Abstract
For over a decade, the field of stem cell research has advanced tremendously and gained new attention in light of novel insights and emerging developments for regenerative medicine. Invariably, multiple considerations come into play, and clinicians and researchers must weigh the benefits of certain stem cell platforms against the costs they incur. Notably, human embryonic stem (hES) cell research has been a source of continued debate, leading to differing policies and regulations worldwide. This article briefly reviews current stem cell platforms, looking specifically at the two existing pluripotent lines available for potential therapeutic applications: hES cells and induced pluripotent stem (iPS) cells. We submit iPS technology as a viable and possibly superior alternative for future medical and research endeavors as it obviates many ethical and resource-related concerns posed by hES cells while prospectively matching their potential for scientific use. However, while the clinical realities of iPS cells appear promising, we must recognize the current limitations of this technology, avoid hype, and articulate ethically acceptable medical and scientific goals.
ES = embryonic stem; hES = human embryonic stem; ICM = inner cell mass; iPS = induced pluripotent stem; IVF = in vitro fertilization; MSC = mesenchymal stem cell; NBAC = National Bioethics Advisory Commission; SCNT = somatic cell nuclear transfer; UCB = umbilical cord blood
Stem cell research has been the focus of public attention for more than a decade as novel developments and insights into cellular therapy have emerged.1 Given the aging US population, the need for targeted interventions for chronic degenerative diseases will become increasingly urgent, spurring further research into treatments and solutions for diseases linked to progressive cellular and tissue destruction.2-4 Stem cell technology is rapidly expanding the field of regenerative medicine, allowing for the de novo production of functional tissue and providing for new diagnostic and therapeutic capabilities that may surpass the risk-benefit profile of conventional reparative methods (eg, solid organ transplant, tissue rejuvenation).5-8
However, like many prospective tools of medicine, stem cell technology is not without ethical implications. This field, in particular, continues to be a source of ongoing discussion, with most of the controversy centered on embryo destruction.9 This debate is informed by the concepts of nonmaleficence (avoiding harm), beneficence (protecting and defending the rights of others, preventing harm, removing existing harm, and promoting good), justice (fair opportunity, entitlement, and distribution of resources), and human dignity (moral status and the ethical definition of personhood).10,11 For research that necessitates embryo destruction, the verdict is still out among clinicians and researchers regarding one of the cardinal rules of medical ethics: “Primum non nocere” (First, do no harm). The principle of nonmaleficence takes into account the moral nature of the act, the agent's intention, the means of the act, the possible adverse consequences, and the proportionality between the good and bad effects.12 The ongoing dispute has worked its way into the global political arena such that an international consensus has not been reached regarding the regulation of human embryonic stem (hES) cell research. Policies and legislation are restrictive in certain countries (Ireland, Italy, Germany, Poland, Austria) and permissive in others (Belgium, United Kingdom, Sweden, Switzerland, Korea), with the United States currently finding itself somewhere in the middle.13 More than a decade of intense debate has failed to resolve deeply entrenched differences in belief about the nature and beginning of human personhood. Because consensus on the ethicality of embryo destruction appears unlikely, experts must weigh the social, ethical, legal, scientific, and medical costs and benefits for each stem cell platform.
The fundamental biological distinction among stem cell platforms is defined by cellular potency; parental cell lines equally capable of self-renewal and asymmetrical cell division have varying capacities for differentiation into target tissues and cell types.14 For example, embryonic stem (ES) cells are pluripotent in that they are capable of giving rise to all tissues of the developing organism across all three embryological germ layers when allowed to differentiate within the appropriate microenvironment.15,16 In contrast, adult stem cells are multipotent in that they give rise to specialized cell types restricted to an embryological germ layer,17 even despite cultivation within a nurturing embryonic environment.18,19 Notably, both of these classifications are distinct from totipotency, which is defined as the ability to autonomously give rise to an entire adult animal by producing both embryonic and extraembryonic tissues and allowing for complete gestational development.20,21 Thus, cellular potency represents a spectrum of biological capacity in which cells that are less differentiated possess a greater degree of malleability and technological ability for biomedical applications22 at the expense of increased ethical concern regarding the sources from which they are derived.
When weighing the advantages and disadvantages of a stem cell platform, clinicians should apply the calculus of beneficence and nonmaleficence as well as consider the availability of resources, the usefulness of the platform for biomedical applications, and current policy. Since first being isolated in 1998,23 hES cells have come to dominate the stem cell landscape and are currently regarded as “the gold standard.”24 In September 1999, the National Bioethics Advisory Commission (NBAC) of the Clinton administration evaluated the ethical concerns of hES cell research and concluded:
In our judgment, the derivation of stem cells from embryos remaining following infertility treatments is justifiable only if no less morally problematic alternatives are available for advancing the research. But as we have noted, ES cells from embryos appear to be different in scientifically important ways from [adult stem] cells and also appear to offer greater promise of therapeutic breakthroughs. The claim that there are alternatives to using stem cells derived from embryos is not, at the present time, supported scientifically. We recognize, however, that this is a matter that must be revisited continually as science advances [italics inserted].25
Indeed, the science has advanced considerably during the past decade, and ground-breaking discoveries necessitate revisiting the matter.
CURRENT STEM CELL PLATFORMS
Adult Stem Cells
Adult stem cells are derived from bone marrow, adipose tissue, and tissue-resident stem cells. This category notably includes multipotent hematopoietic stem cells and mesenchymal stem cells (MSCs), also known as marrow stromal cells.26 In turn, MSCs give rise to lineages such as bone, cartilage, and adipose.27,28 Both site-directed and systemic deliveries of MSCs have been used for treatment in a wide spectrum of disease models.29,30 The multipotency and extensive clinical availability of these progenitor cells have made MSCs a common tool for tissue engineering.31
Perinatal Stem Cells
Perinatal stem cells are derived from umbilical cord blood (UCB) and amniotic fluid–derived stem cells. In addition to hematopoietic tissues, progenitor cells from UCB have also been used to produce non-hematopoietic lineages that enable an expanding field of applications.32 The heterogeneous pool within UCB also contains stem cells that behave similarly to embryonic-like stem cells.33 Like stem cells in UCB, amniotic fluid–derived stem cells give rise to a wide variety of tissues, including those of adipogenic, osteogenic, myogenic, endothelial, neuronal, and hepatic lineages.34 Storage of these multipotent stem cells is becoming more common as a result of long-term biobanking efforts worldwide.35
Embryonic Stem Cells
Embryonic stem cells are derived from the inner cell mass (ICM) of the embryo at the blastocyst stage. Cells of the ICM are pluripotent and give rise to the embryo proper, in contrast to the trophoblast, which gives rise to the extraembryonic tissues present during gestation. As such, ES cells have the potential to give rise to all tissues of the adult body through sequential differentiation of ectoderm, endoderm, and mesoderm germ layers.14 This class of stem cells was first isolated from mice in the 1980s.36 Since this breakthrough, hES cells have been shown to give rise to a comprehensive spectrum of cell types.37 However, therapeutic use of hES cells has not yet been realized, despite promising results in animal studies of Parkinson disease and spinal injury,38 type 1 diabetes,39 and cardiovascular disease.40 In addition, the first-in-man phase 1 clinical trials for hES technology are currently evaluating the therapeutic effect of hES cell–derived oligodendrocyte progenitor cells in spinal cord injury and retinal pigmented epithelial cells in age-related macular degeneration.41,42
Bioengineered Stem Cells
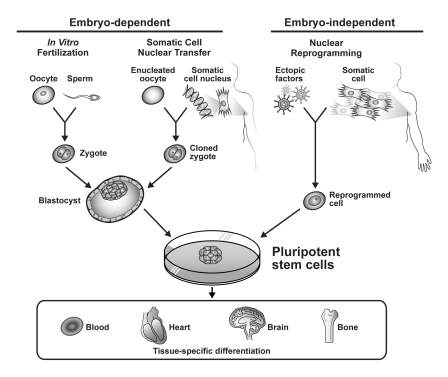
Bioengineered stem cells are derived using techniques that seek to achieve pluripotency in non–stem cells. Such techniques can be classified into two general categories: somatic cell nuclear transfer (SCNT) and pluripotency induced by factor-mediated nuclear reprogramming.37 Better known as cloning, SCNT was first demonstrated in 1997 through the creation of Dolly the sheep.43 As its name would suggest, SCNT is simply the transfer of a somatic cell nucleus into an enucleated oocyte. This gives rise to a cloned zygote from which ES cells can ultimately be derived (and thus it can also be classified under the embryonic stem cell platform; Figure).44 The cytoplasmic environment of the enucleated mammalian oocyte is remarkably conserved across species and allows for reprogramming of the somatic nucleus back to an undifferentiated state.45,46
FIGURE.
Schematic representation of common techniques to procure pluripotent stem cells. Nuclear reprogramming by ectopic factors results in induced pluripotent stem cells that may have functional equivalence to embryonic stem cells derived by conventional embryo-dependent methods and possess therapeutic potential through multilineage differentiation.
One technical variation of SCNT is altered nuclear transfer. In 2006, investigators reported inactivation of the Cdx2 gene in murine fibroblasts using lentiviral RNA interference before somatic nuclear transfer, which resulted in a blastocyst that produced only cells of the ICM.47 These cells were then tested and indeed found to be pluripotent and to function similarly to ES cells (ie, they were able to form postnatal chimeras when injected into diploid blastocysts). This work was based on an earlier study that showed Cdx2 to be necessary for formation of the trophoblast that gives rise to extraembryonic tissues.48 Hence, this work offered a novel alternative to bioengineered pluripotent stem cells that would not necessitate the destruction of viable embryos. However, producing “disabled embryos” incapable of implantation raises ethical concerns and, therefore, this platform is still actively debated.49,50 For technical reasons not yet fully understood, ES cells have not been successfully isolated in humans using any of these methods.44,51
In contrast to nuclear transfer strategies that require an oocytic environment to bioengineer pluripotent stem cells, investigators in 2006 presented a novel technique for nuclear reprogramming of ordinary fibroblasts requiring only the retroviral transduction of four transcription factors (Oct4, Sox2, c-Myc, and Klf4) into the cells, yielding an innovative method to achieve pluripotency of somatic cells known as induced pluripotent stem (iPS) cells.52 One year later, nearly a decade after the Clinton NBAC statement, the first human iPS cells were isolated.53 Ectopic factor–mediated nuclear reprogramming was also found to be achievable with expression of Oct4, Sox2, Nanog, and Lin28.54 Since then, embryo-independent nuclear reprogramming of human iPS cells has been found to be remarkably reproducible (Figure).55 Moreover, nonhuman iPS cells have been found to meet the most stringent of tests for pluripotency, including production of chimeric offspring capable of germline transmission—previously only possible with ES cells.56 In addition, iPS cells injected into tetraploid embryos (forming trophoblasts and giving rise only to extraembryonic tissues) have demonstrated autonomous capacity for embryogenesis.57 Patient-specific iPS cells have also been derived and can be used for pharmacologic testing, diagnostics, and the study of a variety of disease models in vitro.58-61 The regenerative applications of iPS cells for treatment have also been demonstrated in various models that include sickle cell anemia,62 Parkinson disease,63 hemophilia A,64 and ischemic heart disease.65,66
Two months before the initial announcement of iPS, stem cell pioneer Rudolf Jaenisch stated:
The future challenge will be to study alternatives to nuclear transfer in order to recapitulate reprogramming in a Petri dish without the use of oocytes...Will it be possible to fully reprogram a somatic cell into an ES-like cell without exposure of the nucleus to the reprogramming factors of the oocyte?67
Three years of rapidly advancing iPS technology seem to definitively answer, “Yes.” However, the question remains, “As we approach the next step, what will become of the old?”
EMBRYOS vs REPROGRAMMING
Multiple tools exist to create pluripotent stem cells (Figure).44,67 Yet only hES cells derived from donated supernumerary embryos after in vitro fertilization (IVF) procedures and iPS cells are available for pluripotent stem cell technology in humans.13,68-70 In light of the arduous path we have travelled to advance the science of hES cells in this country,9,13,71 as we look ahead we must ask whether ES cells are necessary in light of iPS technology.
Certainly, current iPS technology is far from perfect. As with hES cell derivation, protocols for nuclear reprogramming need further improvement, particularly to address low efficiency and partial reprogramming of cells with transformed or dysplastic progenitor cells that contaminate the stem cell pool.22,72 Although select iPS cells have exhibited certain aspects of hES pluripotency, it is important to recognize that not all iPS cells generated to date have demonstrated longitudinal functional equivalence to hES cells because of the lack of long-term follow-up studies. Additionally, hES cells are still considered by many scientists to be the gold standard for fully understanding the basic mechanisms and environment of pluripotency and for optimizing diagnostic and therapeutic applications of iPS. As noted by iPS researcher Juan Carlos Izpisúa Belmonte, “ES cells are needed to understand the basic mechanism of pluripotency and self-renewal. As such, it is out of the question to even suggest phasing them out. We will be lost without them.”73
The risk of tumorigenesis is another commonly cited potential problem with using iPS cells for therapy. Notably, this is a problem shared by hES cells and any other pluripotent cell models that are capable of multilineage differentiation and teratoma formation.74 However, iPS cells can become transformed with dysplastic growth potential due to the retroviral insertion of reprogramming transcription factors into the genome, a process known as insertional mutagenesis. To address this iPS-specific limitation, studies in the past three years have presented a wide array of preliminary evidence and have introduced successful protocols, including use of nonintegrating viral vectors (eg, adenovirus, plasmid, and episomal vectors), traceless systems to remove the transgenes after induced pluripotency, incubation with recombinant proteins, and transfection with modified RNA.60,75-77 Although these advances are encouraging, producing safe and highly purified iPS cells will remain a serious challenge for future clinical therapeutic application.73
Despite their limitations, bioengineered stem cells offer unique biological and ethical advantages. First, they help address a limitation of many existing stem cell platforms— the risk of immune rejection.78,79 For example, in the first hES phase 1 clinical trial, patients undergoing allogeneic hES cell transplants had to receive immunosuppressive therapy for at least four months and be monitored for 15 years to evaluate for rejection.80 A major advantage of using reprogrammed somatic cells is that they provide the possibility for autologous transplant in regenerative therapy, eliminating the need for immunosuppressive therapy. Second, iPS cells possess an unlimited resource capacity because they require only a tissue biopsy for derivation (that is, anyone can donate and donation is easy), whereas hES cells have severely limited resource capacity because they must be derived from embryos discarded after IVF.81,82 Third, the tissue donation process for iPS production would be a safer and less invasive alternative for patients than egg donation during IVF.83 Finally, and perhaps most importantly, iPS cells avoid the ethical quandaries surrounding embryo destruction. This technology provides a potentially viable alternative to hES cells without invoking sharp dispute about whether we are wrongfully destroying human beings.84,85 These sentiments, which are deeply rooted in the moral and religious consciences of many, will not go away and should not be brushed aside.86-90 Human embryos need never be destroyed to obtain iPS cells, but their destruction is required to obtain hES cells (Figure).
Some might claim that iPS cells, in theory, could be used to create human embryos and thus should be considered equally as problematic as hES cells.91 However, iPS technology does not require or mandate the creation of human embryos. In a Nature interview, Rudolf Jaenisch, a noted SCNT researcher, stated that it would be “possible in principle” to repeat the cloning process for mice (injection of iPS cells into tetraploid blastocysts) in humans, but he also noted that “it would be unrealistic and a ridiculous thing to do.” He also admitted, however, that because “fertilized embryos are easier to get than the fresh eggs used in cloning, some maverick might give it a try.”92 Under current policy in certain institutions and states, “some maverick” could also risk career loss and legal action.93 The solution is not to condemn iPS technology but rather to enact logical, universal policy with severe penalties to discourage potential misuse of iPS. In vitro fertilization technologies have not been forbidden because of their potential contribution to cloning efforts. Likewise, iPS technologies should not be rejected because of a theoretical risk of abuse.
The general consensus among hES and iPS researchers alike is that ES cells are needed for the time being.94 As noted by iPS researcher Konrad Hochedlinger, “...once we have a better understanding of what iPS cells can do and what they cannot do—if anything—it will be worthwhile to revisit the question of whether ES cells have become obsolete. At the moment, this is clearly not the case.” Shinya Yamanaka, one of the founders of iPS technology, has predicted “that iPS cells will eventually replace ES cells in most, if not all, applications in the future,” but he adds that “[e]ven thereafter... ES cells are still expected to have an important role as a control in both experiments and trials.”73 Because hES cells remain the standard of pluripotency, those who claim that iPS cells eliminate the need for hES research must understand that bioengineered cells have a long road of validation and quality control ahead of them to determine their relative risk to benefit profile.92 This will likely require comparison with the highest-quality hES cells available using current technology to reveal subtle functional differences between hES and iPS cells and refine their molecular identity. However, biomedical applications, such as patient-specific drug toxicity and discovery sciences to map disease-causing mutations and molecular pathologies, require relative comparison between iPS cells from healthy and diseased cohorts rather than indirect comparison with hES cells.
An ongoing debate, therefore, is whether we have sufficient hES cell lines to bridge the gap and help us move toward better techniques or whether it is necessary to continue deriving more lines via embryo destruction. In 2009, the availability of hES cell lines increased dramatically, more than doubling, compared with the previous 15 years; many more hES cell lines are under review by the National Institutes of Health.95,96 Although the courts may eventually reach a conclusion about what is permitted or prohibited legally,97,98 the larger ethical and social disagreement will remain. Thus, it would seem prudent practically, ethically, and socially for the scientific community to question the need to derive new hES cell lines both now and as iPS technology advances.
So what does the future look like for stem cell research? More pertinently, what is the ideal at which we are aiming? Is the goal to find better cures that are biologically accessible to everyone and, as much as possible, avoid ethical quandaries? This certainly seemed to be the goal of the NBAC, which first permitted hES research more than 10 years ago. The age-old challenge for science has been to avoid becoming rigid and inflexible. When the question is as truly important and fundamental as the nature of the human being, perhaps it is best not to plow continually ahead in the name of scientific progress. Otherwise, in rigidly adhering to a set course of action, we may be ignoring other considerations that provide a value and context to the process of science.8
As we have already addressed, a major goal of SCNT was to improve on hES research by providing genetically identical cells from any available person, allowing superior versatility compared with picking and choosing from the random leftovers available at the frozen embryo bank. Yet the derivation of stem cells from human cloning has not been successfully performed and remains ethically contentious because it is embryo-dependent (Figure).44,51 As Rudolf Jaenisch has stated, “One potential use of the nuclear cloning approach is the derivation of `customized' embryonic stem (ES) cells for patient-specific cell treatment, but technical and ethical considerations impede the therapeutic application of this technology.”6 In iPS cells, we have a technology that could move us two steps ahead by providing identically matched cells without using enucleated oocytes and embryos. This article has discussed a number of studies that suggest iPS cells have a potential role in spurring advances both in patient-specific therapies and in diagnostic tools and research models for normal development and human disease.59-61,69,70,73,75,82,92
Is iPS technology a viable route in which to direct our efforts over hES cells? Although practice may appear relatively unchanged, at least in the early interim phase, it seems practical and intuitive that we understand where the science is heading and advance our perspective. The discoverer of hES cells, James Thomson, seems to think so. Three weeks after the publication in 2007 by Yamanaka and colleagues deriving human iPS cells, Thomson's group came out with a report in which they also derived human iPS cells. In the conclusion, they state:
The human iPS cells described here meet the defining criteria that we originally proposed for human ES cells, with the notable exception that the iPS cells are not derived from embryos. Similar to human ES cells, human iPS cells should prove useful for studying the development and function of human tissues, for discovering and testing new drugs, and for transplantation medicine....Human ES cells remain controversial because their derivation involves the destruction of human preimplantation embryos and iPS cells remove this concern.54
In a later interview, Thomson remarked, “Only time will tell, but I know where I'm going....If you can't tell the difference between iPS cells and embryonic stem cells, then embryonic stem cells will turn out to be a historical anomaly.”92 We agree.
Footnotes
For editorial comment, see page 600
This work is supported by a grant from the National Institutes of Health (1DP2OD007015) and a generous gift from the Todd and Karen Wanek family.
Dr Mueller is a member of the Boston Scientific Patient Safety Advisory Board and Associate Editor of Journal Watch General Medicine.
This article is freely available on publication, because the authors have chosen the immediate access option.
REFERENCES
- 1. Rosenzweig A. Illuminating the potential of pluripotent stem cells. N Engl J Med. 2010;36(15):1471-1472 [DOI] [PubMed] [Google Scholar]
- 2. Nelson T, Behfar A, Terzic A. Stem cells: biologics for regeneration. Clin Pharmacol Ther. 2008;84(5):620-623 [DOI] [PubMed] [Google Scholar]
- 3. Surani MA, McLaren A. Stem cells: a new route to rejuvenation. Nature. 2006;443(7109):284-285 [DOI] [PubMed] [Google Scholar]
- 4. Klimanskaya I, Rosenthal N, Lanza R. Derive and conquer: sourcing and differentiating stem cells for therapeutic applications. Nat Rev Drug Discov. 2008;7(2):131-142 [DOI] [PubMed] [Google Scholar]
- 5. Nelson TJ, Behfar A, Terzic A. Strategies for therapeutic repair: the ``R(3)'' regenerative medicine paradigm. Clin Transl Sci. 2008;1(2):168-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Atala A. Advances in tissue and organ replacement. Curr Stem Cell Res Ther. 2008;3(1):21-31 [DOI] [PubMed] [Google Scholar]
- 7. Daley GQ, Scadden DT. Prospects for stem cell-based therapy. Cell. 2008;132(4):544-548 [DOI] [PubMed] [Google Scholar]
- 8. Korbling M, Estrov Z. Adult stem cells for tissue repair: a new therapeutic concept? N Engl J Med. 2003;349(6):570-582 [DOI] [PubMed] [Google Scholar]
- 9. Monroe KR, Miller RB, Tobis J, eds. Fundamentals of the Stem Cell Debate: The Scientific, Religious, Ethical, and Political Issues. Los Angeles, CA: University of California Press; 2007. [Google Scholar]
- 10. Beauchamp TL, Childress JF. Principles of Biomedical Ethics. 6th ed. New York, NY: Oxford University Press, Inc.; 2001. [Google Scholar]
- 11. President's Council on Bioethics. Human Dignity and Bioethics: Essays Commissioned by the President's Council on Bioethics. Washington, DC: US Independent Agencies and Commissions; 2008. [Google Scholar]
- 12. Woodward PA, ed. The Doctrine of Double Effect: Philosophers Debate a Controversial Moral Principle. Notre Dame, IN: University of Notre Dame Press; 2001. [Google Scholar]
- 13. Solo P, Pressberg G. The Promise and Politics of Stem Cell Research. Westport, CT: Praeger; 2007. [Google Scholar]
- 14. Lanza R, Gearhart J, Hogan B, et al., eds. Essentials of Stem Cell Biology. 2nd ed. San Diego, CA: Academic Press; 2009. [Google Scholar]
- 15. Silva J, Smith A. Capturing pluripotency. Cell. 2008;132(4):532-536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith KP, Luong MX, Stein GS. Pluripotency: toward a gold standard for human ES and iPS cells. J Cell Physiol. 2009;220(1):21-29 [DOI] [PubMed] [Google Scholar]
- 17. Liechty KW, MacKenzie TC, Shaaban AF, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6(11):1282-1286 [DOI] [PubMed] [Google Scholar]
- 18. Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132(4):631-644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645-660 [DOI] [PubMed] [Google Scholar]
- 20. Rossant J. Stem cells and early lineage development. Cell. 2008;132(4):527-531 [DOI] [PubMed] [Google Scholar]
- 21. Vrtovec KT, Vrtovec B. Commentary: is totipotency of a human cell a sufficient reason to exclude its patentability under the European law? Stem Cells. 2007;25(12):3026-3028 [DOI] [PubMed] [Google Scholar]
- 22. Yamanaka S. Elite and stochastic models for induced pluripotent stem cell generation. Nature. 2009;460(7251):49-52 [DOI] [PubMed] [Google Scholar]
- 23. Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145-1147 [DOI] [PubMed] [Google Scholar]
- 24. Zwaka TP. Stem cells: troublesome memories. Nature. 2010;467(7313):280-281 [DOI] [PubMed] [Google Scholar]
- 25. National Bioethics Advisory Commission Ethical Issues in Human Stem Cell Research. Vol 1 Rockville, MD: National Bioethics Advisory Commission; 1999. http://bioethics.georgetown.edu/nbac/stemcell.pdf Accessed May 2, 2011 [Google Scholar]
- 26. Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116(5):639-648 [DOI] [PubMed] [Google Scholar]
- 27. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341-347 [DOI] [PubMed] [Google Scholar]
- 28. Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair: current views. Stem Cells. 2007;25(11):2896-2902 [DOI] [PubMed] [Google Scholar]
- 29. Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701-705 [DOI] [PubMed] [Google Scholar]
- 30. Horwitz EM, Prockop DJ, Fitzpatrick LA, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5(3):309-313 [DOI] [PubMed] [Google Scholar]
- 31. Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739-2749 [DOI] [PubMed] [Google Scholar]
- 32. van de Ven C, Collins D, Bradley MB, Morris E, Cairo MS. The potential of umbilical cord blood multipotent stem cells for nonhematopoietic tissue and cell regeneration. Exp Hematol. 2007;35(12):1753-1765 [DOI] [PubMed] [Google Scholar]
- 33. McGuckin CP, Forraz N. Potential for access to embryonic-like cells from human umbilical cord blood. Cell Prolif. 2008;41(suppl 1):31-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De Coppi P, Bartsch G, Jr, Siddiqui MM, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25(1):100-106 [DOI] [PubMed] [Google Scholar]
- 35. Hollands P, McCauley C. Private cord blood banking: current use and clinical future. Stem Cell Rev. 2009;5(3):195-203 [DOI] [PubMed] [Google Scholar]
- 36. Solter D. From teratocarcinomas to embryonic stem cells and beyond: a history of embryonic stem cell research. Nat Rev Genet. 2006;7(4):319-327 [DOI] [PubMed] [Google Scholar]
- 37. Nelson TJ, Behfar A, Yamada S, Martinez-Fernandez A, Terzic A. Stem cell platforms for regenerative medicine. Clin Transl Sci. 2009;2(3):222-227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goldman S. Stem and progenitor cell-based therapy of the human central nervous system. Nat Biotechnol. 2005;23(7):862-871 [DOI] [PubMed] [Google Scholar]
- 39. Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443-452 [DOI] [PubMed] [Google Scholar]
- 40. Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453(7193):322-329 [DOI] [PubMed] [Google Scholar]
- 41. Geron initiates clinical trial of human embryonic stem cell-based therapy [news release]. Geron Web site http://www.geron.com/media/pressview.aspx?id=1235 Accessed May 2, 2011
- 42. Advanced Cell Technology Advanced Cell Technology receives FDA clearance for clinical trials using embryonic stem cells to treat age-related macular degeneration [press release]. http://www.advancedcell.com/news-and-media/press-releases/advanced-cell-technology-receives-fda-clearance-for-clinical-trials-using-embryonic-stem-cells-to-tre/ Accessed May 2, 2011
- 43. Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385(6619):810-813 [DOI] [PubMed] [Google Scholar]
- 44. Yamanaka S, Blau HM. Nuclear reprogramming to a pluripotent state by three approaches. Nature. 2010;465(7299):704-712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Beyhan Z, Iager AE, Cibelli JB. Interspecies nuclear transfer: implications for embryonic stem cell biology. Cell Stem Cell. 2007;1(5):502-512 [DOI] [PubMed] [Google Scholar]
- 46. Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132(4):567-582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meissner A, Jaenisch R. Generation of nuclear transfer-derived pluripotent ES cells from cloned Cdx2-deficient blastocysts. Nature. 2006;439(7073):212-215 [DOI] [PubMed] [Google Scholar]
- 48. Strumpf D, Mao CA, Yamanaka Y, et al. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development. 2005;132(9):2093-2102 [DOI] [PubMed] [Google Scholar]
- 49. Hipp J, Atala A. Sources of stem cells for regenerative medicine. Stem Cell Rev. 2008;4(1):3-11 [DOI] [PubMed] [Google Scholar]
- 50. Guenin LM. Wishful thinking will not obviate embryo use. Stem Cell Rev. 2005;1(4):309-315 [DOI] [PubMed] [Google Scholar]
- 51. Yang X, Smith SL, Tian XC, Lewin HA, Renard JP, Wakayama T. Nuclear reprogramming of cloned embryos and its implications for therapeutic cloning. Nat Genet. 2007;39(3):295-302 [DOI] [PubMed] [Google Scholar]
- 52. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663-676 [DOI] [PubMed] [Google Scholar]
- 53. Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861-872 [DOI] [PubMed] [Google Scholar]
- 54. Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917-1920 [DOI] [PubMed] [Google Scholar]
- 55. Park IH, Lerou PH, Zhao R, Huo H, Daley GQ. Generation of human-induced pluripotent stem cells. Nat Protoc. 2008;3(7):1180-1186 [DOI] [PubMed] [Google Scholar]
- 56. Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313-317 [DOI] [PubMed] [Google Scholar]
- 57. Zhao XY, Li W, Lv Z, et al. iPS cells produce viable mice through tetraploid complementation. Nature. 2009;461(7260):86-90 [DOI] [PubMed] [Google Scholar]
- 58. Hanley J, Rastegarlari G, Nathwani AC. An introduction to induced pluripotent stem cells. Br J Haematol. 2010;151(1):16-24 [DOI] [PubMed] [Google Scholar]
- 59. Park IH, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134(5):877-886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nelson TJ, Martinez-Fernandez A, Terzic A. Induced pluripotent stem cells: developmental biology to regenerative medicine. Nat Rev Cardiol. 2010;7(12):700-710 [DOI] [PubMed] [Google Scholar]
- 61. Wichterle H, Przedborski S. What can pluripotent stem cells teach us about neurodegenerative diseases? Nat Neurosci. 2010;13(7):800-804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318(5858):1920-1923 [DOI] [PubMed] [Google Scholar]
- 63. Wernig M, Zhao JP, Pruszak J, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci U S A. 2008;105(15):5856-5861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xu D, Alipio Z, Fink LM, et al. Phenotypic correction of murine hemophilia A using an iPS cell-based therapy. Proc Natl Acad Sci U S A. 2009;106(3):808-813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120(5):408-416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Martinez-Fernandez A, Nelson TJ, Yamada S, et al. iPS programmed without c-MYC yield proficient cardiogenesis for functional heart chimerism. Circ Res. 2009;105(7):648-656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hochedlinger K, Jaenisch R. Nuclear reprogramming and pluripotency. Nature. 2006;441(7097):1061-1067 [DOI] [PubMed] [Google Scholar]
- 68. Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibro blasts into a pluripotent ES-cell-like state. Nature. 2007;448(7151):318-324 [DOI] [PubMed] [Google Scholar]
- 69. Maherali N, Sridharan R, Xie W, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1(1):55-70 [DOI] [PubMed] [Google Scholar]
- 70. Nishikawa S, Goldstein RA, Nierras CR. The promise of human induced pluripotent stem cells for research and therapy. Nat Rev Mol Cell Biol. 2008;9(9):725-729 [DOI] [PubMed] [Google Scholar]
- 71. Kaiser J, Vogel G. Embryonic stem cells. Controversial ruling throws U.S. research into a tailspin. Science. 2010;329(5996):1132-1133 [DOI] [PubMed] [Google Scholar]
- 72. Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467(7313):285-290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Belmonte JC, Ellis J, Hochedlinger K, Yamanaka S. Induced pluripotent stem cells and reprogramming: seeing the science through the hype. Nat Rev Genet. 2009;10(12):878-883 [DOI] [PubMed] [Google Scholar]
- 74. Fong CY, Gauthaman K, Bongso A. Teratomas from pluripotent stem cells: a clinical hurdle. J Cell Biochem. 2010;111(4):769-781 [DOI] [PubMed] [Google Scholar]
- 75. Amabile G, Meissner A. Induced pluripotent stem cells: current progress and potential for regenerative medicine. Trends Mol Med. 2009;15(2):59-68 [DOI] [PubMed] [Google Scholar]
- 76. Warren L, Manos PD, Ahfeldt T, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7(5):618-630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yamanaka S. A fresh look at iPS cells. Cell. 2009;137(1):13-17 [DOI] [PubMed] [Google Scholar]
- 78. Boyd AS, Fairchild PJ. Approaches for immunological tolerance induction to stem cell-derived cell replacement therapies. Expert Rev Clin Immunol. 2010;6(3):435-448 [DOI] [PubMed] [Google Scholar]
- 79. Charron D, Suberbielle-Boissel C, Al-Daccak R. Immunogenicity and allogenicity: a challenge of stem cell therapy. J Cardiovasc Transl Res. 2009;2(1):130-138 [DOI] [PubMed] [Google Scholar]
- 80. Geron Corporation About GRNOPC1 [clinical program]. Geron Web site http://www.geron.com/GRNOPC1Trial/grnopc1-sec4.html Accessed May 2, 2011
- 81. National Institutes of Health (NIH) National Institutes of Health guidelines on human stem cell research. http://stemcells.nih.gov/policy/2009guidelines.htm Accessed May 2, 2011
- 82. Nelson TJ, Martinez-Fernandez A, Yamada S, Ikeda Y, Perez-Terzic C, Terzic A. Induced pluripotent stem cells: advances to applications. Stem Cells Cloning. 2010;3:29-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kallen B. Maternal morbidity and mortality in in-vitro fertilization. Best Pract Res Clin Obstet Gynaecol. 2008;22(3):549-558 [DOI] [PubMed] [Google Scholar]
- 84. Green RM. Benefiting from `evil': an incipient moral problem in human stem cell research. Bioethics. 2002;16(6):544-556 [DOI] [PubMed] [Google Scholar]
- 85. George RP, Gomez-Lobo A. The moral status of the human embryo. Perspect Biol Med. 2005;48(2):201-210 [DOI] [PubMed] [Google Scholar]
- 86. Jafari M, Elahi F, Ozyurt S, Wrigley T. Religious perspectives on embryonic stem cell research. In: Monroe KR, Miller RB, Tobis J, eds. Fundamentals of the Stem Cell Debate: The Scientific, Religious, Ethical, and Political Issues. Los Angeles, CA: University of California Press; 2007. [Google Scholar]
- 87. Pope John Paul., II Pope John Paul II addresses President Bush. American Catholic.Org Web site. http://www.americancatholic.org/news/stemcell/pope_to_bush.asp Accessed May 2, 2011
- 88. Meilaender G. Some protestant reflections. In: Holland S, Lebacqz K, Zoloth L, eds. The Human Embryonic Stem Cell Debate: Science, Ethics, and Public Policy. Cambridge, MA: The MIT Press; 2001. [Google Scholar]
- 89. Pope John Paul., IICongregation for the Doctrine of the Faith: Instruction Dignitas Personae on Certain Bioethical Questions [letter]. http://www.usccb.org/comm/Dignitaspersonae/Dignitas_Personae.pdf Published May 19, 1991 Accessed May 2, 2011
- 90. Nickel PJ. Ethical issues in human embryonic stem cell research. In: Monroe KR, Miller RB, Tobis J, eds. Fundamentals of the Stem Cell Debate: The Scientific, Religious, Ethical, and Political Issues. Los Angeles, CA: University of California Press; 2007. [Google Scholar]
- 91. Lehrman S. Undifferentiated ethics: why stem cells from adult skin are as morally fraught as embryonic stem cells: hailed as a potential alternative to embryonic stem cells, induced pluripotent stem cells (iPS cells) raise their own ethical dilemmas. http://www.scientificamerican.com/article.cfm?id=undifferentiatied-ethics Accessed May 2, 2011
- 92. Cyranoski D. Stem cells: 5 things to know before jumping on the iPS bandwagon. Nature. 2008;452(7186):406-408 [DOI] [PubMed] [Google Scholar]
- 93. National Conference of State Legislatures Human cloning laws. Updated January 2008. http://www.ncsl.org/default.aspx?tabid=14284 Accessed May 2, 2011 [Google Scholar]
- 94. Hyun I, Hochedlinger K, Jaenisch R, Yamanaka S. New advances in iPS cell research do not obviate the need for human embryonic stem cells. Cell Stem Cell. 2007;1(4):367-368 [DOI] [PubMed] [Google Scholar]
- 95. Borrell B. Stem-cell line given the nod. Nature. 2010;463(7280):411 [DOI] [PubMed] [Google Scholar]
- 96. Dolgin E. Embryonic stem cell lines given the go-ahead. Nat Med. 2010;16(1):4 [DOI] [PubMed] [Google Scholar]
- 97. Kaiser J. Court battle: what's next for stem cell research? Science. 2010;330(6001):163 [DOI] [PubMed] [Google Scholar]
- 98. Annas GJ. Resurrection of a stem-cell funding barrier: Dickey-Wicker in court. N Engl J Med. 2010;363(18):1687-1689 [DOI] [PubMed] [Google Scholar]