol population was nonadherence to study drug therapy, which was significantly greater in the duloxetine monotherapy group (P=.02) vs the pregabalin and duloxetine plus gabapentin groups (P=.01).
TABLE S2.
Per-Protocol Population and Reasons for Exclusiona
Efficacy
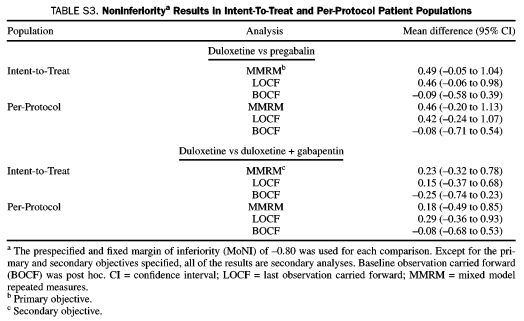
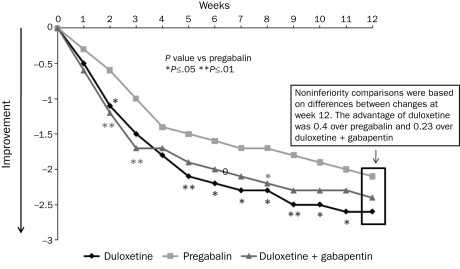
The noninferiority of duloxetine to pregabalin was confirmed by comparing changes in the weekly mean of the daily pain diary ratings in the ITT patient population at end point (week 12) (Figure 2). The estimated mean change in the daily pain severity score at 12 weeks was –2.6 for duloxetine and –2.1 for pregabalin, representing an observed 0.49 advantage for duloxetine. The 97.5% lower confidence limit for the difference between the estimated means was –0.05, far in excess of the protocol-specified MoNI of –0.8, establishing noninferiority. Noninferiority was also demonstrated by additional analyses in the perprotocol population and with use of the LOCF and BOCF approaches (Table S3, Supporting Online Material). Significant superiority vs pregabalin in the mean daily pain diary ratings was observed at weeks 2, 3, and 5 through 11 with duloxetine and with duloxetine plus gabapentin at weeks 2 and 8, but between-treatment differences at the 12-week end point met noninferiority criteria, not statistical superiority (Figure 3). The noninferiority comparison between duloxetine monotherapy and duloxetine plus gabapentin (secondary objective) on the differences between end point mean changes in daily pain diary ratings in the ITT patient population was also met, with further support from each of the 5 additional analyses (Table S3, Supporting Online Material).
FIGURE 2.
Results of the noninferiority test for duloxetine vs pregabalin. Changes from baseline to week 12 in the weekly mean of the daily pain severity for duloxetine- and pregabalin-treated patients were –2.6 and –2.1, respectively (treatment difference, 0.49; 95% confidence interval [CI], –0.05 to 1.04; P=.08).
TABLE S3.
Noninferioritya Results in Intent-To-Treat and Per-Protocol Patient Populations
FIGURE 3.
Changes in weekly means of the daily pain severity ratings from patient diaries. Significant superiority vs pregabalin was seen in some weeks before week 12 for duloxetine monotherapy and for duloxetine + gabapentin combination therapy.
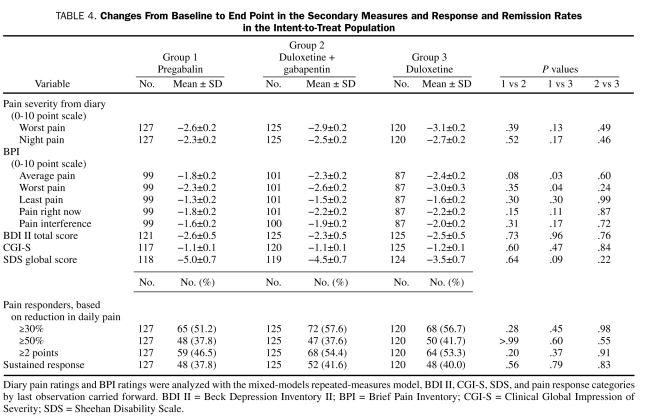
Secondary efficacy outcomes at end point are summarized in Table 4. Reduction from baseline in BPI average pain and BPI worst pain severity ratings was significantly greater with duloxetine monotherapy vs pregabalin monotherapy, but differences between treatments were not significant for the other BPI pain measures, Clinical Global Impression of Severity, depressive symptoms, or the Sheehan Disability Scale global measure. Also, no significant between-treatment differences were found among the various response outcomes (Table 4).
TABLE 4.
Changes From Baseline to End Point in the Secondary Measures and Response and Remission Rates in the Intent-to-Treat Population
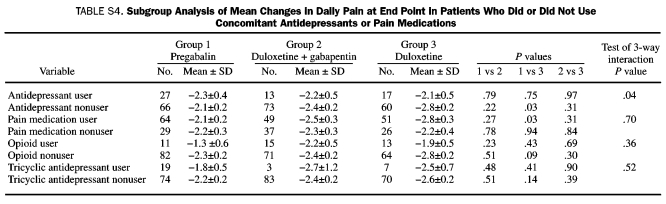
The results of the subgroup analyses of mean changes in daily pain at end point in patients who did or did not use concomitant antidepressants or pain medications are summarized in Table S4 (Supporting Online Material). In antidepressant users, no significant between-treatment group differences were found at end point, whereas there was significantly greater pain reduction with duloxetine treatment vs pregabalin in patients who did not use antidepressants. The result of the test of 3-way interaction (antidepressant subgroup by treatment by week) was significant, which demonstrates a differential treatment effect on pain reduction with concomitant antidepressant use. In patients who did not use antidepressants, pain reduction with duloxetine monotherapy at week 12 was significantly greater than with pregabalin (–2.8 vs –2.1; P=.03), not simply noninferior. The results of the test of 3-way interaction for the other subgroup analyses were not significant, revealing no differential treatment effects based on concomitant use of pain medications, opioids, or TCAs.
TABLE S4.
Subgroup Analysis of Mean Changes in Daily Pain at End Point in Patients Who Did or Did Not Use Concomitant Antidepressants or Pain Medications
The result of the subgroup analysis based on country was not significant (test of 3-way interaction: country by treatment by week; P=.85); thus, differences in pregabalin administration did not confound pain improvement. However, all of the patients who received pregabalin, except for the Canadian patients (n=12), were dosed according to the US prescribing information.
Safety
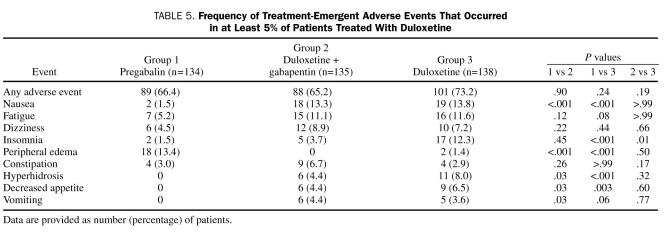
Significantly more discontinuations occurred as a result of adverse events in the duloxetine monotherapy group (n=27, 19.6%; P=.04) vs the pregabalin monotherapy group (n=14, 10.4%) but not vs the duloxetine plus gabapentin group (n=18, 13.3%; P=.19). Peripheral edema associated with pregabalin treatment (n=5, 3.7%) was the only adverse event reported as a reason for discontinuation with significantly greater frequency than for the other treatments (duloxetine, 0%; P=.03; duloxetine plus gabapentin, 0%; P=.03). Rates of discontinuation for other reasons did not differ significantly among the treatment groups.
The TEAEs are summarized in Table 5. Nausea, insomnia, hyperhidrosis, and decreased appetite occurred significantly more frequently with duloxetine monotherapy than with pregabalin monotherapy. The frequency of insomnia was also significantly greater with duloxetine monotherapy than with duloxetine plus gabapentin therapy. The occurrence of peripheral edema was significantly greater with pregabalin monotherapy than with the other 2 treatment groups. Treatment with duloxetine plus gabapentin was associated with significantly more occurrences of nausea, hyperhidrosis, decreased appetite, and vomiting than was treatment with pregabalin monotherapy.
TABLE 5.
Frequency of Treatment-Emergent Adverse Events That Occurred in at Least 5% of Patients Treated With Duloxetine
Baseline to end point changes in weight were significant between treatment groups. Patients treated with pregabalin gained an average of 1 kg, whereas patients in the duloxetine group lost an average of 2.4 kg (P<.001 vs pregabalin), and those in the duloxetine plus gabapentin group lost an average of 1.1 kg (P<.001 vs pregabalin; P=.01 vs duloxetine).
The HbA1c levels did not change significantly during the 12 weeks of the study, and the average HbA1c level was 7.5% or less across the treatment groups. Statistically significant differences in the mean change from baseline in fasting glucose levels were found between the pregabalin group and the duloxetine plus gabapentin group (2.9 vs 12.1 mg/dL [to convert glucose values to mmol/L, multiply by 0.555]; P=.05) but not the duloxetine group (3.4 mg/dL; P=.23). No significant differences were found in fasting glucose levels between the duloxetine group and the duloxetine plus gabapentin group (P=.42). Fasting glucose levels at end point were numerically similar among the treatment groups: pregabalin, 151.0±78.5 mg/dL; duloxetine plus gaba pentin, 155.7±65.0 mg/dL; and duloxetine, 155.7±65.7 mg/dL. Overall, findings on laboratory testing and safety assessment were not clinically relevant.
DISCUSSION
Treatment with duloxetine met criteria for establishing noninferiority to pregabalin in terms of efficacy for pain reduction in patients with diabetic peripheral neuropathy who had less than adequate response to treatment with gabapentin. The primary outcome was also supported by positive noninferiority results by analyses conducted in the per-protocol population and from sensitivity analyses using LOCF and BOCF approaches. In addition, duloxetine monotherapy met criteria for noninferiority to treatment with duloxetine plus gabapentin that was also supported by each of the sensitivity analyses.
The interpretation of the noninferiority outcomes is supported by several features of the trial design. The primary outcome measure in this study is comparable to that used in previous duloxetine18-20 and pregabalin24,25 randomized, double-blind, placebo-controlled efficacy trials of 12 weeks' duration. In addition, both monotherapy groups were cross-tapered from gabapentin and titrated to fixed doses of duloxetine or pregabalin over 2 weeks, so timing of escalation to the therapeutic dose did not vary. Furthermore, the therapeutic dose for each medication was consistent with current dosing guidelines for patients with DPNP, which reduces potential bias against either treatment. However, patient selection was based on not having an adequate response to gabapentin, which has a proposed mechanism of action similar to pregabalin, so this could be considered a potential bias against pregabalin. Although pregabalin and gabapentin share the characteristic of binding to the α2-δ subunit of calcium channels, subtle differences were seen between these agents in other mechanisms of action that differentiate them as analgesics. For example, in an open-label study, pregabalin effectively reduced pain in patients with DPNP or postherpetic neuropathic pain who were refractory to or had experienced intolerable adverse events from treatment with gabapentin, a TCA, or a third medication.26
To our knowledge, this is the first study to investigate adding duloxetine to gabapentin. This combination was efficacious with patients experiencing baseline to end point reductions in pain, similar to treatment with duloxetine monotherapy and pregabalin monotherapy. Although adding duloxetine to gabapentin may provide increased efficacy, further studies of combination therapies in inadequate responders or naive patients are warranted. Duloxetine plus gabapentin was generally well tolerated, with an adverse event profile and rate similar to those of duloxetine monotherapy treatment, with the exception of significantly less insomnia occurring in the combination treatment group. Also, the adverse event profile for duloxetine plus gabapentin was not different from that reported for gabapentin monotherapy.27
The analysis of concomitant pain medication use (including opioids and TCAs) found no differential treatment effect with these agents. However, the analysis of concomitant antidepressant use yielded intriguing results, suggesting that switching to duloxetine instead of pregabalin could provide better pain reduction, particularly in those patients who were not taking antidepressants. Conversely, adding duloxetine to gabapentin in nonresponders who are already using an antidepressant may not provide better pain reduction than switching to pregabalin. Although concomitant antidepressant medications were allowed in this study, the use of multiple serotonergic agents should be avoided or approached with caution because of the risk of serotonin syndrome.
Duloxetine, pregabalin, and duloxetine plus gabapentin were safely administered and well tolerated. No significant differences were found between treatments in the frequency of these events. Although the TEAE profile for each treatment varied, each profile was consistent with previous reports for duloxetine,18-20 pregabalin,24-26,28-30 and gabapentin.27
This study has limitations that should be considered. First, the open-label conduct of the current trial may have influenced the evaluation of efficacy and adverse events by both patients and investigators. However, the magnitude of pain reduction observed in this study is consistent with reports from 12-week, randomized, double-blind, placebo-controlled clinical trials in patients with DPNP treated with duloxetine, 60 mg/d,18-20 or pregabalin, 300 mg/d.24,25 Second, the relatively small number of patients with comorbid major depressive disorder or generalized anxiety disorder (15 of 407, 3.7%) included in the study does not adequately represent the population of patients with diabetes because depression is twice as prevalent in those with vs without diabetes.31 Persons with these comorbid conditions may have been screened for inclusion in this study but opted not to participate, especially if they were responding to their current medication and did not want to risk worsening their symptoms by modifying their treatment. Third, the lack of a gabapentin monotherapy control group in the current study limits the conclusions drawn from any difference observed between duloxetine monotherapy and duloxetine plus gabapentin and must be viewed with caution. Finally, the minimum gabapentin dose allowed for inclusion in the study was less than the recommended efficacious dose of 1800 mg/d.7
CONCLUSION
Findings from the current study indicate that treatment with duloxetine, 60 mg once daily, was noninferior to pregabalin, 300 mg/d, in patients with DPNP who had a suboptimal pain response to gabapentin. Adding duloxetine to gabapentin was efficacious, with patients experiencing a reduction in pain similar to that achieved with duloxetine or pregabalin monotherapy. The magnitude of pain reduction observed in this study, which included patients with comorbid pain conditions, is consistent with reports from double-blind, placebo-controlled DPNP clinical trials of duloxetine and pregabalin in patients without these comorbid conditions.
Supplementary Material
Acknowledgments
This study was funded by Lilly USA, LLC. We thank the clinical operations and data management teams of Lilly USA, LLC, the investigators and staff of the F1J-US-HMEZ study, and the many patients who generously agreed to participate in this trial. In addition, we gratefully acknowledge Indu Lakhani, MS, for expert statistical programming.
Footnotes
This work was supported by Lilly USA, LLC, Indianapolis, IN.
Dr Ahl, Mr Risser, Dr Skljarevski, and Ms Malcolm are employees and/or stockholders of Eli Lilly and Company or Lilly USA, LLC. Dr Tanenberg has received research funding from Medtronic Mini Med; CPEX Pharmaceuticals, Inc; and Lilly USA, LLC. He is on the Speaker's Bureau for sanofi-aventis, Takeda Pharmaceuticals North America, and Pfizer. Dr Irving is a consultant to Eli Lilly and Company, King, Elan, Endo, Xanodyne, and Neurogesx and is on the Speaker's Bureau for Eli Lilly and Company, Primera, and Xanodyne.
This article is freely available on publication, because the authors have chosen the immediate access option.
REFERENCES
- 1. Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain. 2002;18(6):350-354 [DOI] [PubMed] [Google Scholar]
- 2. Vinik AI. Diabetic neuropathy: pathogenesis and therapy. Am J Med. 1999;107(2B):17S-26S [DOI] [PubMed] [Google Scholar]
- 3. Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain. 2002;100(1-2):1-6 [DOI] [PubMed] [Google Scholar]
- 4. Ray WA, Meredith S, Thapa PB, Hall K, Murray KT. Cyclic antidepressants and the risk of sudden cardiac death. Clin Pharmacol Ther. 2004;75(3):234-241 [DOI] [PubMed] [Google Scholar]
- 5. Cohen HW, Gibson G, Alderman MH. Excess risk of myocardial infarction in patients treated with antidepressant medications: association with use of tricyclic agents. Am J Med. 2000;108:2-8 [DOI] [PubMed] [Google Scholar]
- 6. Dworkin RH, O'Connor AB, Backonja M, et al. Pharmacological management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237-251 [DOI] [PubMed] [Google Scholar]
- 7. Backonja M, Glanzman RL. Gabapentin dosing for neuropathic pain evidence from randomized, placebo-controlled clinical trials. Clin Ther. 2003;25(1):81-104 [DOI] [PubMed] [Google Scholar]
- 8. Wong DT, Bymaster FP, Mayle DA, Reid LR, Krushinski JH, Robertson DW. LY248686, a new inhibitor of serotonin and norepinephrine uptake. Neuropsychopharmacology. 1993;8(1):23-33 [DOI] [PubMed] [Google Scholar]
- 9. Bymaster FP, Dreshfield-Ahmad LJ, Threlkeld PG, et al. Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacology. 2001;25(6):871-880 [DOI] [PubMed] [Google Scholar]
- 10. Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the α2-∂ subunit of a calcium channel. J Biol Chem. 1996;271(10):5768-5776 [DOI] [PubMed] [Google Scholar]
- 11. Sleath B, Shih YT. Sociological influences on antidepressant prescribing. Soc Sci Med. 2003;56(6):1335-1344 [DOI] [PubMed] [Google Scholar]
- 12. Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17(11):1281-1289 [DOI] [PubMed] [Google Scholar]
- 13. Lyrica® United States prescribing information. 2007. Ann Arbor, MI: Pfizer; [Google Scholar]
- 14. Lyrica® Canadian product monograph. Kirkland, Quebec, Canada: Pfizer Canada; 2007. [Google Scholar]
- 15. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129-138 [PubMed] [Google Scholar]
- 16. Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -I and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588-597 [DOI] [PubMed] [Google Scholar]
- 17. Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol. 1996;11(suppl 3):89-95 [DOI] [PubMed] [Google Scholar]
- 18. Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain. 2005;116(1-2):109-118 [DOI] [PubMed] [Google Scholar]
- 19. Raskin J, Pritchett YL, Wang F, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med. 2005;6(5):346-356 [DOI] [PubMed] [Google Scholar]
- 20. Wernicke JF, Pritchett YL, D'Souza DN, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006;67(8):1411-1420 [DOI] [PubMed] [Google Scholar]
- 21. European Medicines Agency Statistical principles for clinical trials: ICH harmonised tripartite guideline (CPMP/ICH/363/96). Published September 1989 http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002928.pdf Accessed March 24, 2011
- 22. Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJW; CONSORT group Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT Statement. JAMA. 2006;295(10):1152-1160 [DOI] [PubMed] [Google Scholar]
- 23. Gore M, Brandenburg NA, Dukes E, Hoffman DL, Tai KS, Stacey B. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J Pain Symptom Manage. 2005;30(4):374-385 [DOI] [PubMed] [Google Scholar]
- 24. Freynhagen R, Strojek K, Griesing T, Whalen E, Balkenohl M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomized, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain. 2005;115(3):254-263 [DOI] [PubMed] [Google Scholar]
- 25. Tölle T, Freynhagen R, Versavel M, Trostmann U, Young JP Jr. Pregabalin for relief of neuropathic pain associated with diabetic neuropathy: a randomized, double-blind study. Eur J Pain. 2008;12(2):203-213 [DOI] [PubMed] [Google Scholar]
- 26. Stacey BR, Dworkin RH, Murphy K, Sharma U, Emir B, Griesing T. Pregabalin in the treatment of refractory neuropathic pain: results of a 15-month open-label trial. Pain Med. 2008;9(8):1202-1208 [DOI] [PubMed] [Google Scholar]
- 27. Backonja M, Beydoun A, Edwards K, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus. a randomized controlled trial. JAMA. 1998;280(21):1831-1836 [DOI] [PubMed] [Google Scholar]
- 28. Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology. 2004;63(11):2104-2110 [DOI] [PubMed] [Google Scholar]
- 29. Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain. 2005;6(4):253-260 [DOI] [PubMed] [Google Scholar]
- 30. Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004;110(3):628-638 [DOI] [PubMed] [Google Scholar]
- 31. Chapman DP, Perry GS, Strine TW. The vital link between chronic disease and depressive disorders [serial online]. Prev Chronic Dis. January 2005. http://www.cdc.gov/pcd/issues/2005/jan/04_0066.htm Accessed March 24, 2011 [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.