Abstract
Renal artery stenosis (RAS) is characterized by a heterogeneous group of pathophysiologic entities, of which fibromuscular dysplasia and atherosclerotic RAS (ARAS) are the most common. Whether and which patients should undergo revascularization for ARAS is controversial. The general consensus is that all patients with ARAS should receive intensive medical treatment. The latest randomized clinical trials have increased confusion regarding recommendations for revascularization for ARAS. Although revascularization is not indicated in all patients with ARAS, experts agree that it should be considered in some patients, especially those with unstable angina, unexplained pulmonary edema, and hemodynamically significant ARAS with either worsening renal function or with difficult to control hypertension. A search of the literature was performed using PubMed and entering the search terms renal artery stenosis, atherosclerotic renal artery stenosis, and renal artery stenosis AND hypertension to retrieve the most recent publications on diagnosis and treatment of ARAS. In this review, we analyze the pathways related to hypertension in ARAS, the optimal invasive and noninvasive modalities for evaluating the renal arteries, and the available therapies for ARAS and assess future tools and algorithms that may prove useful in evaluating patients for renal revascularization therapy.
AHA = American Heart Association; ARAS = atherosclerotic renal artery stenosis; CORAL = Cardiovascular Outcomes in Renal Atherosclerotic Lesions; CTA = computed tomographic angiography; FMD = fibromuscular dysplasia; MRA = magnetic resonance angiography; PTRA = percutaneous transluminal renal angioplasty; RAAS = renin-angiotensin-aldosterone system; RAS = renal artery stenosis; RRI = renal resistive index
Renal artery stenosis (RAS), narrowing of the renal arteries, is caused by a heterogeneous group of conditions, including atherosclerosis, fibromuscular dysplasia (FMD), vasculitis, neurofibromatosis, congenital bands, and extrinsic compression, and radiation.1 Atherosclerosis accounts for approximately 90% of the lesions that obstruct blood flow to the renal arteries. Atherosclerotic renal artery stenosis (ARAS) typically involves the ostium and/or proximal one-third of the renal artery and often the adjacent aorta.2 However, segmental and diffuse intrarenal atherosclerosis may also be observed, especially in advanced cases.3
We reviewed the literature using PubMed to search for relevant recent publications with the terms renal artery stenosis, atherosclerotic renal artery stenosis, and renal artery stenosis AND hypertension. This review highlights salient points of the pathophysiology, diagnosis, and treatment of ARAS.
The prevalence of ARAS increases with advancing age and with the presence of traditional cardiovascular risk factors. Among patients with hypertension, ARAS is observed in only 1% to 6%,4-6 whereas the incidence of ARAS is more than 30% in patients undergoing cardiac catheterization7,8 and more than 50% in elderly patients with known atherosclerotic disease.9,10 In a study of 170 patients with ARAS who were followed up with serial duplex scans, the cumulative incidence of disease progression was 51% 5 years after diagnosis.11 In a pooled review of 5 trials using serial arteriography, 49% of all renal arteries examined demonstrated progression of stenosis during follow-up ranging from 6 to 180 months.12
Atherosclerotic renal artery stenosis results in a progressive loss of renal mass and function over time. In a subgroup of patients with renovascular hypertension and 60% obstruction, renal atrophy occurred in 21%.13,14 Historical data suggest that up to 27% of patients with ARAS will develop chronic renal failure within 6 years.15 A prospective angiographic study revealed that ARAS was the cause of end-stage renal disease in 14% of patients in whom dialysis was newly initiated7; thus, early detection and appropriate treatment of ARAS could have important economic consequences.
The presence of ARAS is known to predict adverse coronary events. In the Cardiovascular Health Study, patients diagnosed as having ARAS had a higher incidence of hospitalization for angina, myocardial infarction, and coronary revascularization.16 In a cohort of patients with ARAS detected at the time of coronary angiography, the 4-year survival rate was 65% for those with vs 86% for those without ARAS.17
PATHOPHYSIOLOGY
The pathophysiology of hypertension in patients with RAS due to FMD was well-described in the seminal work on animal models of hypertension by Goldblatt18 in the 1930s. This model describes renin-dependent hypertension in patients with FMD, but it does not adequately describe the etiology of hypertension in patients with
Article Highlights
Renal artery stenosis (RAS) is caused by a heterogeneous group of conditions that lead to narrowing of the renal arteries; ARAS produces 90% of the lesions that obstruct blood flow
The mechanism of hypertension and cardiac morbidity in patients with ARAS is complex and often is not strictly renin-dependent; the interplay between the direct vasculotoxic effects of renin, the proinflammatory and neurohormonal effects of circulating angiotensin II, and the endocrinologic effects of aldosterone leads to an increase in total blood volume
Ultrasonography is widely accepted as the first-line diagnostic imaging test because of its availability and cost
Invasive renal angiography can be useful in evaluating ARAS and can be used in combination with adjunctive invasive tools, such as fractional flow reserve to measure translesional pressure gradients
All patients with ARAS require intensive antihypertensive agents and lipid-lowering agents; they should stop smoking, and antiplatelet therapy should be considered
Renal revascularization therapy is controversial; the only class I indication for renal revascularization under current guidelines is for hemodynamically significant ARAS in the setting of recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema
Patients should undergo an evaluation of renal ischemia and of baseline renal function before undergoing renal revascularization therapy
ARAS, in whom the mechanisms of hypertension are more complex.
Well-established animal studies have clearly shown that decreased renal artery perfusion leads to a cascade of events, starting with the production of renin.1,19,20 Renin promotes conversion of angiotensinogen to angiotensin I, which is converted to angiotensin II by angiotensin I converting enzyme, which also inactivates kinins that promote hypotension.21 The largest store of angiotensin I converting enzyme is found in the pulmonary vasculature, where it plays an important role in the regulation of systemic blood pressure.22 In addition to causing hypertension by being directly vasoconstrictive, angiotensin II promotes hypertension by increasing total blood volume through its effect on aldosterone and by potentiating the vasoconstrictor response to circulating norepinephrine.23
Goldblatt's work has been expounded on to suggest 3 phases of renovascular hypertension as demonstrated in animal models. In stage I (acute occlusion) and stage II (occlusion for days/weeks), the blood pressure and plasma renin/angiotensin II levels are elevated, and elimination of the obstruction leads to normalization of both blood pressure and plasma renin/angiotensin II levels. In stage III, the occlusion is prolonged for months, plasma renin/angiotensin levels are no longer elevated, and elimination of the obstruction does not lead to normalization of blood pressure.24 Although these stages were described in animal models, stage III may reflect hypertension seen in patients with ARAS who do not appear to have strict renin-dependence. Patients with ARAS and low renin/angiotensin II levels may have improvement in hypertension after renal revascularization, but the results are unreliable. In summary, activation of the sympathetic and central nervous systems, increasing total blood volume via aldosterone, and the direct pressor effects of angiotensin II in the setting of ARAS are thought to contribute to hypertension.25,26
Several studies have suggested that ARAS results in cardiac morbidity that is disproportionate to the degree of hypertension.15,27,28 Multiple pathways account for this because angiotensin II has been associated with a range of proinflammatory and toxic cardiovascular effects, including myocardial fibrosis,29 arterial medial hypertrophy, smooth muscle cell proliferation, endothelial cell dysfunction, and plaque rupture.30,31 Renin has also been associated with vasculotoxic and nephrotoxic effects.32 Oxidative stress has been implicated in the ischemic and hypertensive parenchymal renal injury related to ARAS. 33,34
CLINICAL EVALUATION AND SCREENING
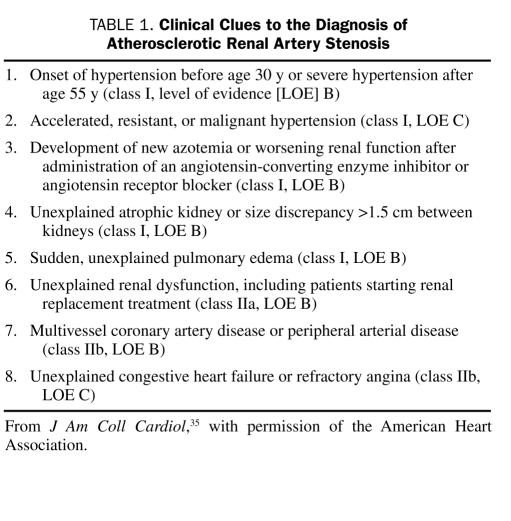
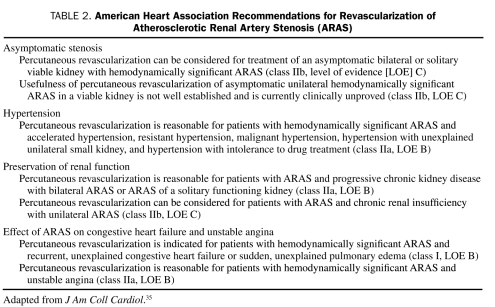
Clinical clues to the presence of ARAS are listed in Table 1.35 On the basis of the American Heart Association (AHA) guidelines, screening and revascularization should be considered in patients who present in the setting of the clinical scenarios outlined in Table 2.35,36 The Joint National Committee stated that more extensive testing in patients with identifiable causes of ARAS is typically not necessary unless blood pressure control is not achieved while the patient is receiving maximal antihypertensive therapy.37
TABLE 1.
Clinical Clues to the Diagnosis of Atherosclerotic Renal Artery Stenosis
TABLE 2.
American Heart Association Recommendations for Revascularization of Atherosclerotic Renal Artery Stenosis (ARAS)
DIAGNOSIS
Magnetic resonance angiography (MRA), helical computed tomographic angiography (CTA), Doppler ultrasonography, renal scintigraphy (ie, captopril scan), invasive angiography, peripheral renin levels, and renal vein renin sampling have all been used as screening tests to detect ARAS. Renal vein renin sampling, peripheral renin levels, and renal scintigraphy are not generally recommended for ARAS screening because of their low sensitivity and low specifity.38-40
For an imaging study to be considered optimal, the following 4 objectives must be met: (1) ARAS must be detected and characterized on the basis of anatomic and hemodynamic severity; (2) anatomic consequences of ARAS on the artery itself and on the kidney must be assessed (eg, severe ARAS can result in poststenotic dilatation of the artery, which can be detected by CTA and MRA, and also in shrinkage of the renal parenchyma, with the kidney being <8 cm); (3) functional and cellular consequences of ARAS on the kidney must be evaluated (eg, functional data can be obtained via the abnormal intrarenal transit of gadolinium during magnetic resonance imaging with use of captopril, future studies are assessing the ability of diffusion-weighted magnetic resonance imaging to determine the cellular viability of renal parenchyma tissue in patients with chronic kidney disease; and (4) criteria associated with renal impairment related to renovascular disease must be identified).41
Ultrasonography
Ultrasonography is widely available, safe, and inexpensive and consequently is typically the first imaging study used to detect ARAS. However, results are operator dependent, with accuracy ranging from 60% to 90%; the entire length of the renal artery or an accessory renal artery can be overlooked, and thus the stenotic lesion will be missed.42
Information on size of the kidneys, renal functional reserve, and renal resistive index (RRI [defined as peak systolic velocity – end-diastolic velocity/peak systolic velocity]) can be obtained with ultrasonography.43 A high renal artery end-diastolic velocity (>90 cm/s) and low RRI (<75-80) indicate no microvascular disease or increased resistance.39,44
Spectral broadening and increased velocity on ultrasonography are markers of hemodynamically significant stenoses. For example, a renoaortic velocity ratio (defined as the renal artery peak systolic velocity/aortic peak systolic velocity) greater than 3.5 has been correlated to 60% stenosis,45 whereas a renal artery peak systolic velocity greater than 150 cm/s correlates to 50% stenosis, and a velocity greater than 180 cm/s correlates to 60% stenosis.45-48 A literature review found that the sensitivity and specificity of ultrasonography were 85% and 92%, respectively, in detecting hemodynamically significant ARAS.49
Severe stenoses can produce tardus-parvus spectral changes on Doppler ultrasonography, revealed as a slowed systolic acceleration with a decreased resistive index.50,51 Quantitative criteria proposed for the diagnosis of distal stenoses include blunting of early systolic peak acceleration (<3 m/s2), an acceleration index greater than 4 m/s2, increase in time to systolic peak (>0.07 s), or greater than 5% difference in RRI between kidneys. However, because of the difficulty in interpretating these complex waveforms, these criteria are seldom used.52-54
Computed Tomographic Angiography
The possibility of 3-dimensional reconstructions has made CTA an important tool in the diagnosis of ARAS. Because CTA involves use of ionizing radiation and iodinated contrast medium, it is contraindicated in patients with contrast allergy. Patients with impaired renal function can develop contrast-induced nephropathy if iodinated contrast is used, but generous fluid hydration before contrast administration can effectively prevent this complication. For detection of ARAS, the sensitivity of CTA is 94%; the specificity varies between 60% and 90%.55,56
Compared to MRA, CTA can detect small accessory renal arteries because of its high spatial resolution. It is also preferred for patients who have implanted devices, for patients with limited breath-hold capacity (requiring shorter acquisition times), and for patients with claustrophobia. However, CTA has less specificity than MRA for detecting hemodynamically significant ARAS; it cannot be used safely in patients with borderline renal dysfunction because of the necessity of iodinated contrast agents; images obtained with CTA are difficult to interpret in heavily calcified arteries, and CTA requires use of ionizing radiation.57
Magnetic Resonance Angiography
Magnetic resonance angiography has a reported sensitivity and specificity of 90% to 100%55,56 and does not require use of iodinated contrast or radiation. Gadolinium-based contrast medium should be avoided in patients with moderate to end-stage renal failure because of the risk of nephrogenic systemic fibrosis. Additionally, MRA should not be used in patients with certain implanted devices (ie, pacemakers, defibrillators, cochlear implants, and spinal cord stimulators) or in claustrophobic patients. Unlike CTA, MRA has no calcification artifact, neither iodinated contrast medium nor radiation is used, and contrast reaction rates are lower.1
Angiography
Invasive renal arteriography is helpful in evaluating ARAS. In addition to assessing the severity of ARAS, angiography can detect intrarenal vascular abnormalities and anatomic abnormalities of the kidneys, renal arteries, and aorta. Digital subtraction angiography improves contrast resolution and may decrease the volume of contrast needed to as little as 15 mL. However, because renal angiography is invasive, there are risks associated with arterial puncture and manipulation of the catheter/wire, which can result in arterial trauma, spasm, or thromboembolic phenomenon.58 In patients with renal impairment or contrast allergy, carbon dioxide can be used as a nonnephrotoxic contrast agent.
The early work by White et al59 established that there is substantial intra- and interobserver variability in the visual estimation of coronary stenoses, which likely also applies to the visual estimation of ARAS. Therefore, relying solely on angiography to visually estimate the severity of ARAS is suboptimal, and adjunctive tools should be used to determine whether renal ischemia is present.
Translesional pressure gradients can be measured across areas of stenosis to determine hemodynamic significance (if there is doubt) before performing therapeutic procedures such as percutaneous transluminal renal angioplasty (PTRA) or stenting. In a small case series, Mangiacapra et al60 measured translesional pressure gradients using papaverine and dopamine to induce renal hyperemia in 53 consecutive patients before PTRA. They found that patients with the most substantial improvement in hypertension were those with a translesional gradient greater than 20 mm Hg (corresponding to a distal-proximal pressure ratio of 0.79 as the optimal cutoff). De Bruyne et al61 demonstrated that stenoses with a distal to proximal renal artery pressure decrease greater than 10% were associated with increased renin production, suggesting that measurement of translesional pressure gradients might help identify hemodynamically significant ARAS.
TREATMENT
Medical Therapy
There is widespread agreement that all patients with ARAS require intensive medical therapy. Use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers to inhibit the sympathetic and renin-angiotensin systems, respectively, is recommended for controlling hypertension and for reducing clinical events in those with known cardiovascular disease. A decline in renal function after initiation of an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker is often associated with bilateral RAS but is neither a sensitive nor a specific finding.62
Aggressive use of statins, optimal glycemic control, and smoking cessation counseling are of paramount importance. No randomized controlled study has analyzed the effects of different medical regimens on the treatment of hypertension associated with ARAS because such patients often have refractory hypertension and require multiple antihypertensive medications. Medications that block the renin-angiotensin-aldosterone system (RAAS) are frequently used to treat hypertension in patients with ARAS, but their use can lead to acute renal failure, particularly in patients with severe bilateral RAS, high-grade unilateral stenosis in the presence of a solitary kidney or an atrophic contralateral kidney, or advanced chronic kidney disease. In such patients, renal failure is related to a decrease in renal perfusion pressure caused by the RAAS inhibitor or through the intrarenal effects of these medications on chronically diseased kidneys. Renal function should be monitored carefully in these patients after initiation of a RAAS inhibitor because prompt discontinuation of the offending agent can frequently reverse acute impairment in renal function.63
In patients with ARAS and advanced renal disease (ie, chronic renal failure, proteinuria [>1 g/d]), diffuse intrarenal vascular disease, and renal atrophy), medical therapy is preferred to revascularization.64 Some argue that patients with a high RRI (>80) benefit more from medical therapy than revascularization; however, using the RRI as a stand-alone indicator of medical therapy is controversial, and expert consensus varies.
Despite the importance of medical therapy, it is wellknown that such medications have an array of adverse effects that often affect quality of life and limit adherence. Adherence to treatment of hypertension per published guidelines is poor, and use of 50% to 70% of medications is discontinued or changed within 6 months.
Renal Artery Revascularization
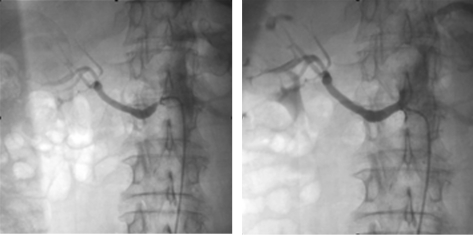
Whether patients with ARAS and hypertension should undergo therapeutic revascularization is less clear and far more controversial. Even patients with severe ostial stenosis of the renal artery (Figure, left) who undergo successful percutaneous revascularization (Figure, right) do not always obtain clinical benefit. Notably, the ACC/AHA definition of hemodynamically significant RAS is as follows: (1) stenosis of 50% to 70% diameter by visual estimation with a peak translesional gradient of at least 20 mm Hg or a mean gradient of at least 10 mm Hg (measured with a 5F or smaller catheter or pressure wire); (2) angiographic stenosis of at least 70% diameter; or (3) stenosis greater than 70% diameter by intravascular ultrasound measurement.65 However, current ACC/AHA guidelines do not incorporate these measures and recommend revascularization of ARAS only when it is complicated by certain medical comorbidities (Table 2).
FIGURE.
Renal angiograms. Left, Severe ostial stenosis of the right renal artery. Right, After percutaneous transluminal renal angioplasty and stent implantation in the right renal artery.
When renal artery revascularization is being considered, care must be taken to determine the severity of underlying nephropathy. Patients with advanced nephropathy receive less benefit from renal revascularization, even in the presence of documented renal ischemia. The most predictive measures of advanced nephropathy are proteinuria (>1 g/d), renal length less than 10 cm, RRI greater than 0.8, and renal biopsy confirming pathologic changes consistent with advanced nephropathy; the serum creatinine level is a less reliable predictor of nephropathy.35
Percutaneous Transluminal Renal Angioplasty
The largest randomized trial that compared drug treatment and PTRA was the Dutch Renal Artery Stenosis Intervention Cooperative (DRASTIC) study.66 In that study, 106 patients were randomly assigned to PTRA or medical therapy. The study design was such that patients in the drug treatment group whose condition was refractory to medical therapy were allowed to undergo balloon angioplasty if their blood pressure control was inadequate. Major limitations of that study included enrollment of patients with insignificant ARAS, a 44% crossover from medical therapy to PTRA, and low use of stents (20%). Despite the authors' assertion that PTRA in addition to drug therapy provided “little benefit,” patients in the PTRA group were less likely to have deterioration of their blood pressure control or renal artery occlusion during 12 months of follow-up.
Renal Artery Stenting
Results from observational studies have demonstrated that renal stenting is safe and effective in reducing blood pressure.67,68 The problem of elastic recoil is alleviated by using stents, which provide mechanical scaffolding. In a meta-analysis of 1322 patients, stent placement had a significantly higher technical success rate and lower restenosis rate than did PTRA (98% vs 77% and 17% vs 26%, respectively) and higher cure rates for hypertension.69
A randomized trial demonstrated the superiority of renal stenting vs PTRA for immediate procedural success (88% vs 57%, respectively) and lower restenosis rates (14% vs 48%, respectively).70 The limitation of that study appears to be the complication rates, although no significant differences in complications were noted between either study arm. The authors identified bleeding as a complication in 19% of patients in both arms (although the definition of bleeding was unclear) and cholesterol embolism as a complication in 10% of both arms. Other studies have demonstrated improvement or stabilization of renal function after unilateral or bilateral renal stenting in patients with ARAS and progressive renal insufficiency.71,72 In patients with ARAS and hypertension (blood pressure >140/90 mm Hg) despite treatment with at least 2 antihypertensive medications, renal stenting resulted in systolic blood pressure reduction of 20 mm Hg and use of 1 less antihypertensive medication.73
Two important randomized trials of renal artery stenting vs medical therapy have recently been reported. In the Stent Placement in Patients with Atherosclerotic Renal Artery Stenosis and Impaired Renal Function (STAR) trial,74 140 patients with a creatinine clearance of less than 80 mL/min/m2, RAS greater than 50%, and well-controlled hypertension were randomized to either renal artery stenting plus medical therapy or medical therapy alone. The primary end point was a 20% or greater decrease in creatinine clearance, and secondary end points included safety and cardiovascular morbidity and mortality. The authors concluded that stent placement with medical treatment did not clearly affect progression of impaired renal function but led to a few serious procedure-related complications.75 However, this study had a number of important limitations. Foremost, as noted by the editors of Annals of Internal Medicine, the study was “underpowered to provide a definitive estimate of efficacy.”75 Several patients were incorrectly identified as having ARAS greater than 50% by noninvasive imaging and did not require stenting, yet they were analyzed by intention to treat in the stent group. Also, 33% of the study participants had only mild RAS (50%-70%), and more than half of the patients had unilateral disease. Because the primary end point was a change in renal function, it is not surprising that patients with unilateral disease and stenosis of less than 70% had no benefit from revascularization.
In the Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) trial, 806 patients with ARAS were randomized to undergo stent-based renal revascularization plus medical therapy or medical therapy alone. The primary outcome was renal function, as measured by the reciprocal of the serum creatinine level, and secondary outcomes were blood pressure, time to renal and major cardiovascular events, and mortality. After a median follow-up of 34 months, the authors found “substantial risks but no evidence of a worthwhile clinical benefit from revascularization in patients with ARAS.”74 This study had a number of limitations that might affect interpretation of the results. By limiting study participation to patients in whom the treating physicians had to be undecided about the appropriate treatment strategy (ie, patients were excluded if physicians were sure stenting was necessary), selection bias was introduced into the trial design. Of course, this selection bias is also reflective of clinical practice because many physicians will often refer patients for renal revascularization if they are unsure of the appropriate course of management. In addition, 25% of patients had normal renal function, a significant number had unilateral disease, and 41% had a stenosis of less than 70%. A subgroup analysis of the cohort with bilateral disease also failed to show clinical benefit, downplaying the notion that the negative results were largely affected by the high enrollment of patients with unilateral disease who would benefit less from renal revascularization. Importantly, there was no core laboratory to adjudicate the imaging studies and ensure their accurate and unbiased interpretation. During the 7 years of recruitment, more than half of the centers enrolled fewer than 1 patient per year, perhaps explaining the high adverse event rate. In summary, as the authors of the trial have stated, the results of ASTRAL appear to indicate that renal stenting does not provide a significant net clinical benefit for patients with RAS and may inflict harm because 2 deaths and 3 amputations were attributed to complications of the procedure.
Importantly, in both the ASTRAL and the STAR trials, creatinine clearance equations used to estimate glomerular filtration rate have not been validated in patients with ARAS. Patients with advanced nephropathy (who would be less likely to benefit from revascularization) were included in both trials, and neither trial used an adjunctive measurement of renal ischemia, such as translesional pressure gradients, which adds variability to the assessment of lesion severity.
The CORAL (Cardiovascular Outcomes in Renal Atherosclerotic Lesions) study is a large randomized, prospective multicenter trial funded by the National Institutes of Health that is comparing the effects of angioplasty with stenting and optimal medical therapy to medical therapy alone on a composite of adverse cardiovascular and renal events.76 Enrollment ended on January 31, 2010, and study data will not be available for several years. The CORAL investigators realized that previous trials of ARAS have lacked rigorous medical treatment that could prevent the progression of cardiovascular and renal disease pervasive in this population. Thus, this trial focuses on strict antihypertensive therapy, smoking cessation, aggressive treatment of dyslipidemia and diabetes, administration of an antiplatelet agent, and complications of renal insufficiency. The CORAL treatment algorithm is based on current evidence-based practice guidelines even though the effect of these medical interventions on outcomes has not yet been well defined in this population.
In the CORAL trial, randomization to the revascularization or medical treatment arm was performed at the time of the invasive assessment. Patients in the stent therapy arm underwent implantation of a Genesis stent (Cordis, Warren, NJ). The study was designed to have more than 80% power to detect a threshold effect size of 25% with a sample of 1080 randomized patients. This is the pivotal study of stent therapy for ARAS, on par with large randomized clinical trials of carotid artery or coronary artery bypass surgery (NASCET [North American Symptomatic Carotid Endarterectomy Trial] and CASS [Coronary Artery Surgery Study], respectively).
We anticipate that the CORAL study will greatly clarify the controversy regarding renal artery stenting. However, the strict crossover requirements may have led some centers to avoid enrolling patients in whom the indication to treat was weak.
Additional Interventional Procedures
Although brachytherapy and cutting balloon atherotomy have been used successfully for renal artery in-stent restenosis,77,78 long-term outcomes are unknown. Use of coronary drug-eluting stents has also been described for small renal arteries,79 but well-designed studies to determine the adequate dosing of the eluting drug for this vessel are lacking. The largest drug-eluting stent is only 3.5 mm in diameter, an inadequate size for stenting of a renal artery (with a normal diameter of 4-7 mm). Distal embolic protection devices have also been used to capture atherosclerotic debris and prevent it from distal embolization during renal stenting,80 which may help preserve renal function.
Surgery
Surgical revascularization is effective for treating ARAS; however, morbidity and mortality are higher with surgery vs stenting.59 In one of the few studies that compared surgical to percutaneous revascularization for ostial ARAS, Balzer et al81 found no significant difference in long-term morbidity or mortality, a significant improvement in durability of the result in the surgical arm, and no significant difference in blood pressure reduction (although blood pressure improved significantly from baseline in both study arms). These results suggest that surgical revascularization may be at least equivalent to PTRA for ostial ARAS.
CONCLUSION
The general consensus is that all patients with ARAS should undergo aggressive medical treatment. The pathophysiology of hypertension in ARAS is complex, and multiple pharmacological agents may be needed for effective control. Early recognition and effective medical treatment of ARAS may prevent onset of future cardiovascular events. Revascularization for ARAS remains controversial; the latest randomized clinical trials have not only failed to clarify this conundrum but also have resulted in further confusion regarding treatment recommendations. Clearly, revascularization is not indicated in all patients with ARAS but should be considered in some patients, especially those with unstable angina, unexplained pulmonary edema, and hemodynamically significant ARAS with worsening renal function or difficult to control hypertension (while taking at least 3 medications, 1 of which is a diuretic). These recommendations should be taken into account if a patient has bilateral disease or has unilateral disease and only 1 kidney. Although the results of trials such as CORAL are eagerly awaited to shed further light on this controversy, certain sensible steps can be taken to ensure that renal artery stenting is used in the correct patient population. Revascularization therapy has an important role in treatment of ARAS, but available data suggest that it should be limited to patients who have renal ischemia with viable underlying renal function because they will ultimately receive the greatest clinical benefit.
REFERENCES
- 1. Dubel GJ, Murphy TP. The role of percutaneous revascularization for renal artery stenosis. Vasc Med. 2008;13(2):141-156 [DOI] [PubMed] [Google Scholar]
- 2. Kaatee R, Beek FJ, Verschuyl EJ, et al. Atherosclerotic renal artery stenosis: ostial or truncal? Radiology. 1996;199(3):637-640 [DOI] [PubMed] [Google Scholar]
- 3. Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344(6):431-442 [DOI] [PubMed] [Google Scholar]
- 4. Simon N, Franklin SS, Bleifer KH, Maxwell MH. Clinical characteristics of renovascular hypertension. JAMA. 1972;220(9):1209-1218 [PubMed] [Google Scholar]
- 5. Ram CV. Renovascular hypertension. Curr Opin Nephrol Hypertens. 1997;6(6):575-579 [DOI] [PubMed] [Google Scholar]
- 6. Vokonas PS, Kannel WB, Cupples LA. Epidemiology and risk of hypertension in the elderly: the Framingham Study. J Hypertens Suppl. 1988;6(1):S3-S9 [PubMed] [Google Scholar]
- 7. Harding MB, Smith LR, Himmelstein SI, et al. Renal artery stenosis: prevalence and associated risk factors in patients undergoing routine cardiac catheterization. J Am Soc Nephrol. 1992;2(11):1608-1616 [DOI] [PubMed] [Google Scholar]
- 8. Buller CE, Nogareda JG, Ramanathan K, et al. The profile of cardiac patients with renal artery stenosis. J Am Coll Cardiol. 2004;43(9):1606-1613 [DOI] [PubMed] [Google Scholar]
- 9. Miralles M, Corominas A, Cotillas J, Castro F, Clara A, Vidal-Barraquer F. Screening for carotid and renal artery stenoses in patients with aortoiliac disease. Ann Vasc Surg. 1998;12(1):17-22 [DOI] [PubMed] [Google Scholar]
- 10. Swartbol P, Parsson H, Thorvinger B, Norgren L. To what extent does peripheral vascular disease and hypertension predict renal artery stenosis? Int Angiol. 1994;13(2):109-114 [PubMed] [Google Scholar]
- 11. Caps MT, Perissinotto C, Zierler RE, et al. Prospective study of atherosclerotic disease progression in the renal artery. Circulation. 1998;98(25):2866-2872 [DOI] [PubMed] [Google Scholar]
- 12. Greco BA, Breyer JA. The natural history of renal artery stenosis: who should be evaluated for suspected ischemic nephropathy? Semin Nephrol. 1996;16(1):2-11 [PubMed] [Google Scholar]
- 13. Dean RH, Kieffer RW, Smith BM, et al. Renovascular hypertension: anatomic and renal function changes during drug therapy. Arch Surg. 1981;116(11):1408-1415 [DOI] [PubMed] [Google Scholar]
- 14. Tollefson DF, Ernst CB. Natural history of atherosclerotic renal artery stenosis associated with aortic disease. J Vasc Surg. 1991;14(3):327-331 [PubMed] [Google Scholar]
- 15. Wollenweber J, Sheps SG, Davis GD. Clinical course of atherosclerotic renovascular disease. Am J Cardiol. 1968;21(1):60-71 [DOI] [PubMed] [Google Scholar]
- 16. Edwards MS, Craven TE, Burke GL, Dean RH, Hansen KJ. Renovascular disease and the risk of adverse coronary events in the elderly: a prospective, population-based study. Arch Intern Med. 2005;165(2):207-213 [DOI] [PubMed] [Google Scholar]
- 17. Conlon PJ, Athirakul K, Kovalik E, et al. Survival in renal vascular disease. J Am Soc Nephrol. 1998;9(2):252-256 [DOI] [PubMed] [Google Scholar]
- 18. Goldblatt H. Experimental hypertension induced by renal ischemia: Harvey Lecture, May 19, 1938. Bull N Y Acad Med. 1938;14(9):523-553 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1911248/pdf/bullnyacadmed00606-0004.pdf Accessed May 3, 2011 [PMC free article] [PubMed] [Google Scholar]
- 19. Gomez RA, Sequeira Lopez ML. Who and where is the renal baroreceptor? The connexin hypothesis. Kidney Int. 2009;75(5):460-462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Skinner SL, McCubbin JW, Page IH. Control of renin secretion. Circ Res. 1964;15:64-76 [DOI] [PubMed] [Google Scholar]
- 21. Erdos EG, Angiotensin I converting enzyme. Circ Res. 1975;36(2):247-255 [DOI] [PubMed] [Google Scholar]
- 22. Aiken JW, Vane JR. Renin–angiotensin system: inhibition of converting enzyme in isolated tissues. Nature. 1970;228(5266):30-34 [DOI] [PubMed] [Google Scholar]
- 23. Reid IA. Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am J Physiol. 1992;262:E763-E778 [DOI] [PubMed] [Google Scholar]
- 24. Glorioso N, Laragh JH, Rappelli A, eds. Renovascular Hypertension: Pathophysiology, Diagnosis, and Treatment. New York, NY: Raven Press Books, Ltd; 1987. [Google Scholar]
- 25. Johansson M, Elam M, Rundqvist B, et al. Increased sympathetic nerve activity in renovascular hypertension. Circulation. 1999;99(19):2537-2542 [DOI] [PubMed] [Google Scholar]
- 26. Mathias CJ, Kooner JS, Peart S. Neurogenic components of hypertension in human renal artery stenosis. Clin Exp Hypertens A. 1987;9(suppl 1):293-306 [DOI] [PubMed] [Google Scholar]
- 27. Conlon PJ, Little MA, Pieper K, Mark DB. Severity of renal vascular disease predicts mortality in patients undergoing coronary angiography. Kidney Int. 2001;60(4):1490-1497 [DOI] [PubMed] [Google Scholar]
- 28. Isles C, Main J, O'Connell J, et al. Survival associated with renovascular disease in Glasgow and Newcastle: a collaborative study. Scott Med J. 1990;35(3):70-73 [DOI] [PubMed] [Google Scholar]
- 29. Vasan RS, Evans JC, Benjamin EJ, et al. Relations of serum aldosterone to cardiac structure: gender-related differences in the Framingham Heart Study. Hypertension. 2004;43(5):957-962 [DOI] [PubMed] [Google Scholar]
- 30. Meyrier A. Vascular mechanisms of renal fibrosis: vasculonephropathies and arterial hypertension [article in French]. Bull Acad Natl Med. 1999;183(1):33-45 [PubMed] [Google Scholar]
- 31. Nakashima H, Suzuki H, Ohtsu H, et al. Angiotensin II regulates vascular and endothelial dysfunction: recent topics of angiotensin II type-1 receptor signaling in the vasculature. Curr Vasc Pharmacol. 2006;4(1):67-78 [DOI] [PubMed] [Google Scholar]
- 32. Volpe M, Camargo MJ, Mueller FB, et al. Relation of plasma renin to end organ damage and to protection of K+ feeding in strokeprone hypertensive rats. Hypertension. 1990;15(3):318-326 [DOI] [PubMed] [Google Scholar]
- 33. Lerman LO, Nath KA, Rodriguez-Porcel M, et al. Increased oxidative stress in experimental renovascular hypertension. Hypertension. 2001;37(2, pt 2):541-546 [DOI] [PubMed] [Google Scholar]
- 34. Parildar M, Parildar Z, Oran I, Kabaroglu C, Memis A, Bayindir O. Nitric oxide and oxidative stress in atherosclerotic renovascular hypertension: effect of endovascular treatment. J Vasc Interv Radiol. 2003;14(7):887-892 [DOI] [PubMed] [Google Scholar]
- 35. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease). J Am Coll Cardiol. 2006;47(6):1239-1312 [DOI] [PubMed] [Google Scholar]
- 36. Rundback JH, Sacks D, Kent KC, et al. ; AHA Councils on Cardiovascular Radiology, High Blood Pressure Research, Kidney in Cardiovascular Disease, Cardio-Thoracic and Vascular Surgery, and Clinical Cardiology; Society of Interventional Radiology FDA Device Forum Committee; American Heart Association Guidelines for the reporting of renal artery revascularization in clinical trials. Circulation. 2002;106(12):1572-1585 [DOI] [PubMed] [Google Scholar]
- 37. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206-1252 [DOI] [PubMed] [Google Scholar]
- 38. Napoli V, Pinto S, Bargellini I, et al. Duplex ultrasonographic study of the renal arteries before and after renal artery stenting. Eur Radiol. 2002;12(4):796-803 [DOI] [PubMed] [Google Scholar]
- 39. Radermacher J, Chavan A, Bleck J, et al. Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N Engl J Med. 2001;344(6):410-417 [DOI] [PubMed] [Google Scholar]
- 40. Grenier N, Trillaud H, Combe C, et al. Diagnosis of renovascular hypertension: feasibility of captopril-sensitized dynamic MR imaging and comparison with captopril scintigraphy. AJR Am J Roentgenol. 1996;166(4):835-843 [DOI] [PubMed] [Google Scholar]
- 41. Grenier N, Hauger O, Cimpean A, Perot V. Update of renal imaging. Semin Nucl Med. 2006;36(1):3-15 [DOI] [PubMed] [Google Scholar]
- 42. Zhang HL, Sos TA, Winchester PA, Gao J, Prince MR. Renal artery stenosis: imaging options, pitfalls, and concerns. Prog Cardiovasc Dis. 2009;52(3):209-219 [DOI] [PubMed] [Google Scholar]
- 43. Ohta Y, Fujii K, Arima H, et al. Increased renal resisitive index in atherosclerosis and diabetic nephropathy assessed by Doppler sonography. J Hypertens. 2005;23:1905-1911 [DOI] [PubMed] [Google Scholar]
- 44. Mukherjee D, Bhatt DL, Robbins M, et al. Renal artery end diastolic velocity and renal artery resistance index as predictors of outcome after renal stenting. Am J Cardiol. 2001;88(9):1064-1066 [DOI] [PubMed] [Google Scholar]
- 45. Olin JW, Piedmonte MR, Young JR, DeAnna S, Grubb M, Childs MB. The utility of duplex ultrasound scanning of the renal arteries for diagnosing significant renal artery stenosis. Ann Intern Med. 1995;122(11):833-838 [DOI] [PubMed] [Google Scholar]
- 46. Kohler TR, Zierler RE, Martin RL, et al. Noninvasive diagnosis of renal artery stenosis by ultrasonic duplex scanning. J Vasc Surg. 1986;4(5):450-456 [DOI] [PubMed] [Google Scholar]
- 47. Taylor DC, Kettler MD, Moneta GL, et al. Duplex ultrasound scanning in the diagnosis of renal artery stenosis: a prospective evaluation. J Vasc Surg. 1988;7(2):363-369 [PubMed] [Google Scholar]
- 48. Helenon O, el Rody F, Correas JM, et al. Color Doppler US of renovascular disease in native kidneys. Radiographics. 1995;15(4):833-854 [DOI] [PubMed] [Google Scholar]
- 49. Williams GJ, Macaskill P, Chan SF, et al. Comparative accuracy of renal duplex sonographic parameters in the diagnosis of renal artery stenosis: paired and unpaired analysis. AJR Am J Roentgenol. 2007;188(3):798-811 [DOI] [PubMed] [Google Scholar]
- 50. Stavros AT, Parker SH, Yakes WF, et al. Segmental stenosis of the renal artery: pattern recognition of tardus and parvus abnormalities with duplex sonography. Radiology. 1992;184(2):487-492 [DOI] [PubMed] [Google Scholar]
- 51. Kliewer MA, Tupler RH, Carroll BA, et al. Renal artery stenosis: analysis of Doppler waveform parameters and tardus-parvus pattern. Radiology. 1993;189(3):779-787 [DOI] [PubMed] [Google Scholar]
- 52. Conkbayir I, Yucesoy C, Edguer T, Yanik B, Yasar Ayaz U, Hekimoglu B. Doppler sonography in renal artery stenosis: an evaluation of intrarenal and extrarenal imaging parameters. Clin Imaging. 2003;27(4):256-260 [DOI] [PubMed] [Google Scholar]
- 53. Schwerk WB, Restrepo IK, Stellwaag M, Klose KJ, Schade-Brittinger C. Renal artery stenosis: grading with image-directed Doppler US evaluation of renal resistive index. Radiology. 1994;190(3):785-790 [DOI] [PubMed] [Google Scholar]
- 54. Baxter GM, Aitchison F, Sheppard D, et al. Colour Doppler ultrasound in renal artery stenosis: intrarenal waveform analysis. Br J Radiol. 1996;69(825):810-815 [DOI] [PubMed] [Google Scholar]
- 55. Eklof H. A prospective comparsion of duplex ultrasonography, captopril renography, MRA and CTA in assessing renal artery stenosis. Acta Radiol. 2006;47(8):764-774 [DOI] [PubMed] [Google Scholar]
- 56. Rountas C, Vlychou M, Vassiou K, et al. Imaging modalities for renal artery stenosis in suspected renovascular hypertension: prospective intraindividual comparison of color Doppler US, CT angiography, GD-enhanced MR angiography, and digital substraction angiography. Ren Fail. 2007;29(3):295-302 [DOI] [PubMed] [Google Scholar]
- 57. Glockner JF, Vrtiska TJ. Renal MR and CT angiography: current concepts. Abdom Imaging. 2007;32(3):407-420 [DOI] [PubMed] [Google Scholar]
- 58. Thadhani RI, Camargo CA, Jr, Xavier RJ, Fang LS, Bazari H. Atheroembolic renal failure after invasive procedures: natural history based on 52 histologically proven cases. Medicine (Baltimore). 1995;74(6):350-358 [DOI] [PubMed] [Google Scholar]
- 59. White CW, Wright CB, Doty DB, et al. Does visual interpretation of the coronary arteriogram predict the physiologic importance of a coronary stenosis. N Engl J Med. 1984;310(13):819-824 [DOI] [PubMed] [Google Scholar]
- 60. Mangiacapra F, Trana C, Sarno G, et al. Translesional pressure gradients to predict blood pressure response after renal artery stenting in patients with renovascular hypertension. Circ Cardiovasc Interv. 2010;3(6):537-542 [DOI] [PubMed] [Google Scholar]
- 61. De Bruyne B, Manoharan G, Pijls NH, et al. Assessment of renal artery stenosis severity by pressure gradient measurements. J Am Coll Cardiol. 2006;48(9):1851-1855 [DOI] [PubMed] [Google Scholar]
- 62. Hricik DE, Browning PJ, Kopelman R, Goorno WE, Madias NE, Dzau VJ. Captopril-induced functional renal insufficiency in patients with bilateral renal-artery stenoses or renal-artery stenosis in a solitary kidney. N Engl J Med. 1983;308(7):373-376 [DOI] [PubMed] [Google Scholar]
- 63. Dworkin LD, Cooper CJ. Renal-artery stenosis. N Engl J Med. 2009;361(20):1972-1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bokhari SW, Faxon DP. Current advances in the diagnosis and treatment of renal artery stenosis. Rev Cardiovasc Med. 2004;5(4):204-215 [PubMed] [Google Scholar]
- 65. Olin JW. Role of duplex ultrasonography in screening for significant renal artery disease. Urol Clin North Am. 1994;21:215-226 [PubMed] [Google Scholar]
- 66. van Jaarsveld BC, Krijnen P, Pieterman H, et al. ; Dutch Renal Artery Stenosis Intervention Cooperative Study Group The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. N Engl J Med. 2000;342(14):1007-1014 [DOI] [PubMed] [Google Scholar]
- 67. Blum U, Krumme B, Flugel P, et al. Treatment of ostial renal-artery stenoses with vascular endoprostheses after unsuccessful balloon angioplasty. N Engl J Med. 1997;336(7):459-465 [DOI] [PubMed] [Google Scholar]
- 68. Dorros G, Jaff M, Mathiak L, et al. Four-year follow-up of Palmaz-Schatz stent revascularization as treatment for atherosclerotic renal artery stenosis. Circulation. 1998;98(7):642-647 [DOI] [PubMed] [Google Scholar]
- 69. Leertouwer TC, Gussenhoven EJ, Bosch JL, et al. Stent placement for renal arterial stenosis: where do we stand? A meta-analysis. Radiology. 2000;216(1):78-85 [DOI] [PubMed] [Google Scholar]
- 70. van de Ven PJ, Kaatee R, Beutler JJ, et al. Arterial stenting and balloon angioplasty in ostial atherosclerotic renovascular disease: a randomised trial. Lancet. 1999;353(9149):282-286 [DOI] [PubMed] [Google Scholar]
- 71. Dorros G, Jaff M, Mathiak L, He T. Multicenter Palmaz stent renal artery stenosis revascularization registry report: four-year follow-up of 1,058 successful patients. Catheter Cardiovasc Interv. 2002;55(2):182-188 [DOI] [PubMed] [Google Scholar]
- 72. Rocha-Singh KJ, Ahuja RK, Sung CH, Rutherford J. Long-term renal function preservation after renal artery stenting in patients with progressive ischemic nephropathy. Catheter Cardiovasc Interv. 2002;57(2):135-141 [DOI] [PubMed] [Google Scholar]
- 73. Rocha-Singh K, Jaff MR, Rosenfield K. Evaluation of the safety and effectiveness of renal artery stenting after unsuccessful balloon angioplasty: the ASPIRE-2 study. J Am Coll Cardiol. 2005;46(5):776-783 [DOI] [PubMed] [Google Scholar]
- 74. Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009;361(20):1953-1962 [DOI] [PubMed] [Google Scholar]
- 75. Bax L, Woittiez AJ, Kouwenberg HJ, et al. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med. 2009;150(12):840-848, W150-W151 [DOI] [PubMed] [Google Scholar]
- 76. Cooper CJ, Murphy TP, Matsumoto A, et al. Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: rationale and design of the CORAL trial. Am Heart J. 2006;152(1):59-66 [DOI] [PubMed] [Google Scholar]
- 77. Jahraus CD, Meigooni AS. Vascular brachytherapy: a new approach to renal artery in-stent restenosis. J Invasive Cardiol. 2004;16(4):224-227 [PubMed] [Google Scholar]
- 78. Otah KE, Alhaddad IA. Intravascular ultrasound-guided cutting balloon angioplasty for renal artery stent restenosis. Clin Cardiol. 2004;27(10):581-583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Granillo GA, van Dijk LC, McFadden EP, Serruys PW. Percutaneous radial intervention for complex bilateral renal artery stenosis using paclitaxel eluting stents. Catheter Cardiovasc Interv. 2005;64(1):23-27 [DOI] [PubMed] [Google Scholar]
- 80. Henry M, Henry I, Klonaris C, et al. Renal angioplasty and stenting under protection: the way for the future? Catheter Cardiovasc Interv. 2003;60(3):299-312 [DOI] [PubMed] [Google Scholar]
- 81. Balzer KM, Pfeiffer T, Rossbach S, et al. Prospective randomized trial of operative vs. interventional treatment for renal artery ostial occlusive disease (RAOOD). J Vasc Surg. 2009;49(3):667-675 [DOI] [PubMed] [Google Scholar]